Abstract
Autophagy is a highly regulated intracellular degradation process by which cells remove cytosolic long-lived proteins and damaged organelles. The mitochondrial permeability transition (MPT) results in mitochondrial depolarization and increased reactive oxygen species production, which can trigger autophagy. Therefore, we hypothesized that the MPT may have a role in signaling autophagy in cardiac cells. Mitochondrial membrane potential was lower in HL-1 cells subjected to starvation compared to cells maintained in full medium. Mitochondrial membrane potential was preserved in starved cells treated with cyclosporin A (CsA), suggesting the MPT pore is associated with starvation-induced depolarization. Starvation-induced autophagy in HL-1 cells, neonatal rat cardiomyocytes and adult mouse cardiomyocytes was inhibited by CsA. Starvation failed to induce autophagy in CypD-deficient murine cardiomyocytes, whereas in myocytes from mice overexpressing CypD the levels of autophagy were enhanced even under fed conditions. Collectively, these results demonstrate a role for CypD and the MPT in the initiation of autophagy. We also analyzed the role of the MPT in the degradation of mitochondria by biochemical analysis and electron microscopy. HL-1 cells subjected to starvation in the presence of CsA had higher levels of mitochondrial proteins (by Western blot), more mitochondria and less autophagosomes (by electron microscopy) then cells starved in the absence of CsA. Our results suggest a physiologic function for CypD and the MPT in the regulation of starvation-induced autophagy. Starvation-induced autophagy regulated by CypD and the MPT may represent a homeostatic mechanism for cellular and mitochondrial quality control.
Keywords: autophagy, cardiac myocyte, cyclophilin D, mitochondrial permeability transition
Introduction
Mitochondrial permeability transition (MPT) is a common response to ischemia-reperfusion injury, particularly to stresses such as reactive oxygen species (ROS) and calcium overload. MPT makes the inner mitochondrial membrane permeable to solutes of up to 1,500 Da,1 resulting in depolarization due to dissipation of the electrochemical gradient, which in turn causes ATP synthase to operate in reverse, consuming ATP.2 The immunosuppressant cyclosporin A (CsA) blocks the formation of or conductance through MPT pores by inhibiting cyclophilin D (CypD).3,4 CsA inhibition of the MPT occurs independent of its inhibition of calcineurin, which mediates CsA’s immunosuppressive effects.5,6,7,8
Originally, the MPT pore was proposed to be composed of the voltage-dependent anion channel in the outer membrane,9,10 the adenine nucleotide translocase in the inner membrane,11,12 plus CypD in the matrix.13,14 Of the three components, genetic evidence has only supported a role for CypD.15,16,17,18,19 CypD is a member of the peptidylprolyl isomerase family, which catalyze the rotation of proline peptide bonds, thereby inducing a conformational change in the target protein.20 Inhibition of CypD’s isomerase activity by CsA, or its non-immunosuppressive analogs, inhibits MPT and cell death in numerous cell culture systems.3,17,21,22 CypD-deficient mitochondria and cells are resistant to Ca2+ and oxidative stress-induced MPT and cell death.15,16,17,18 Mitochondria of hepatocytes from CypD knockout mice still undergo MPT, but only at substantially higher concentrations of calcium and the addition of CsA did not extend the calcium tolerance.15,16 CypD-null mice are significantly more resistant to myocardial ischemia-reperfusion injury than their wild type counterparts.15,17,23
Autophagy is now well recognized as a major intracellular pathway for degrading long-lived cytosolic proteins and damaged organelles.24,25,26 When autophagy is initiated, cytoplasmic constituents are sequestered in the autophagosome, a closed double membrane vacuole. The autophagosome then fuses with a lysosome, forming an autolysosome in which the contents are degraded and recycled for metabolism or protein synthesis.27 Genetic studies have demonstrated a vital role for autophagy in physiological and pathological events.28,29,30 At the basal level, autophagy is necessary to control the quality of proteins and organelles in order to maintain cellular functions. It also has a role in cell differentiation and development,31,32 as well as in cellular responses to a variety of stresses.27,33 Knockout of autophagy genes leads to multiple cellular abnormalities including formation of concentric membranous structures and deformed mitochondria, and accumulation of ubiquitin-positive aggregates during starvation.34,35 Mitochondria are known to be degraded by the autophagosomal-lysosomal pathway, but the basis on which individual mitochondria are targeted for autophagy is unknown. Although some mitochondria may be selected at random for autophagy,36 non-random selection also appears to occur. Induction of autophagy in rat hepatocytes by serum deprivation and glucagon causes an increase of spontaneously depolarizing mitochondria, and these mitochondria are sequestered by autophagosomes.7,8 Also, in pancreatic beta cells, fission generates asymmetric daughter mitochondria: one subpopulation has increased membrane potential and high probability of fusion, while the other has decreased potential and reduced probability of fusion.37 Fission followed by selective fusion segregates dysfunctional mitochondria and permits their removal by autophagy. One of the primary events of mitochondrial permeability transition is mitochondrial depolarization; therefore, it is possible that the MPT is the trigger that signals mitochondria to be removed by autophagy. Given this, the aim of our work was to investigate the role of the MPT in the induction of autophagy in cardiac cells.
Materials and methods
Materials
Chloroquine, rapamycin, and bafilomycin A1 were acquired from EMD Biosciences. CsA was acquired from Sigma. BODIPY® TR cadaverine and rhodamine 123 were from Invitrogen. Liberase blendzyme was from Roche.
Generation of Ppif null mice and cyclophilin D transgenic mice
The mice were a kind gift of Jeffery D. Molkentin. Ppif null mice and CypD overexpressing mice were generated previously.15 Ppif null mice (Ppif−/−, CypD knock out) were generated in C75/BL6 background and wild type controls for this group were C75/BL6 (Ppif+/+). CypD overexpressing mice were generated in FVB/N background and the corresponding wild types were FVB/N. All animal procedures conformed with the “Principles of laboratory animal care” (NIH publication No. 86-23, revised 1985) and were approved by the Animal Care Committee of the San Diego State University in San Diego, CA.
HL-1 cell culture
The murine atria derived cell line, HL-1, was kindly provided by Professor William C. Claycomb.38 HL-1 cells were maintained in flasks coated with gelatin/fibronectin, at 37°C in an atmosphere of 5% CO2 in Claycomb medium (Sigma) supplemented with 2 mM L-glutamine, 10% fetal bovine serum, 0.1 mM norepinephrine, 100 U/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL amphotericin B.
Plasmid construction
ECFP was excised from pECFP-N1 at restriction sites BamHI and NotI. DsRed2 was removed from the pDsRED2-Mito vector (Clontech) at restriction sites BamHI and NotI, and replaced with ECFP to generate the mito-ECFP vector. The fluorescent protein ECFP is fused to the COX IV targeting sequence, thus targeting it to the mitochondrial inner membrane.39
Cell transfection
HL-1 cells were transfected with mito-ECFP, using Effectene (Qiagen) according to the manufacturer's instructions, achieving approximately 40% transfection efficiency.
Neonatal cardiomyocyte culture
Neonatal rat cardiomyocytes were prepared by collagenase digestion as previously described,40 with the following changes: 100 µM bromodeoxyuridine was added to inhibit proliferation of non myocytes. Cells were plated on fibronectin-coated chamber slides. After 24 h, they were infected with adenovirus for GFP-LC3 (MOI=10).
Adult mouse cardiomyocyte isolation
Mice (Ppif null mice, cyclophilin D transgenic mice, and wild type mice of corresponding strains) were anesthetized with sodium pentobarbital (50 mg/kg, i.p.), and injected with heparin (350 U/kg, i.p.). After a surgical plane of anesthesia was reached, the heart was rapidly excised and placed in ice-cold Ca2+-free perfusion solution (in mM: 113 NaCl, 4.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4.7H2O, 0.032 phenol red, 12 NaHCO3, 10 KHCO3, 10 HEPES, 30 taurine, 5.5 glucose, 10 2,3-butanedione monoxime). The aorta was quickly cannulated and the heart was perfused retrogradely with Ca2+-free perfusion solution followed by perfusion with digestion buffer containing 0.25 mg/mL liberase blendzyme 1 (Roche Applied Science, USA), 0.14 mg/mL trypsin (Invitrogen, USA) and 12.5 µM CaCl2 in perfusion buffer. After tissue digestion, the heart was removed from the perfusion system, the ventricles were minced in digestion buffer, and then filtered through a cell strainer (Sigma). Stop solution 1 (10% bovine calf serum, 12.5 µM CaCl2) was added to the cardiomyocytes, and cells were pelleted by gravity for 10 minutes. The resulting cell pellet was resuspended in stop solution 2 (5% bovine calf serum, 12.5 µM CaCl2), and calcium was gradually reintroduced up to a concentration of 1 mM. The cells were centrifuged at 100xg for 1 min, and suspended in plating medium (minimum essential medium, 5% bovine calf serum, 10 mM 2,3-butanedione monoxime, 100 U/mL penicillin, and 2 mM L-glutamine). The cardiomyocytes (6 × 104) were plated in permanox chamber slides coated with mouse laminin (10 µg/mL). After 2 hr, the plating medium was removed and replaced by culture medium (minimum essential medium, 0.1 mg/mL bovine serum albumin, 100 U/mL penicillin, and 2 mM L-glutamine).
Mitochondrial potential
Cells were incubated in Claycomb media supplemented with 1µg/mL rhodamine 123, for 30 minutes, at 37°C, after which they were washed twice with media and incubated for 30 minutes, at 37°C, in the absence of rhodamine 123. Cells were washed once with PBS and fluorescence levels were read in a SPECTRAmax fluorimeter (Molecular Devices) (λex=490 nm, λem=533 nm).
Quantification of autophagy
1. GFP-LC3 adenovirus infection
Formation of autophagosomes was quantified via fluorescence imaging of GFP LC3 in cells.41 Cells were infected for 2h with adenovirus encoding GFP-LC3 at a concentration of 10 plaque-forming units/cell, and 18 hr later cells were subjected to the indicated experimental conditions. Cells were fixed with 4% formaldehyde in phosphate buffered saline, pH 7.4, for 15 min. To quantify the number of GFP LC3 puncta in a single cell, Z stack images of single GFP LC3 expressing cells were captured using 60X oil objective. Maximal projection images were produced, and the number of GFP LC3 puncta per cell was determined using IMAGEJ software (NIH, Bethesda, MD). For population analysis, cells were inspected at 60X magnification and classified as either having predominantly diffuse GFP-LC3 fluorescence (0–30 GFP-LC3 dots/cell) or having numerous punctate GFP-LC3 structures (>30 GFP-LC3 dots/cell), representing autophagosomes. Previous studies established a clear bimodal distribution, allowing us to use the threshold of 30 dots per cell as a cutoff.42,43 At least 200 cells were scored in each of three or more independent experiments.
2. BODIPY TR cadaverine assay
We used a cadaverine derivative (BODIPY TR cadaverine, Invitrogen) to quantify autophagy in cells, as described previously, with slight changes.44 Cells were incubated with 125 nM BODIPY TR cadaverine for 10 minutes, washed four times with PBS and lysed in 10 mM Tris-Cl pH 8, 0.1 % Triton X-100. Fluorescence levels were read in a SPECTRAmax fluorimeter (Molecular Devices) (λex=588 nm, λem=616 nm). Fluorescence levels were normalized for cell number by adding 0.2 mM ethidium bromide and reading DNA fluorescence (λex=530 nm, λem=590 nm).
Widefield fluorescence microscopy
Cells were observed through a Nikon TE300 fluorescence microscope (Nikon, Melville, NY) equipped with a x60 Plan Apo objective (1.4 NA oil immersion lenses; Nikon, Japan), a motorized Z-stage (ProScanII, Prior Scientific, Rockland, MA), a cooled CCD camera (Orca-ER, Hamamatsu, Bridgewater,7NJ), and automated excitation and emission filter wheels controlled by a LAMBDA 10-2 (Sutter Instrument, Novato, CA) operated by MetaMorph 6.2r4 (Molecular Devices Co., Downington, PA). Fluorescence was excited through an excitation filter for fluorescein isothiocyanate (HQ480/x40). Fluorescent light was collected via a polychroic beamsplitter (61002 bs) and an emission filter for fluorescein isothiocyanate (HQ535/50 m). All filters were from Chroma.
For high-resolution microscopy, Z-stacks of representative cells were acquired at 60X magnification with 0.3 µm increments. Acquired wide-field Z-stacks were routinely deconvolved using a 3D blind deconvolution algorithm (AutoQuant) to maximize spatial resolution. Representative cells are shown as maximum projections of 3D-deconvoluted Z-stacks, unless stated otherwise.
Immunoblotting
Cells were harvested by scraping and centrifugation at 250xg for 5 minutes at 4°C. To prepare whole cell lysates, cell pellets were resuspended in lysis buffer [50 mM Tris-Cl pH 7.4, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100, and protease inhibitor cocktail (Roche)] and left on ice for 30 minutes. The cell extracts were centrifuged at 20,000xg for 10 minutes to remove cellular debris. After addition of sample buffer and reducing agent (BioRad) samples were incubated at 95°C for 5 minutes. Samples were resolved in 10–20% Tris-glycine SDS-PAGE and transferred to nitrocellulose membranes. Immunodetection was performed using antibodies against GFP (1:1000, Sigma), cytochrome c oxidase IV (1:1000, Invitrogen) and actin (1:1000, Sigma).
Electron microscopy
Cells were prepared for electron microscopy as previously described.45 Briefly, cells were incubated and fixed on ice in 2.5% formaldehyde, 2.5% glutaraldehyde, 0.1 M sodium cacodylate, 5 mM Ca+2, 2.5 % sucrose, pH 7.4. Cells were washed three times in ice-cold 0.1 M sodium cacodylate containing 2.5 % sucrose and 5 mM Ca+2 for 15 min and post-fixed in Palade's OsO4 46 for one hour, on ice, in the dark. After washing three times for 10 min with 0.06 M veronol acetate, and two times for 3 min with distilled water, the fixed cell pellets were stained and stabilized en bloc with Kellenberger's uranyl acetate at room temperature in the dark and dehydrated in an ice-cold ethanol series of 30%, 50%, 70%, 95% and 100% for 10 min each. The cells were then treated three times for 15 min in fresh 100% acetone at room temperature and infiltrated in a well-mixed Epon-acetone resin series of 33% for 3h, 66% for 6h and 100% at least overnight with agitation at room temperature. Afterwards, the samples were allowed to polymerize in 100 % Epon blocks at 60–80°C for at least 48 hours. Thin sections of approximately 70 nm were cut using a diamond knife and post-stained with 2% uranyl acetate and Sato lead before examination in an FEI Tecnai 12 transmission electron microscope operated at 120 kV. Images were recorded on a Tietz 214 CCD camera after which images were assembled in Adobe Photoshop CS version 8 with only linear adjustments in brightness and contrast.
ATP quantification
ATP was quantified using the CellTiter-Glo® assay kit according to the manufacturer’s instruction. Briefly, the cells were incubated with CellTiter-Glo Reagent for 2 minutes under constant agitation to induce cell lysis and then for 10 minutes at 37°C. Luminescence was read in a LMax II384 luminometer (Molecular Devices).
Treatment protocols
Cells were treated for 3h 30min, at 37°C in an atmosphere of 5% CO2, as follows:
Control − fully supplemented medium;
Control + cyclosporin A − media supplemented with 5 µM CsA;
Control + rapamycin − media supplemented with 5 µM Rap;
Starvation − starvation media (in mM: 20 HEPES, 110 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.25 MgSO4, 1.2 CaCl2, 25 NaHCO3);
Starvation + cyclosporin A − starvation media supplemented with 5 µM CsA;
Starvation + bafilomycin A1 − starvation media supplemented with 50 nM Bf;
Starvation + cyclosporin A + bafilomycin A1 − starvation media supplemented with 5 µM CsA and 50 nM Bf;
Starvation + chloroquine − starvation media supplemented with 3 µM Cq.
Starvation + cyclosporin A + chloroquine − starvation media supplemented with 5 µM CsA and 3 µM Cq.
Statistics
Values are expressed as means ± SEM of at least three independent experiments unless stated otherwise. Significance (p<0.05) was calculated using Student t-test or ANOVA, with the help of GraphPad Prism, GraphPad Software®.
Results
Cyclosporin A prevented mitochondrial depolarization induced by starvation
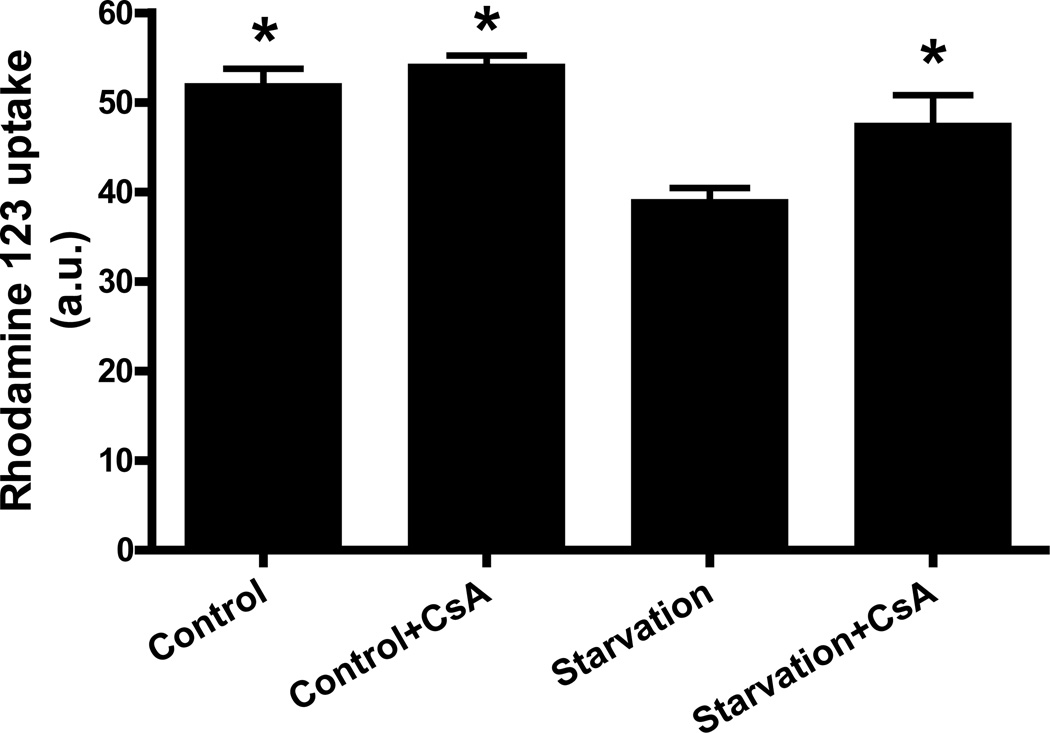
Lemasters’ group7,8 demonstrated that in rat hepatocytes MPT initiates mitochondrial depolarization after autophagic stimulation and the subsequent sequestration of mitochondria into autophagosomes. In the cardiac context the relationship between mitochondrial permeability transition and autophagy has not been established. Therefore, in order to investigate the role of MPT in the induction of autophagy in a murine atria derived cell line, we measured mitochondrial membrane potential in HL-1 cells subjected to starvation, a well known inducer of autophagy,47,48 in the presence or absence of an inhibitor of the pore, CsA. We observed that starvation caused a decrease in mitochondrial membrane potential. CsA prevented the decrease in mitochondrial membrane potential caused by starvation, suggesting the pore is associated with starvation-induced mitochondrial depolarization (Fig. 1). CsA had no effect on the mitochondrial membrane potential of cells maintained in full medium (Fig. 1).
Figure 1. Cyclosporin A prevented mitochondrial depolarization induced by starvation.
HL-1 cells were incubated in Claycomb media, Claycomb media supplemented with 5 µM CsA, starvation media or starvation media supplemented with 5 µM CsA. After the treatment, the cells were incubated in Claycomb media supplemented with 1µg/mL rhodamine 123, after which they were washed twice with media and incubated in the absence of rhodamine 123. Cells were washed once with PBS and fluorescence levels were read in a SPECTRAmax fluorimeter (Molecular Devices) (λex=490 nm, λem=533 nm). * p < 0.05 vs. starvation.
Cyclosporin A prevented starvation-induced increase in BODIPY TR cadaverine fluorescence
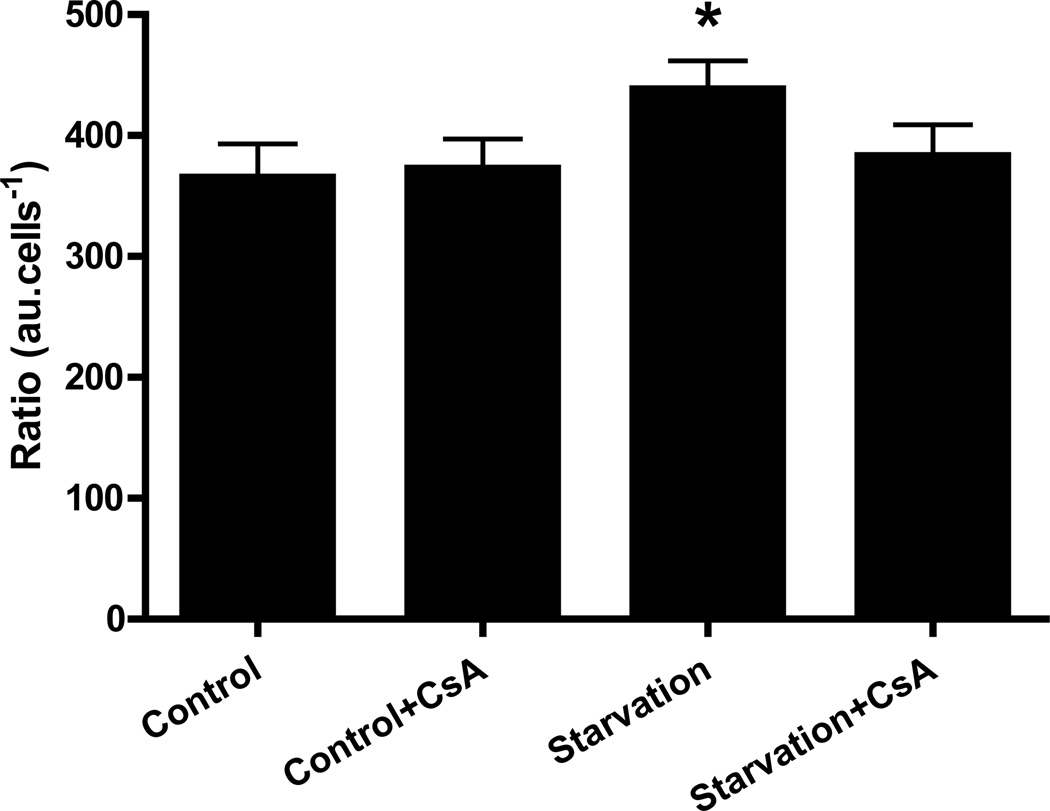
To determine if starvation-induced autophagy required function of cyclophilin D and the MPT, we subjected HL-1 cells to starvation in the presence or absence of CsA and measured autophagy using BODIPY TR cadaverine, a fluorescent dye taken up by autophagosomes and acidic compartments (Fig. 2). BODIPY TR cadaverine dye binding was higher in cells subjected to starvation than in cells maintained in full medium, confirming that autophagy was stimulated. Interestingly, when starvation was induced in the presence of CsA, dye binding was similar to that of control cells, indicating a suppression of autophagy. CsA had no effect in cells maintained in full medium, i.e. it did not affect basal levels of autophagy. These results suggest that cyclosporine-sensitive mitochondrial depolarization (presumably the MPT) is important in starvation-induced autophagy.
Figure 2. Cyclosporin A prevented starvation-induced increase in BODIPY TR cadaverine fluorescence.
HL-1 cells were incubated in Claycomb media, Claycomb media supplemented with 5 µM CsA, starvation media or starvation media supplemented with 5 µM CsA. After the treatment, the cells were incubated with 125 nM BODIPY TR cadaverine, lysed and fluorescence levels were read in a SPECTRAmax fluorimeter (λex=588 nm, λem=616 nm). * p < 0.05 vs. control.
Cyclosporin A reduced starvation-induced autophagy in HL-1 cells and isolated neonatal rat cardiomyocytes
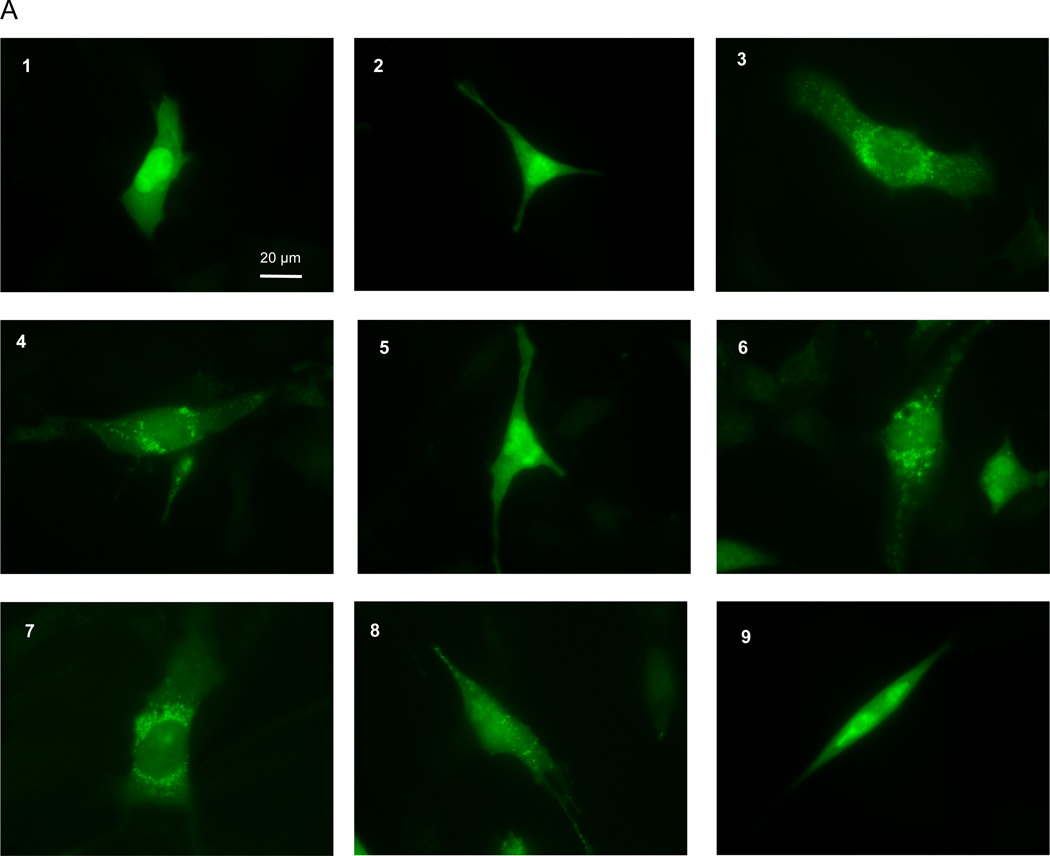
Upon induction of autophagy, LC3-I is conjugated with phosphatidylethanolamine, generating LC3-II, which is localized to the nascent autophagosome membrane at the initiation of autophagy.41 The amount of LC3-II is closely correlated with the number of autophagosomes, serving as a good indicator of autophagosome formation.41 Autophagosomes can be quantified via fluorescence imaging of GFP-LC3 in cells. In the presence of full media, HL-1 cells displayed cytosolic diffuse GFP-LC3 (Fig. 3A1), whereas in cells deprived of amino acids and glucose (starved) we observed numerous membrane-associated GFP-LC3 puncta (Fig. 3A4). When cells were starved in the presence of CsA, we observed a cytosolic diffuse GFP-LC3 distribution similar to control cells (Fig. 3A5). CsA had no effects on the basal levels of autophagy (Fig. 3A2). Rapamycin, which activates autophagy by blocking the mTOR pathway,49,50,51 caused the appearance of autophagosomes even in cells cultured in full medium (Fig. 3A3).
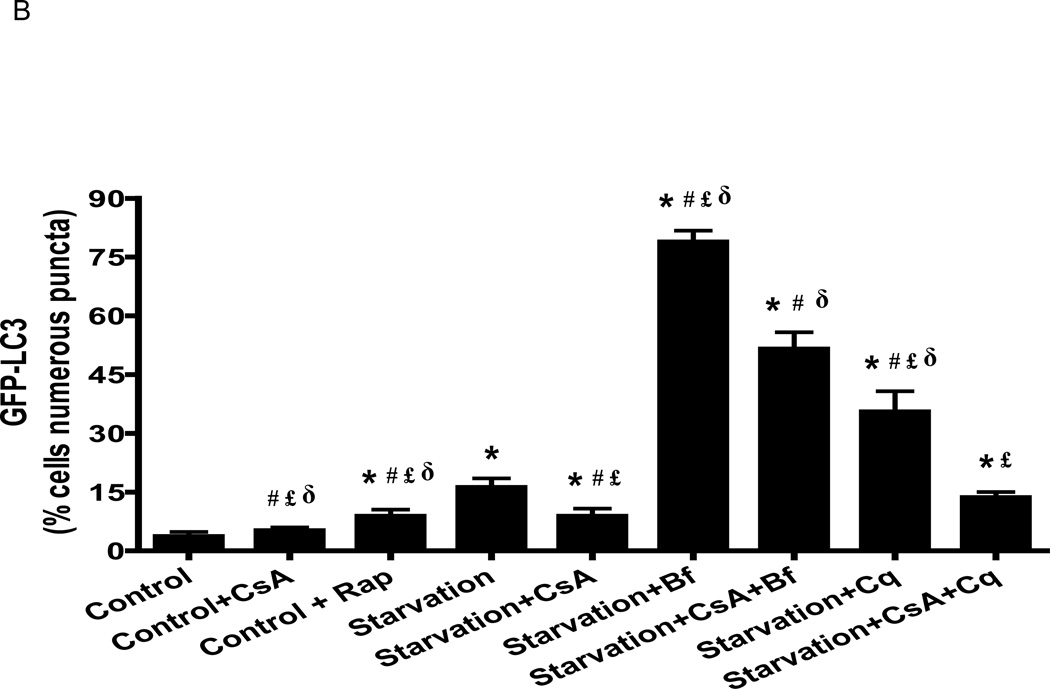
Figure 3. Cyclosporin A reduced starvation-induced autophagy in HL-1 cells.
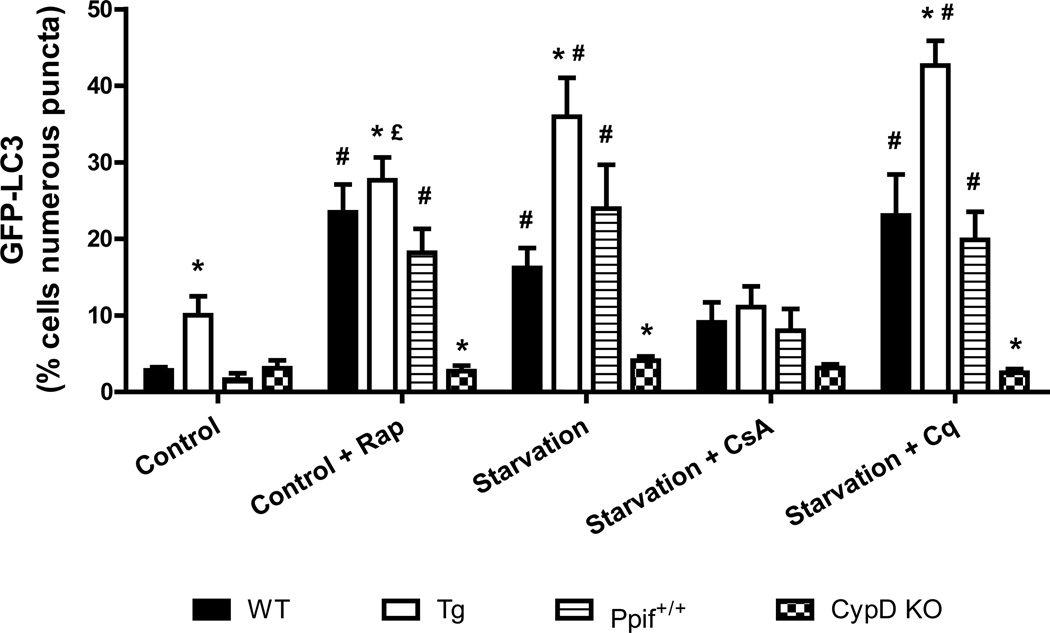
A) After infection with adenovirus encoding GFP-LC3, HL-1 cells were incubated in: 1) Claycomb media; 2) Claycomb media supplemented with 5µM CsA; 3) Claycomb media supplemented with 5 µM Rap; 4) starvation media; starvation media supplemented with: 5) 5 µM CsA; 6) 50 nM Bf; 7) 5 µM CsA and 50 nM Bf; 8) 3 µM Cq; 9) 5 µM CsA and 3 µM Cq. HL-1 cells were visualized in a Nikon TE300 fluorescence microscope (Nikon), using an excitation and emission filter for FITC. B) GFP-LC3 puncta quantification. * p < 0.05 vs. control. # p < 0.05 vs. starvation. £ p < 0.05 vs. starvation+CsA+Bf. δ p < 0.001 vs. starvation+CsA+Cq.
To determine whether CsA interfered with autophagic flux, we evaluated the effects of bafilomycin and chloroquine, which block the fusion of autophagosomes with lysosomes. CsA did not increase the percentage of cells with numerous puncta under fed conditions (5.3±1.4% vs. 3.8±1.0%, p=n.s.), while rapamycin and starvation increased it (9.0±1.6% and 16.3±2.2%, respectively). Bafilomycin and chloroquine increased the accumulation of autophagosomes in cells subjected to starvation (79.0±2.8%, 35.6±5.1%, respectively). Consistent with the dye binding assay described above, CsA decreased the percentage of cells with numerous puncta under starved conditions (reduced from 16.3±2.2% to 9.0±1.8%, p<0.05), and this effect was maintained in the presence of bafilomycin (79.0±2.8% vs 51.7±4.2%, p<0.05) and chloroquine (35.6±5.1% vs. 13.8±1.5, p<0.001) (Fig. 3B). The reduction in autophagosome abundance by CsA in starved cells both with and without bafilomycin or choloquine indicates that CsA suppresses autophagosome formation rather than accelerating flux (Fig. 3B). To investigate whether the results in HL-1 cells would be similar in primary cardiac cells, we performed the same experiment in isolated neonatal rat cardiomyocytes (Fig. 4) and adult mouse cardiomyocytes (Fig. 5), and obtained identical results.
Figure 4. Cyclosporin A reduced starvation-induced autophagy in isolated neonatal rat cardiomyocytes.
After infection with adenovirus encoding GFP-LC3, the cardiomyocytes were incubated in culture media, culture media supplemented with 5 µM Rap, starvation media, starvation media supplemented with: 5 µM CsA; 50 nM Bf; 5 µM CsA and 50 nM Bf; 3 µM Cq; 5 µM CsA and 3 µM Cq. The cardiomyocytes were visualized in a Nikon TE300 fluorescence microscope (Nikon), using an excitation and emission filter for FITC. * p < 0.01 vs. control. # p < 0.002 vs. starvation. £ p < 0.002 vs. starvation+CsA. δ p < 0.002 vs. starvation+CsA+Bf and starvation+CsA+Cq.
Figure 5. Cyclophilin D knock out mice do not exhibit starvation induced autophagy.
After infection with adenovirus encoding GFP-LC3, the cardiomyocytes were incubated in culture media, culture media supplemented with 5 µM Rap, starvation media, starvation media supplemented with: 5 µM CsA, 3 µM Cq. The cardiomyocytes were visualized in a Nikon TE300 fluorescence microscope (Nikon), using an excitation and emission filter for FITC. * p < 0.05 vs. respective wild type group. # p < 0.05 vs. control and starvation+CsA. £ p < 0.05 vs. starvation+Cq.
Reduction of cyclophilin D expression inhibited starvation-induced autophagy
To rule out a secondary effect of CsA on calcineurin or other cyclophilins, and to confirm the results obtained previously we used a genetic model. We isolated cardiomyocytes from transgenic mice overexpressing cyclophilin D, from cyclophilin D knockout mice, and from the respective wild type mice. The cardiomyocytes were infected with adenovirus encoding GFP-LC3 and were subjected to the same treatment as the other cell lines.. Wild type cardiomyocytes responded to starvation and rapamycin with an increase in the number of autophagosomes, and chloroquine further increased the number of autophagosomes in starved cells (Fig. 5 and supplemental Fig. S1). In contrast, cardiomyocytes isolated from CypD KO mice did not increase autophagy in response to starvation or rapamycin. The addition of chloroquine also failed to increase the number of autophagosomes in these cells. Interestingly, cardiomyocytes isolated from CypD overexpressing mice exhibited enhanced levels of autophagy even in full media compared to wild types (Fig. 5 and supplemental Fig. S1). These results implicate cyclophilin D and the MPT pore in the induction of autophagy during starvation.
Cyclosporin A prevented mitochondrial protein degradation induced by starvation
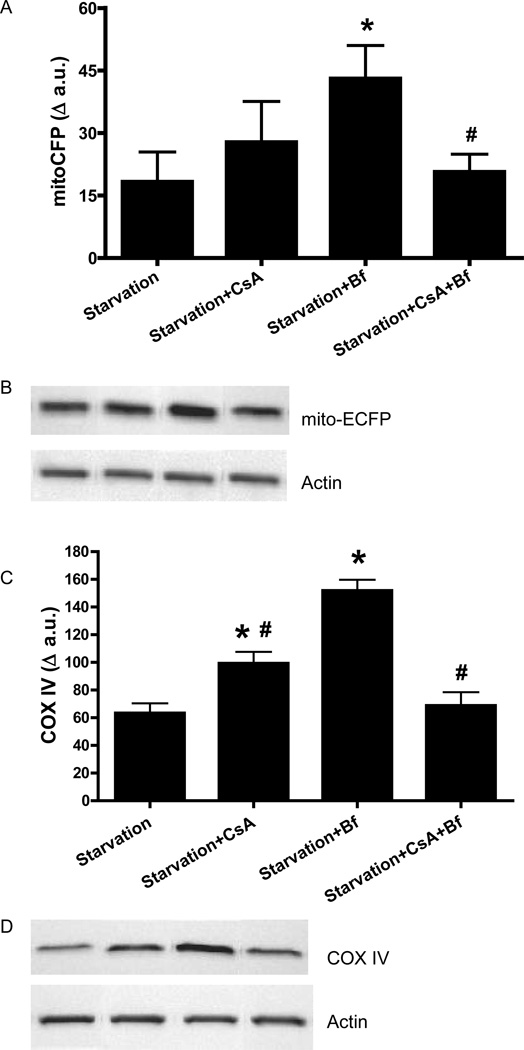
To determine whether the suppression of autophagy mediated by cyclophilin D affected mitochondrial protein degradation, HL-1 cells were transfected with mito-targeted ECFP and subjected to starvation in the absence or presence of CsA. There was less degradation of inner mitochondrial membrane proteins (cytochrome c oxidase IV and mito-ECFP) in cells starved in the presence of CsA (Fig. 6). Bafilomycin A1 also attenuated degradation of mitochondrial proteins (Fig. 6). Intriguingly, the combination of CsA and bafilomycin during starvation had no effect on levels of either mitoECFP or COX IV in comparison to starvation alone (Fig. 6). These results indicate that starvation induces mitochondrial protein degradation that is dependent upon cyclophilin D.
Figure 6. Cyclosporin A prevented mitochondrial protein degradation induced by starvation.
HL-1 cells were incubated in Claycomb media or starvation media in the presence of 5 µM CsA, 50 nM Bf A1 or 5 µM CsA and 50 nM Bf. After the treatment, immunoblots were performed for mito-ECFP and COX IV. A) mito-ECFP; B) Representative immunoblot for mito-ECFP - from left to right: starvation, starvation+CsA, starvation+Bf, starvation+CsA+Bf. C) COX IV; D) Representative immunoblot for COX IV - from left to right: starvation, starvation+CsA, starvation+Bf, starvation+CsA+Bf. Actin was used as a loading control. * p < 0.05 vs. starvation; # p < 0.05 vs. Starvation+bafilomycin A1.
Cyclosporin A prevented starvation-induced decrease in mitochondrial content and increase in autophagosome formation
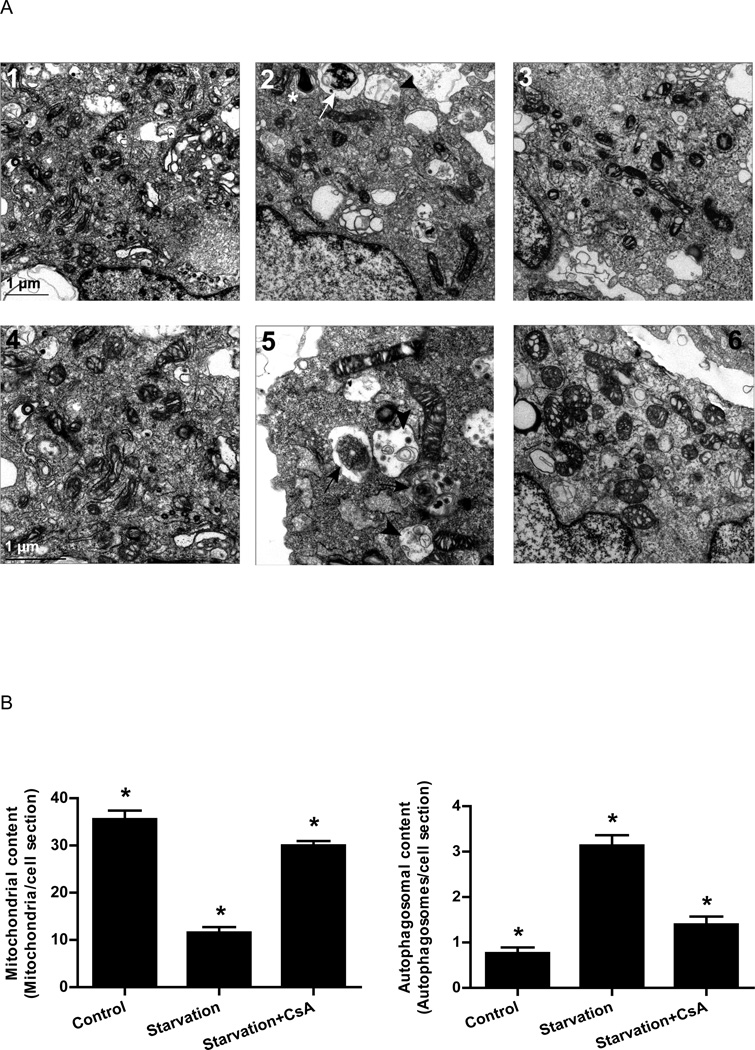
To determine whether the change in mitochondrial protein levels corrrelated with loss of mitochondrial mass, we used electron microscopy to assess the abundance of mitochondria in HL-1 cells cultured in full medium or subjected to starvation in the presence or absence of CsA. Cells were selected at low magnification based on presence of a central nucleus and complete perimeter of plasma membrane to avoid tangential sections. As shown in Fig. 7, we observed an average of 35 mitochondria per transverse cell section in full medium. However, after starvation, the number of mitochondria decreased dramatically to 11 per cell section, while the size of individual mitochondria did not change appreciably, arguing against a shift in fusion or fission. Starvation in the presence of CsA prevented the mitochondrial depletion, with an average of 30 mitochondria per cell section (Fig. 7).
Figure 7. Cyclosporin A prevented starvation-induced decrease in mitochondrial content and increase in autophagosome formation.
A) Electron micrographs of HL-1 cells incubated in: 1 and 4) Claycomb media; 2 and 5) starvation media; 3 and 6) starvation media supplemented with 5 µM CsA. Images 1, 2 and 3 are the same magnification. Images 4, 5 and 6 are higher magnification for better visualization of structures. Phagophores (*), autophagosomes (white arrow) and autolysosomes (black arrowhead) were observed in cells subjected to starvation. Black arrows indicate autophagosomes and black arrowheads indicate autolysosomal vesicles. B) Quantification of mitochondria and autophagosomes in thin slices from 30 different cells per experimental condition. * p < 0.05.
Consistent with the results obtained by GFP-LC3 adenoviral infection and fluorescence microscopy, and by cadaverine dye binding assay, electron microscopy confirmed that starvation caused an increase in the number of autophagosomes, which was attenuated when starvation was performed in the presence of CsA (Fig. 7). These results suggest that starvation triggers autophagy through a process dependent on cyclophilin D.
Cyclosporin A did not alter starvation-induced decrease in cellular ATP levels
Starvation decreased ATP cellular levels, effect that was not prevented by CsA (Fig. 8). Inhibiting the fusion of autophagosomes with lysosomes, with either chloroquine or bafilomycin, decreased ATP levels probably due to the accumulation of defective mitochondria which are not being removed by autophagy and whose ATP synthase is operating in reverse consuming ATP. The combination of CsA and bafilomycin during starvation caused an extreme decrease in cellular ATP levels. Given that protein synthesis rate is dependent on ATP levels, this result might explain why the combination of CsA and bafilomycin during starvation had no effect on the levels of either mitoECFP or COX IV in comparison to starvation alone, while separately they increased proteins levels (Fig. 6). Starvation in the presence of bafilomycin also caused a decrease in ATP levels which, according to our rationale, could cause a decrease in protein synthesis. However, bafilomycin did not decrease mitoECFP or COX IV levels, which is probably due to the fact that bafilomycin-induced decrease in ATP levels was not as profound as the one observed for starvation in the presence of both bafilomycin and CsA (Fig. 8).
Figure 8. Cyclosporin A did not prevent starvation-induced decrease in cellular ATP levels.
ATP was quantified using the CellTiter-Glo® assay kit according to the manufacturer’s instruction. * p < 0.0001 vs. control and control+Rap. # p < 0.0001 vs. starvation and starvation+CsA. £ p < 0.001 vs. starvation+Bf. δp < 0.01 vs. starvation+Bf and starvation+CsA+Bf.
Discussion
The basis by which individual mitochondria are targeted for autophagy is unknown. Although some mitochondria may be selected at random for autophagy,36 nonrandom selection also appears to occur. Lemasters’ group7,8 demonstrated that induction of autophagy in rat hepatocytes by serum deprivation and glucagon causes an increase in the number of spontaneously depolarizing mitochondria, and these mitochondria are sequestered by autophagosomes. Also, in pancreatic beta cells fission generates asymmetric mitochondria: some have high membrane potential and can proceed to successive rounds of fusion and fission, while others have low membrane potential and are targeted for autophagic destruction.37 One of the primary events of mitochondrial permeability transition is mitochondrial depolarization; therefore we hypothesized that MPT may serve as a signaling mechanism for mitochondrial removal by autophagy in cardiac cells.
In yeast cells, studies show that autophagic activity can result from impairing the mitochondrial transmembrane potential. Furthermore, mitochondrial damage results in the preferential removal of photodamaged mitochondria by autophagy.52 In mammals, Lemasters’ group53 demonstrated that in cultured rat hepatocytes remodeling is associated with increased levels of autophagy, and that the MPT plays a role in the remodeling. The authors have also shown that induction of autophagy in rat hepatocytes by serum deprivation and glucagon causes an increase in the number of spontaneously depolarizing mitochondria, and these mitochondria are sequestered by autophagosomes.7,8 CsA prevents this depolarization and blocks autophagosome proliferation.7,8 The authors concluded that MPT initiates mitochondrial depolarization after autophagic stimulation and the subsequent sequestration of mitochondria into autophagosomes. Other authors obtained similar results using nicotinamide-treated human fibroblasts.54 Nicotinamide-activated autophagy selectively removes mitochondria with low membrane potential, which is attenuated by treatment with cyclosporin A.54 However, studies performed by Twig and colleagues,37 using pancreatic beta cells and a lower concentration of CsA in comparison to the previous authors, argued against the MPT pore as the cause of the depolarization, because the depolarization was still observed in the presence of 1 µM cyclosporin A.
In accordance with previous results from Lemasters’ group in rat hepatocytes7,8 and contrast to the results from Twig et al. in pancreatic beta cells,37 we observed that in cardiac cells, starvation-induced autophagy causes mitochondrial depolarization which is prevented by cyclosporin A. These results suggest that the MPT pore underlies starvation-induced mitochondrial depolarization. Furthermore, our results demonstrate that cyclophilin D, a component of the MPT pore, is required for autophagy induced by starvation given that cyclosporin A prevented mitochondrial protein degradation, mitochondrial elimination and autophagy in starved myocytes. Moreover, cardiomyocytes from cyclophilin D deficient mice did not upregulate autophagy when subjected to starvation, in contrast to cardiomyocytes from wild type mice. One concern when using CsA, is that it blocks calcineurin, a cytoplasmic Ca2+/calmodulin-dependent protein phosphatase. CsA, as a calcineurin inhibitor, is capable of inhibiting DRP1 (dynamin related protein 1), which in turn will block autophagy, not by blocking the pore, but by blocking fission, which has been shown to be necessary for autophagy.37 However, several reports show that CsA inhibition of the MPT pore occurs independent of its inhibition of calcineurin because tacrolimus, another calcineurin inhibitor, does not block the MPT.5,6,7,8 Moreover, hepatocytes subjected to autophagic stimulation in the presence of tacrolimus do not show less autophagy, in contrast to CsA.7,8 And finally, NIM811, an analog of CsA that blocks the MPT, but does not inhibit calcineurin,55 blocks autophagic stimulation during nutrient deprivation to the same degree as CsA.8 The effect of CsA on autophagy observed in our experiments is not mediated by calcineurin, because we obtain the same results when using cardiomyocytes isolated from CypD deficient mice.
Chen et al.56 recently reported that superoxide is the form of reactive oxygen species required for induction of autophagy in response to starvation, and that modulation of Mn superoxide dismutase (SOD2), which is the mitochondrial isoform, or use of inhibitors of mitochondrial electron transfer complexes to increase superoxide production, could regulate autophagy. This implicates mitochondrial ROS in the regulation of starvation-induced autophagy, and supports a role for cyclophilin D and the MPT in regulating starvation-induced autophagy.
Twig et al.37 observed that mitochondrial fusion is followed by fission in which resulting daughter mitochondria are asymmetric with respect to membrane potential. Those with high membrane potential can proceed to successive rounds of fusion and fission, while those with low membrane potential are targeted for autophagic destruction. Hoppel’s group57 observed that mitochondrial dysfunction in heart failure is associated with loss of respirasomes (assembly of electron transfer complexes into functional supercomplexes). We hypothesize that supercomplex assembly and disassembly is a dynamic process, resulting in exclusion of damaged and dysfunctional components. This sorting process could result in spatial segregation of supercomplexes from damaged isolated subunits or complexes and may underlie asymmetric fission. More work will be required to test this notion and also how mitochondria with low membrane potential are recognized and selectively targeted for autophagic destruction.
MPT can lead to the release of apoptotic factors from the intermembrane space, including cytochrome c, apoptosis inducing factor, and Smac/Diablo.58 It is possible that the proteins released may signal a particular mitochondrion to be removed by mitophagy. This connection between autophagy and apoptosis may explain why many known inducers of apoptosis have been shown to also activate autophagy, such as etoposide in mouse embryonic fibroblasts,59 ceramide in breast and colon carcinoma,60 activation of the TRAIL receptor-2 in cancer cells,61 tumor necrosis factor alpha,43,62,63,64 serum/growth factor deprivation,65,66 staurosporin67 and lipopolysaccharide.43,64 Of special interest is Bnip3, which has been shown to be important for autophagy.68–72 In mouse embryo fibroblasts, HIF-1 induces expression of Bnip3, which triggers selective mitochondrial autophagy.71,72 Given that the factors released from the mitochondria when MPT occurs can also lead to apoptosis, there must be a very delicate balance between the extent of MPT and of apoptotic factors release and the ocurrence of autophagy versus apoptosis. In fact, the MPT pore has two conductance states: low and high. The low-conductance state allows the diffusion of small ions like Ca2+, and has a physiological role in the maintenance of cellular calcium homeostasis, while the high-conductance state allows the unselective diffusion of big molecules (up to 1500 Da) and is associated with cell death.73 With mild stresses, limited MPT onset may only increase mitophagy to rid cells of damaged mitochondria as a repair mechanism. With increasing stress, mitophagy may not be able to remove the majority of the dysfunctional mitochondria which release apoptotic factors, and apoptosis occurs. Finally, stresses which trigger wholesale MPT onset will result in rapid ATP depletion. Because of bioenergetic failure, neither autophagy nor apoptosis can progress, and only necrotic cell death ensues.74
The degree of mitochondrial depletion that we observed after just 3.5 hr of starvation was dramatic (although each image reflects only a slice through the cell and obviously underestimates the total number of mitochondria per cell). The observed 68% reduction in mitochondrial number suggests that mitochondrial turnover is rapid and robust, and that starvation triggers the selective removal of mitochondria by mitophagy.. However, it is possible that starvation through the regulation of cyclophilin D and the MPTP, triggers autophagy that is nonspecific, and in which mitochondria are simply innocent bystanders and not the selective target of autophagy. Although these cell-based studies of mitochondrial depletion will need to be confirmed in vivo, the findings are consistent with the study by Young’s group, who showed that nuclear-encoded mitochondrial gene expression varies over the circadian cycle, consistent with episodic starvation during the sleep phase.75 The reduction in the number of mitochondria during periods of inactivity would lower the amount of oxygen consumption, as well as the amount (typically 1–2%) that is converted to superoxide and then to hydrogen peroxide, thereby lowering oxidative stress.76 Removal of dysfunctional mitochondria prevents further cellular injury due to mitochondrial ROS formation, calcium overloading, futile ATP consumption, etc. The opportunity to cull out the mitochondria with the lowest threshold for MPT pore opening would support mitochondrial quality control, enabling replacement of electron transfer complexes with newly synthesized components. These findings provide strong evidence in support of the concept of mitochondrial quality control, and reveal a physiologic role for cyclophilin D and the MPT in maintaining cellular and mitochondrial homeostasis.
Supplementary Material
ACKNOWLEGEMENTS
This work was supported by R01 HL087023 (ABG), NIH R01 HL60590 and NIH R01 AG33283 (RAG), and a postdoctoral fellowship from the Portuguese Foundation for Science and Technology of Portugal (SFRH/BPD/40454/2007) (RSC). We acknowledge San Diego State University electron microscopy facility for their support.
Abbreviations
- Bf
bafilomycin A1
- COX
cytochrome c oxidase IV
- Cq
chloroquine
- CypD
cyclophilin D
- CsA
cyclosporin A
- DRP1
dynamin related protein 1
- MOI
multiplicity of infection
- MPT
mitochondrial permeability transition
- Rap
rapamycin
- ROS
reactive oxygen species
Footnotes
DISCLOSURES: None of the authors have any conflicts of interest relevant to this manuscript. RAG is CEO of Radical Therapeutix, Inc.
References
- 1.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 2.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93(4):292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 3.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+- dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- 4.Fournier N, Ducet G, Crevat A. Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr. 1987;19:297–303. doi: 10.1007/BF00762419. [DOI] [PubMed] [Google Scholar]
- 5.Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J Biol Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths EJ, Halestrap AP. Further evidence that cyclosporin A protects mitochondria from calcium overload by inhibiting a matrix peptidyl-prolyl cis-trans isomerase. Implications for the immunosuppressive and toxic effects of cyclosporin. Biochem J. 1991;274:611–614. doi: 10.1042/bj2740611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–2278. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Enriquez S, Kim I, Currin RT, Lemasters JJ. Tracker dyes to probe mitochondrial autophagy (mitophagy) in rat hepatocytes. Autophagy. 2006;2:39–46. doi: 10.4161/auto.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szabo I, De Pinto V, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules II. The electrophysiological properties of VDAC are compatible with those of the mitochondrial megachannel. FEBS Lett. 1993;330(2):206–210. doi: 10.1016/0014-5793(93)80274-x. [DOI] [PubMed] [Google Scholar]
- 10.Szabo I, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. I. Binary structure and voltage dependence of the pore. FEBS Lett. 1993;330(2):201–205. doi: 10.1016/0014-5793(93)80273-w. [DOI] [PubMed] [Google Scholar]
- 11.de Macedo DV, Nepomuceno ME, Pereira-da-Silva L. Involvement of the ADP/ATP carrier in permeabilization processes of the inner mitochondrial membrane. Eur J Biochem. 1993;215(3):595–600. doi: 10.1111/j.1432-1033.1993.tb18070.x. [DOI] [PubMed] [Google Scholar]
- 12.Brustovetsky N, Klingenberg M. Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+ Biochemistry. 1996;35(26):8483–8488. doi: 10.1021/bi960833v. [DOI] [PubMed] [Google Scholar]
- 13.Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J. 1990;268(1):153–160. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connern CP, Halestrap AP. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem J. 1994;302(Pt 2):321–324. doi: 10.1042/bj3020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 16.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 18.Schinzel A, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res Cardiol. 2009;104(2):181–188. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Heitman J. The cyclophilins. Genome Biol. 2005:6–226. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- 22.Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Lim SY, Davidson SM, Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res. 2007;75:530–535. doi: 10.1016/j.cardiores.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klionsky DJ, Ohsumi Y. Vacuolar import of proteins and organelles from the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:1–32. doi: 10.1146/annurev.cellbio.15.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- 26.Levine B. Autophagy in development, tumor suppression, and innate immunity. Harvey Lect. 2003;99:47–76. [PubMed] [Google Scholar]
- 27.Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14:70–77. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 31.Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- 32.Juhasz G, Csikos G, Sinka R, Erdelyi M, Sass M. The Drosophila homolog of Aut1 is essential for autophagy and development. FEBS Lett. 2003;543:154–158. doi: 10.1016/s0014-5793(03)00431-9. [DOI] [PubMed] [Google Scholar]
- 33.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306(5698):990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto A, Cremona ML, Rothman JE. Autophagy-mediated clearance of huntingtin aggregates triggered by the insulin-signaling pathway. J Cell Biol. 2006;172:719–731. doi: 10.1083/jcb.200510065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kopitz J, Kisen GO, Gordon PB, Bohley P, Seglen PO. Nonselective autophagy of cytosolic enzymes by isolated rat hepatocytes. J Cell Biol. 1990;111:941–953. doi: 10.1083/jcb.111.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27(2):433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claycomb WC, Lanson NA, Jr., Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, et al. Proc Natl Acad Sci U S A. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizzuto R, Brini M, Pizzo P, Murgia M, Pozzan T. Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells. Curr Biol. 1995;5(6):635–642. doi: 10.1016/s0960-9822(95)00128-x. [DOI] [PubMed] [Google Scholar]
- 40.Karwatowska-Prokopczuk E, Nordberg JA, Li HL, Engler RL, Gottlieb RA. Effect of vacuolar proton ATPase on pHi, Ca2+, and apoptosis in neonatal cardiomyocytes during metabolic inhibition/recovery. Circ Res. 1998;82(11):1139–1144. doi: 10.1161/01.res.82.11.1139. [DOI] [PubMed] [Google Scholar]
- 41.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized on autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brady NR, Hamacher Brady A, Yuan H, Gottlieb RA. The autophagic response to nutrient deprivation in the HL 1 cardiac myocytes is modulated by Bcl 2 and sarco/endoplasmic reticulum calcium stores. FEBS J. 2007;274:3184–3197. doi: 10.1111/j.1742-4658.2007.05849.x. [DOI] [PubMed] [Google Scholar]
- 43.Yuan H, Perry CN, Huang C, Iwai-Kanai E, Carreira RS, Glembotski CC, Gottlieb RA. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am J Physiol Heart Circ Physiol. 2009;296(2):H470–H479. doi: 10.1152/ajpheart.01051.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vázquez CL, Colombo MI. Assays to assess autophagy induction and fusion of autophagic vacuoles with a degradative compartment, using monodansylcadaverine (MDC) and DQ-BSA. Methods Enzymol. 2009;452:85–95. doi: 10.1016/S0076-6879(08)03606-9. [DOI] [PubMed] [Google Scholar]
- 45.Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20(15):3525–3532. doi: 10.1091/mbc.E09-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perkins EM, McCaffery JM. Conventional and immunoelectron microscopy of mitochondria. Methods Mol Biol. 2007;372:467–483. doi: 10.1007/978-1-59745-365-3_33. [DOI] [PubMed] [Google Scholar]
- 47.Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 1992;119(2):301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maruyama R, Goto K, Takemura G, Ono K, Nagao K, Horie T, et al. Morphological and biochemical characterization of basal and starvation-induced autophagy in isolated adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295(4):H1599–H1607. doi: 10.1152/ajpheart.91449.2007. [DOI] [PubMed] [Google Scholar]
- 50.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100(6):914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 51.Kondomerkos DJ, Kalamidas SA, Kotoulas OB, Hann AC. Glycogen autophagy in the liver and heart of newborn rats. The effects of glucagon, adrenalin or rapamycin. Histol Histopathol. 2005;20(3):689–696. doi: 10.14670/HH-20.689. [DOI] [PubMed] [Google Scholar]
- 52.Priault M, Salin B, Schaeffer J, Vallette FM, di Rago JP, Martinou JC. Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ. 2005;12:1613–1621. doi: 10.1038/sj.cdd.4401697. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez-Enriquez S, Kai Y, Maldonado E, Currin RT, Lemasters JJ. Roles of mitophagy and the mitochondrial permeability transition in remodeling of cultured rat hepatocytes. Autophagy. 2009;5(8):1099–1106. doi: 10.4161/auto.5.8.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang HT, Hwang ES. Nicotinamide enhances mitochondria quality through autophagy activation in human cells. Aging Cell. 2009;8:426–438. doi: 10.1111/j.1474-9726.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- 55.Waldmeier PC, Feldtrauer JJ, Qian T, Lemasters JJ. Inhibition of the mitochondrial permeability transition by the nonimmunosuppresive cyclosporin derivative NIM811. Mol Pharmacol. 2002;62:22–29. doi: 10.1124/mol.62.1.22. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16(7):1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- 57.Rosca MG, Hoppel CL. New aspects of impaired mitochondrial function in heart failure. J Bioenerg Biomembr. 2009;41(2):107–112. doi: 10.1007/s10863-009-9215-9. [DOI] [PubMed] [Google Scholar]
- 58.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341(Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- 59.Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci USA. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pattingre S, Bauvy C, Carpentier S, Levade T, Levine B, Codogno P. Role of JNK1- dependent Bcl-2 phosphorylation in ceramide induced macroautophagy. J Biol Chem. 2008;284:2719–2728. doi: 10.1074/jbc.M805920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park K, Lee S, Kim T, Lee H, Lee C, Kim E, et al. A human scFv antibody against TRAIL receptor 2 induces autophagic cell death in both TRAIL-sensitive and TRAIL-resistant cancer cells. Cancer Res. 2007;67:7327–7334. doi: 10.1158/0008-5472.CAN-06-4766. [DOI] [PubMed] [Google Scholar]
- 62.Jia L, Dourmashkin RR, Allen PD, Gray AB, Newland AC, Kelsey SM. Inhibition of autophagy abrogates tumour necrosis factor alpha induced apoptosis in human T-lymphoblastic leukaemic cells. Br J Haematol. 1997;98:673–685. doi: 10.1046/j.1365-2141.1997.2623081.x. [DOI] [PubMed] [Google Scholar]
- 63.Prins JB, Ledgerwood EC, Ameloot P, Vandenabeele P, Faraco PR, Bright NA, et al. Tumor necrosis factor-induced cytotoxicity is not related to rates of mitochondrial morphological abnormalities or autophagy-changes that can be mediated by TNFR-I or TNFR-II. Biosci Rep. 1998;18:329–340. doi: 10.1023/a:1020261316486. [DOI] [PubMed] [Google Scholar]
- 64.Comstock KL, Krown KA, Page MT, Martin D, Ho P, Pedraza M, et al. LPS-induced TNF-alpha release from and apoptosis in rat cardiomyocytes: obligatory role for CD14 in mediating the LPS response. J Mol Cell Cardiol. 1998;30:2761–2775. doi: 10.1006/jmcc.1998.0851. [DOI] [PubMed] [Google Scholar]
- 65.Ohsawa Y, Isahara K, Kanamori S, Shibata M, Kametaka S, Gotow T, et al. An ultrastructural and immunohistochemical study of PC12 cells during apoptosis induced by serum deprivation with special reference to autophagy and lysosomal cathepsins. Arch Histol Cytol. 1998;61:395–403. doi: 10.1679/aohc.61.395. [DOI] [PubMed] [Google Scholar]
- 66.Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci. 1999;14:180–198. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- 67.Christensen ST, Chemnitz J, Straarup EM, Kristiansen K, Wheatley DN, Rasmussen L. Staurosporine-induced cell death in Tetrahymena thermophila has mixed characteristics of both apoptotic and autophagic degeneration. Cell Biol Int. 1998;22:591–598. doi: 10.1006/cbir.1998.0320. [DOI] [PubMed] [Google Scholar]
- 68.Hamacher-Brady A, Brady NR, Gottlieb RA, Gustafsson AB. Autophagy as a protective response to Bnip3-mediated apoptotic signaling in the heart. Autophagy. 2006;2(4):307–309. doi: 10.4161/auto.2947. [DOI] [PubMed] [Google Scholar]
- 69.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14(1):146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 70.Kubli DA, Quinsay MN, Huang C, Lee Y, Gustafsson AB. Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2008;295(5):H2025–H2031. doi: 10.1152/ajpheart.00552.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Semenza GL. Mitochondrial autophagy: life and breath of the cell. Autophagy. 2008;16(4):534–536. doi: 10.4161/auto.5956. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283(16):10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Ichas F, Mazat JP. From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim Biophys Acta. 1998;1366:33–50. doi: 10.1016/s0005-2728(98)00119-4. [DOI] [PubMed] [Google Scholar]
- 74.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, et al. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294(2):H1036–H1047. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 76.Nohl H. Generation of superoxide radicals as byproduct of cellular respiration. Ann Biol Clin (Paris) 1994;52(3):199–204. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.