Abstract
Year 2011 noted the first definable ozone “hole” in the Arctic region, serving as an indicator to the continued threat of dangerous ultraviolet radiation (UVR) exposure caused by the deterioration of stratospheric ozone in the northern hemisphere. Despite mandates of the Montreal Protocol to phase out the production of ozone depleting chemicals (ODCs), the relative stability of ODCs validates popular notions of persistent stratospheric ozone for several decades. Moreover, increased UVR exposure through stratospheric ozone depletion is occurring within a larger context of physiological stress and climate change across the biosphere. In this review, we provide commentaries on stratospheric ozone depletion with relative comparisons between the well-known Antarctic ozone hole and the newly defined ozone hole in the Arctic. Compared to the Antarctic region, increased UVR exposure in the Northern Hemisphere poses a threat to denser human populations across North America, Europe and Asia. In this context, we discuss emerging targets of UVR exposure that can potentially offset normal biological rhythms in terms of taxonomically conserved photoperiod dependent seasonal signaling and entrainment of circadian clocks. Consequences of seasonal shifts during critical life history stages can alter the fitness and condition, while circadian disruption is increasingly becoming associated as a causal link to increased carcinogenesis. We further review the significance of genomic alterations via UVR induced modulations of phase I and phase II transcription factors, the aryl hydrocarbon receptor (AhR) and the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2), with emphasis on mechanism that can lead to metabolic shifts and cancer. While concern for adverse health consequences due to increased UVR exposure are longstanding, recent advances in biochemical research suggest that AhR and Nrf2 transcriptional regulators are likely targets for UVR mediated dysregulations of rhymicity and homeostasis among animals, including humans.
Keywords: biological rhythmicity, seasonal cycling, circadian rhythms, AhR& Nrf2 transcription factors, carcinogenicity, climate change
Introduction
Anthropogenic induced climate change poses a significant risk to long term ecological sustainability and human health. Depletion of stratospheric ozone at the poles is recognized as one of the most significant environmental threats characterized in the past several decades. Moreover, the response and establishment of the Montreal Protocol in 1987 (1) with the goal to curtail this glooming threat is championed as a success story of international environmental policy; however, the threat of detrimental exposure to ultraviolet radiation (UVR) still persists.
Until 2011, the depletion of stratospheric ozone associated with the Antarctic polar vortex was the largest and most characterized ozone threat. During the period associated with the spring equinox (March) of 2011, Arctic ozone depletion was recorded at a record high and comparable to that of the Antarctic ozone hole. National Aeronautics and Space Administration (NASA) research has indicated that record cold temperatures in the arctic stratosphere during the winter of 2011 prompted a record breaking amount of ozone depletion in the arctic north (2). The 2011 arctic ozone layer was approximately ~2 million kilometers2 (~ 2x size of Ontario, Canada).
The atmosphere encircles the earth, allowing photons of light energy to exergonically fuel the biosynthesis of earth’s primary chemical food source (i.e. glucose), while filtering the sun’s most harmful UVR from entering the biosphere. Gases in the upper atmosphere absorb virtually all of the sun’s UVC waves (100 to 290 nm), while the ozone layer, located in the stratosphere (~ 10–50 kilometers above sea level) absorbs a majority of the UVB (290 to 320 nm) and UVA (320 to 400 nm) waves. Industrial sourced nitrogen oxides, halogenated hydrocarbons, chloroflorocarbons (CFCs), and bromines released into the atmosphere have been identified as leading ozone depleting chemicals (ODCs) in the stratosphere, causing increased UVR penetration in recent decades (Kyoto Protocol 1997, Montreal Protocol 1987) (1, 3).
Numerous ecological and environmental health risks are associated with excessive UVR exposure, particularly UVB. At the ecological scale, increased exposure of UVR is attributed to global shifts in primary production (4, 5). While rainforests are the most productive ecosystems, oceans dominate the earth’s surface area and therefore validate concerns of UVR mediated alterations of marine ecosystems (4). At the organismal level for aquatic and terrestrial species, both plants and animals are at risk, and the most pronounced physiological alterations induced by excessive UVR exposure are the production of reactive oxygen species (ROS) (6, 7, 8), DNA mutations (6), and elevated carcinogenicity (9, 10). Gene expression can be altered by UVR in both plants (7, 11, 12) and animals (13). Overall, from a life history standpoint, any UVR mediated biochemical or physiological alteration has the potential to reduce individual fitness, thereby leading to reduced populations and negatively impacting ecological integrity.
Herein, we will combine a brief comparative characterization of the Antarctic and Arctic scenarios regarding the global threat to loss of stratospheric ozone and the subsequent increased in UVR exposure, with a commentary on emerging routes of genomic dysregulation and their potential deleterious physiological effects. Specifically, we will consider UVR modulation of phase I and phase II transcription factors, the aryl hydrocarbon receptor (AhR) and the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2), and address the potential for dysregulation of coordinated physiological processes, including cross talk between AhR and Nrf2. We underscore the reliance of sunlight among biological systems for the adaptive timing of seasonal and circadian rhythms, and emphasize the potential for UVR exposure to cause a loss of fitness among target organisms and address risks to human health.
Historical Context of Ozone Depletion and Characterization of its Mechanisms
The ozone layer is formed naturally by two possible reactions in the lower altitudes of the stratosphere 1) (O2 + (UV-C λ <242.4 nm) → O + O; 2) O + O2 + (N2, or O2) → O3 + N2 or O2). Stratospheric ozone levels from Antarctica have been monitored by British run Antarctic Survey stations since the late 1950’s and trend analyses of these data reported a weight of evidence demonstrating the occurrence of springtime (September) depletions of stratospheric ozone in the Antarctic region (14, 15). Methyl bromides and halogenated hydrocarbons, particularly chlorofluorocarbons (CFCs) which were historically characterized to undergo photodissociation via a series of reactions (CF2Cl2 + UV → Cl. + CCIF.2) (16) are identified as the main exogenous sources of ozone depletion.
The industrial utility of CFCs and various halogenated hydrocarbons is due to their stability. CFC compounds found great utility as refrigerants and solvents, while bromines were heavily used as fumigants (17, 18); however, their stability is a major threat due to environmental persistence. (> 100 years). Due to the threat of halogenated hydrocarbon mediated ozone destruction, the Montreal Protocol (1987, and later amendments) formulated as an international treaty to differentially delineate guidelines for developed and developing countries to eradicate the production of halogenated hydrocarbons known to cause stratospheric ozone destruction (17). While existing CFCs and other ozone depleting substances will persist for decades, the effort of the Montreal Protocol is regarded as a victorious achievement of environmental global policy.
The ability of CFCs and other ODCs to induce ozone destruction at the poles is driven by multiple factors, including the coupling of extreme cold winter temperatures, followed by springtime solar radiance (19). Stratospheric ozone accumulation at the poles is largely driven by Brewer-Dobson circulation patterns that naturally draw ozone from the tropics towards the polar regions (20). During cold winter months, circular wind patterns at the poles lead to the formation polar stratospheric clouds (PSC) and the formation of stratospheric polar vortices (SPV) (21) which further concentrate reactive atmospheric chemicals such as nitric acid, and provide a binding substrate for the parent ozone depleting chemicals (ODCs) such as CFCs (ClO + NO2 → ClONO2 ; ClONO2 + H2O → Cl2 + HNO3 ; Cl2 + hv → 2Cl.). When the sun begins to rise in the springtime, these ODCs photo dissociate forming chlorine free radicals that being to attach ozone in a series of chain reactions (Cl. + O3 → ClO. + O2 ;ClO. + ClO.→ ClOO. + Cl. ; ClOO. + M (N2) → Cl. + O2 + M (N2) ; 2Cl.+ 2O3 → 2ClO.+ 2O2 ; 2O3 → 3O2 (Net reaction)). The final reactions of Cl free radical destruction of ozone occur in a cascade with one Cl having the potential to attack thousands of O3 molecules.
Persistent ozone depletion
Measurements of ozone depletion are quantified in Dobson units (DU), whereas one DU refers to a layer of ozone that would be 10 µm thick under standard temperature and pressure (i.e. 273.15 K (0 °C, 32 °F) and an absolute pressure of 100 kPa (14.504 psi, 0.986 atm). A semi-arbitrary marker of any value <220 DUs is set to designate an “ozone hole”. From the commencement of stratospheric monitoring in the 1950’s up until 1979, there were no values of <200 DU. Trends of historical and contemporary data indicate that magnitude of ozone depletion in the Antarctic region has been the greatest recorded. Between 1979–2008, 30 ozone “holes” were documented (22) during the Antarctic spring time, where ozone has been observed to undergo >90% decrease (23). The spring of 2000 is verified as the greatest loss of ozone recorded in the Antarctic region, measuring total column ozone of approximately 93.4 DU (15 day average ozone hole minimum) over a 29.8 million km2maximum area (22). Comparatively, 2008 was the 5th largest ozone depletion on record for the Antarctic region, measuring 99.9 DU over an expanse of 27.1km2 (22).
The persistence of ozone depletion in the stratosphere is apparent despite efforts of the Montreal Protocol (20). Continued ozone depletion “post Montreal Protocol” is not absolutely surprising given the extended lifespan of ozone depleting chemicals; in fact it is predicted that ozone depletion will continue throughout much of the 21st century (19). Moreover, climate change is considered a viable burden to ozone recovery (20), and it is noteworthy that 2011 marked the first time a true “ozone hole” was recorded in the Arctic stratosphere (2). During one week of the Arctic spring (March 2011), the ozone column of the Arctic was between 220–230 DU over a range of approximately 2 million km2 (2). For a period of 27 consecutive days, the 2011 Arctic ozone layer was > 250 DU. The Arctic vortex and area of ozone depletion is roughly the size of Germany, California, or 2x size of Ontario. Although the Arctic vortex is approximately 40% smaller than the Antarctic, the region of Arctic ozone depletion tends be displaced in its position relative to the center of the North Pole (2, 24, 25, 26) posing a viable threat of harmful UVR exposure for dense populations across 3 northern continents (i.e. North America, Europe, and Asia).
Interactions between Ozone Depletion and Climate Change
Polar stratospheric ozone depletion is a dynamic and complex phenomenon that is the subject of rigorous character analysis and predictive modeling which is beyond the scope of this review. Nevertheless, a few points are worth highlighting and from both functional and policy standpoints that suggest ozone depletion and climate change are not mutually exclusive phenomena. Because ODCs are also greenhouse gases, the Montreal Protocol also works toward the aim of the Kyoto Protocol (19, 27) (Figure 1), and the goals of Kyoto may help reduce ozone depletion. Furthermore, persistent ozone depletion also functions as a positive feedback for stratospheric cooling and subsequent ozone depletion (24), which can influence tropospheric conditions in both the Antarctic (28) and Arctic poles (29). Bidirectional coupling between the stratosphere and troposphere is paramount for climatic research focused on unraveling the relationships between ozone depletion and climate change (21, 28, 29).
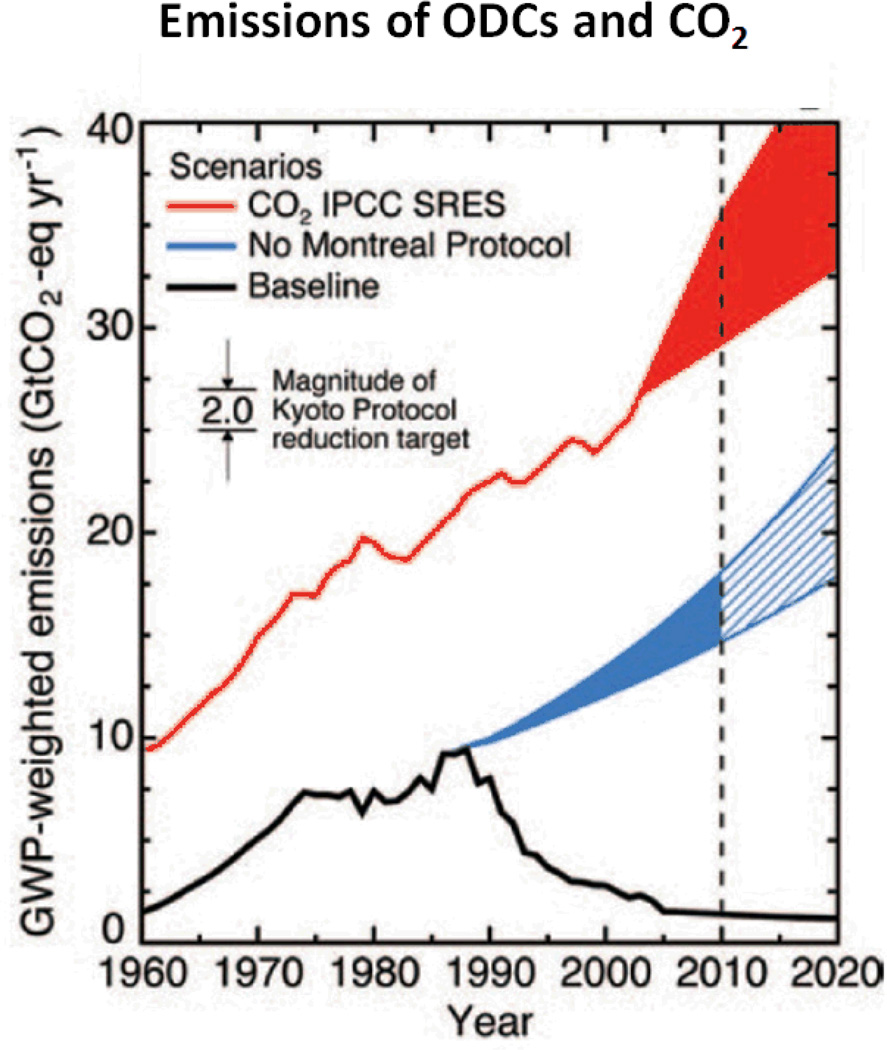
Figure 1.
Graph of the global warming potential of industrial sourced ozone depleting chemicals (ODCs) and emissions in the atmosphere. Blue area shows the projection in the absence of the Montreal Protocol. The Red line and red shared area projects carbon dioxide emissions. Dashed black line indicates Kyoto Protocol Target Evaluation Period. Abbreviations; IPCC (International Panel on Climate change), SRES (Special Report on Emission Standards), GWP (Global Warming Potential), GtCO2 (Gigatonne carbon dioxide equivelant). (Figure published in (19) Solomon and Chanin 2011).
Greenhouse gases (GHGs) have long been modeled as a causal factor in stratospheric cooling (26), and carbon dioxide emissions can exasperate the conditions that promote ozone depletion through several processes. Recent studies indicate GHGs cause an increased temperature differential between the troposphere and stratosphere which is linked to the acceleration of Brewer-Dobson circulation patterns (30). In this model of Arctic SPV enhancement, interannual variability of thermal gradients between sea and air is correlated with the GHGs influence on stratospheric forcing by Rossby waves (31). Additionally, modeling suggests that carbon dioxide may influence the tropospheric coupling with the SPV through tropical quasi-biennial oscillation and the Holten-Tan mechanism (32, 33, 34).
Surface climates in the polar regions illustrate a time shifted summer warming trend since the 1970’s, correlated with more contracted and intensified SPVs and increased wintertime stratospheric cooling (21, 35). In both hemispheres, the stratospheric influences on surface conditions are largely mediated through polar Annular Modes (21, 28, 29), while in the Arctic region, the Annual Mode shows significantly larger interannual variability (21, 36). In addition to various factors of interannual climatic variability (21), fluxes in the Annular Mode appear to be contributing to the shape irregularities and displacement of the vortex from the pole (24, 26, 36). Overall, differential cooling and patterns of interannual variability between the Arctic and Antarctic regions are suspected factors of their divergent patterns of ozone depletion (23, 24, 37). In the Arctic, atypical stratospheric cooling in the presence of ozone depletion is occurring in both the winter and spring months (24), versus just during the spring in Antarctica, which is naturally colder in the winter.
In both the northern and southern hemisphere, the SPV and interannual variations in the Annual Modes are important drivers for predictive modeling of surface climatic trends (28, 29). Nevertheless, the complexity and dynamics associated with ozone depletion and global climate change challenge consensus. Interestingly, 2011 summer ice sheet melts were the second greatest on record for the Arctic region (most ice sheet melt in 2007) (37, 38), while in Antarctica, sea ice has expanded in some areas and receded in others (37, 39). An undefined combination of factors drives these differential patterns, though the variations in the Annular Modes are likely contributors (37, 39, 40). If greenhouse gas emissions are not hindered, Antarctic ice advances are predicted to retreat later in the 21st century as ozone depletion begins to be minimized (37).
Achieving the goals of the Montreal and Kyoto Protocols are not likely to be met concurrently, and the dynamics between ozone depletion and climate change remain elusive and controversial. Additionally, it remains to be seen what types of indirect benefits may arise from long-term storage of GHGs in geological sequestrations, or the development of renewable green energy, including biofuel (41, 42, 43).
In the context of this review, further dialog is encouraged in an attempt to incorporate and model the interrelationships between ozone depletion driven UVR exposures and GHG mediated climate change that may further intensify modulations of environmental health endpoints (44). Physiological dysregulations induced by UVR exposures will be in the context of other physiological stresses sourced from climate change, including fluxes of short and long wave radiations, and warmer temperatures (45, 46). Physiological stress associated with global warming induces the expression of antioxidant enzymes that are elevated in response to UVR (47, 48), further suggesting that UVR mediated physiological responses should be considered in the context of global climate change.
Photoperiod as the universal driver of biological timing
Periodicity and timing are critical to the life history strategy of virtually all sexually reproducing species. The primary timing strategy is driven by photoperiod, which works as a zeitgeber “time giver”, scheduling life history strategies according to predictable environmental climatic patterns. The life histories of plants and animals have evolved in the context of photoperiod as the primary ecological timing signal triggering hormonal regulation of an organisms’ physiology and coordinating downstream endocrine pathways. This signaling system provides environmental context for the timing of development, growth, feeding, migration and reproduction, translating into seasonal patterns of blood plasma levels for several hormone systems, receptors, and metabolizing enzymes (49, 50, 51, 52, 53, 54, 55).
Recent data indicates a molecular basis for photoperiod based timing (56, 57) among both plant and animal lineages, in which the light responsive pathways are tryptophan dependent, utilizing functionally similar tryptophan monooxygenase enzymes. The reliance of tryptophan dependent pathways mediating the initial photoperiod driven cues among plants and animals is noteworthy considering its ratio of occurrence of tryptophan within the genetic code, where it is the least abundant amino acid along, and along with methionine, it the only amino acid that is coded by a single codon. From an evolutionary standpoint, eukaryotic plants and eukaryotic animals are not sister taxa (meaning they are not considered to share the same common ancestor). These observations suggest a profoundly specific functional role of tryptophan in light sensitive biochemistry (58), herein emphasizing the dependence of tryptophan within specific light driven pathways that meet the demands to be in “ecological time”. Among plants, photoperiod is the initial signal for the synthesis of auxin, the primary plant development hormone (59, 60, 61), while melatonin synthesis in response to light cycles for invertebrates and vertebrates acts as the initial cue of the neuroendocrine system, via tryptophan dependent pathways (62, 63, 64).
Among extant vertebrates, melatonin is produced in the pineal body and the hypothalamus is the primary interface between the central nervous system and physiological activities throughout the body (65). Historical significance for photoperiod environmental signaling is further evident in the fossil record of some Paleozoic fishes (66). The Placoderms and Ostracoderms fossils possess a “third eye” on their dorsum, which has been hypothesized to be a light sensing organ, homologous to the external pineal body of extant lampreys. Among lampreys, the pineal body is an external version of the pineal gland that is generally located within the cranial cavity of most vertebrates.
The neuroendocrine system of vertebrates is very complex and interacts with downstream pathways through processes of positive and negative feedback. The light dependent hypothalamus maintains proper function of the central clock within the suprachiasmatic nucleus (SCN). The biological timing strategy synchronizes the central clock and circadian rhythmic function of normal homeostatic physiology. Melatonin levels that vary according to daily light cycles entrains the central clock of invertebrate and vertebrates, by synchronizing peripheral circadian oscillators within various organs through a negative feedback loop of genetic transcription (Figure 2) (67, 68). The circadian clock drives daily rhythms of gene expression for nuclear receptors, metabolizing enzymes, and hormones associated with reproduction, blood glucose levels, and energy metabolism (67, 68, 69, 70, 71).
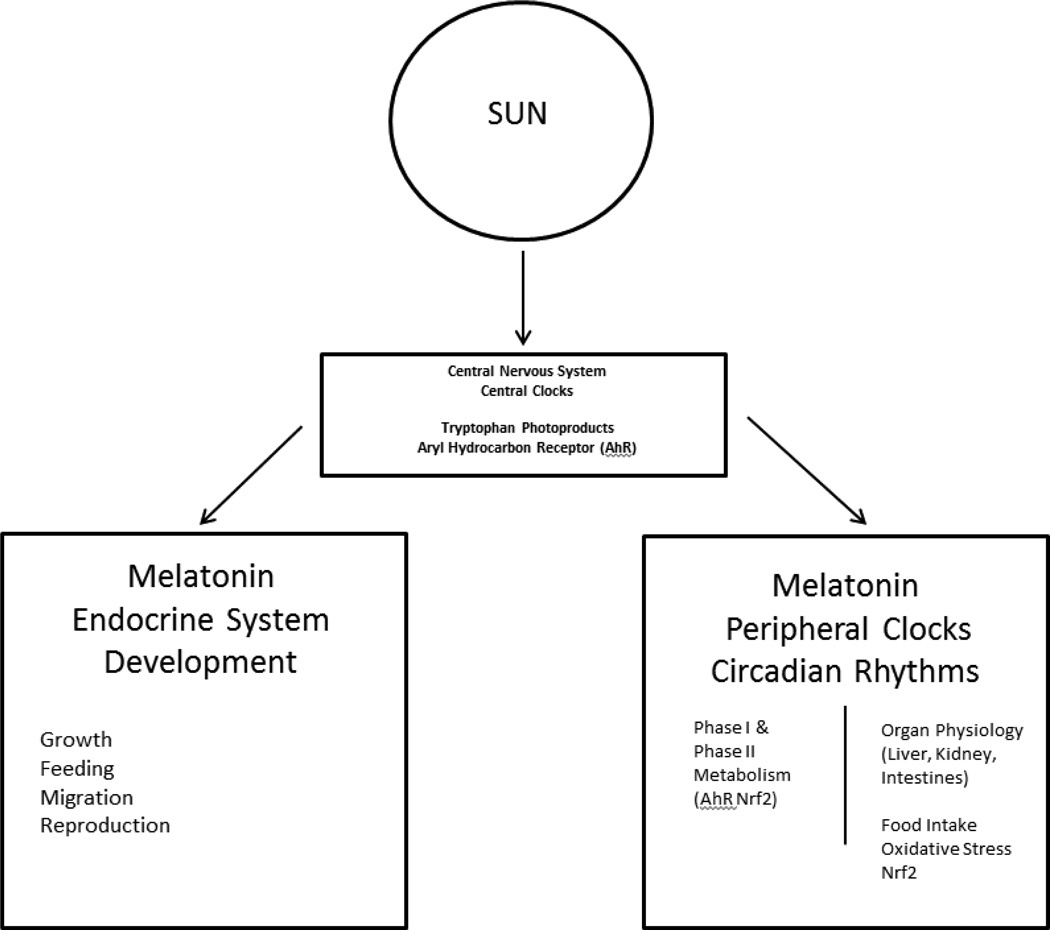
Figure 2.
Schematic of Light Driven hierarchy for the central and peripheral clocks regulation of detoxification. Photoperiod is the primary time keeper for the central clock that signals the peripheral clock in conjunction with other factors, including food. Among vertebrates, melatonin is the primary light sensitive hormone that regulates endocrine control of timed life history events, and circadian regulation of the central and peripheral clocks.
Circadian rhythms share a common adaptive value across major taxonomic lineages (72, 73), whereas selective pressure acting over the course of geologic time led to UVR sensitive processes being scheduled and coordinated according to light/day cycles (74). Central clock mediated gene expression translates into circadian patterns of molecular signaling pathways that maximize physiological efficiency (75, 76), minimize DNA damage (77), oxidative stress (78) and metabolism (71, 79, 80). Variations in the overall oscillator feedback pathways suggest independent evolutionary origins of specific circadian mechanisms among algae, plants, and animals (74, 81), however the occurrence of the basic Helix-Loop-Helix (bHLH) - Per-ARNT-SIM (PAS) related family of transcription factors supports a hypothesis of homology among select circadian genes across multiple kingdoms (72, 74).
Homologous clusters of bHLH-PAS circadian genes including CLOCK, cycle, and Period (Per) have been found throughout the Bilateria (82). The mammalian PAS domain shares structural homology to the prokaryotic environmental sensor protein, photoactive yellow proteins (PYP) (83, 84, 85). The PAS domain is primarily limited to proteins directly involved in signal transduction pathways (84) and among prokaryotes, environmental cues including light (86) and oxygen (84) stimulate PYP, suggesting ancestral origins for light as a signal for PAS mediated genetic transcription. Among fungi (Neurospora), the white collar (wc-1, wc-2) genes are circadian regulators with bHLH-PAS domains, and in plants (Arabidopsis) the PIF-3 phytochrome is a PAS containing regulator of flowering time (74, 87).
UV Mediated Homeostatic Dysregulations via AhR and Nrf2 Transcription Factors
Oxidative stress generated by the production of ROS, including free radicals and oxidants such as hydrogen peroxides, are common physiological effects of UVR exposure among plants and animals (88, 89). The biotransformation of ROS and xenobiotics through phase I and phase II reactions is a ubiquitous regulatory mechanism among organisms that functions in part to maintain relative homeostasis in response to adverse environmental exposures, including UVR. In preparation for phase II translocation from cells, phase I reactions produce metabolites that are more water soluble than the binding toxin, while phase II reactions produce conjugate bases (90, 91). The aryl hydrocarbon receptor (AhR) and the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) are transcription factors that are emerging as significant targets of UVR mediated dysregulations leading to potentially adverse health effects (92, 93). The AhR and Nrf2 transcription factors provide a primary means of response to UVR induced stress, and their modulations are linked to various UVR skin diseases and effects of photoaging caused by ROS (94).
In addition to seasonal and circadian patterns of expression among metabolic enzymes (95, 96), the activities of phase I and phase II enzymes are often synchronized via response elements of target genes, including those expressed by the AhR and Nrf2 transcription factors (97, 98). Phase I and phase II enzymes are induced by AhR in association with the xenobiotic responsive element (XRE) promotor, while phase II enzymes and antioxidant enzymes are more commonly regulated through Nrf2 in association with the antioxidant response element (ARE) promotor (91, 98). The phase II induction by AhR pathways occurs for enzymes such as glutathione S-transferase (GST) and NAD(P)H quinone oxidase (NQO), which are identified to possess both the XRE and the ARE promotor (99, 100). Furthermore, bi-directional crosstalk between AhR and Nrf2 dependent gene transcription has been verified by the identification of XRE promoters on Nrf2 gene and ARE promoters on AhR genes (101, 102, 103). Accordingly, the coordination of phase I and phase II enzymes are regulated by ligands of Ahr, oxidative stressors including phase I metabolites that regulate AhR and or Nrf2 mediated phase I and phase II activities and transcriptional crosstalk between AhR and Nrf2 (91, 98, 104).
AhR-
Phylogenetic studies indicate that the vertebrate AhR gene is basal to a pair of reciprocally monophyletic AhR clades; denoted as AhR1 and AhR 2 (105). The AhR protein is a member of the bHLH–PAS family of transcription factors (83) that occurs throughout the metazoa (Figure 3). When unbound by a ligand, the AhR transcription factor is suppressed in the cytoplasm by association with heat shock protein 90 (hsp90), the co-chaperone protein (p23), and the X-associated protein (XAP 2) that is also known as AhR-interacting protein (AIP) (106, 107, 108). Lipid soluble ligands that can pass through the cell membrane and bind with the AhR transcription factor while positioned in the cytosol, in association with the chaperone complex (109). The ligand binding domain (LBD) of AhR is located within the PAS core of the PAS-B domain.
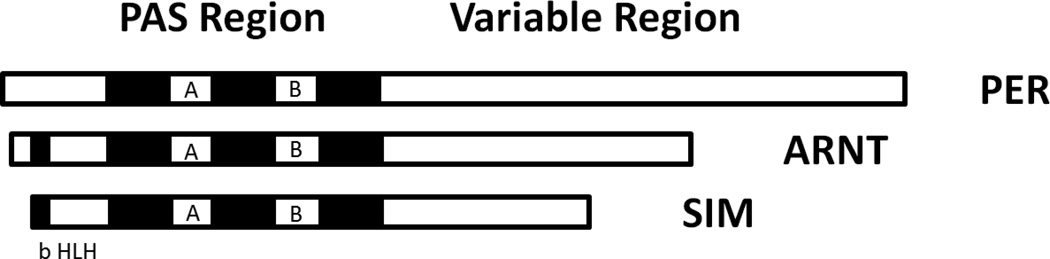
Figure 3.
Nominal members of the PAS Superfamily, named Period (PER), Aryl hydrocarbon receptor nuclear translocator (ARNT), and the Single-Minded Protein (SIM).
The Ah receptor is also known as the “dioxin receptor”, as the exogenous halogenated aromatic hydrocarbon (HAH) 2’,3’,7’,8’-Tetrachlorodibenzo-p-dioxin (TCDD; or dioxin) (Figure 4) has historically demonstrated the strongest binging affinity for AhR (109, 110). Other xenobiotics, including polycyclic aromatic hydrocarbons (PAHs) also induce AhR mediated gene expression. The continued quest for endogenous ligands for AhR most recently implicates tryptophan photoproduct, 6-Formylindolo [3,2-b] carbazole (FICZ) (Figure 4) as the most probable natural ligand in vivo (111, 112, 113). Indeed, endogenous FICZ shows the strongest affinity for AhR, exceeding the binding affinity of exogenous TCDD (111, 112). Numerous genes are expressed via the AhR pathway, with the cytochrome p450 metabolizing enzyme CYP1A1 being the most thoroughly characterized. Recent findings of strong affinity of FICZ to AhR support earlier in vitro studies that showed UVR regulates AhR dependent gene expression of CYP1A1 in mammalian keratinocytes and lymphocytes through tryptophan photoproducts (114), and substantiates a role for AhR in skin related environmental response.
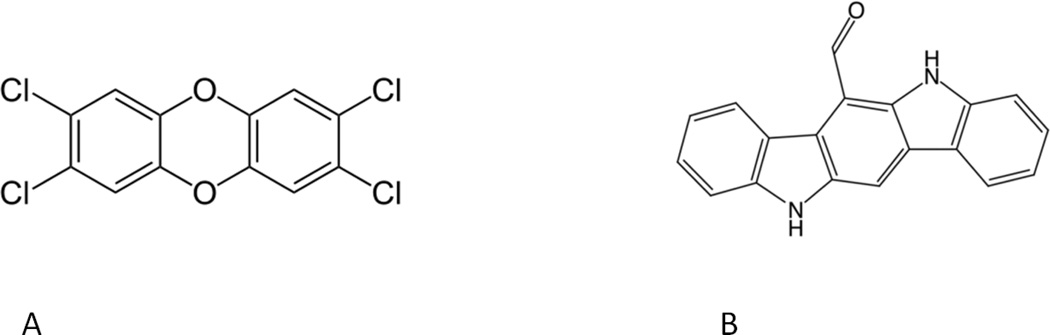
Figure 4.
Structure of high affinity ligands for the aryl hydrocarbon receptor; A) the exogenous halogenated aromatic hydrocarbon (HAH), 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD; Dioxin); B) the endogeous tryptophan photoproduct 6-Formylindolo[3,2-b]carbazole (FICZ).
The AhR is a highly likely target for UVR mediated alterations of gene expression in the context of ozone depletion. Mice studies indicate that UVB exposed AhR knock out mice failed to express CYP1A1 in keratinocytes, as opposed to AhR+ mice (115) and AhR dependent cytochrome genes failed to be expressed in AhR knockout chicken embryos treated with tryptophan metabolites produced by exposing tryptophan to natural sunlight (116). Similarly, zebrafish embryos demonstrate CYP1 gene expression in an AhR dependent manner when exposed to UVB (117) or FICZ (118), indicating that a positive relationship between tryptophan photoproducts and AhR mediated gene expression occurs in a taxonomically diverse array of vertebrates.
The propensity for skin diseases such as melanomas and psoriasis, as a result of UVR modulations of AhR signaling pathways is gaining increased attention within the environmental health sciences (9, 119, 120) including AhR dependent photoresponsive immune responses (121, 122, 123), speculatively linked to psoriasis. The activation of AhR by FICZ promotes the differentiation of CD4+ cells into Th17 lymphocytes and downstream secretion of IL-22 cytokines (121, 122, 124), both of which are major inflammatory components of psoriasis (125, 126). Some evidence suggests that TCDD treatments differentially induce the CD4+ into Treg cells (123). TCDD treatments of human keratinocytes demonstrate a suppression of this pathway, possibly through cross-talk between transforming growth factor (TGF-β) and AhR (123).
Among humans, there are polymorphic variants of AhR dependent phase I and II enzymes that either provide protection against or increase the risk of psoriasis (127). UVR signaling of AhR induces multiple CYP genes within the epidermis, and their expression exhibits cross-sectional stratification (128). In human keratinocytes exposed to UVB, CYP1A1 was expressed basally to CYP1B1 (128). AhR dependent enzyme expression in response to UVR appears varied across tissue boundaries and the complete role of AhR mediated skin disorders is not fully characterized.
The AhR receptor clearly plays a role in melanogenic pathways of tanning among mammals. Both FICZ and UVB exposures to murine keratinocyte cell lines have identified a facultative AhR–CYP1A1 pathway of melanin synthesis that causes an upregulation of tyrosinase, which is known as the rate limiting step of melanogenesis (92). The AhR pathway of melanogenesis appears restricted to melanocytes and has been verified in human cells in vitro, with TCDD induction of AhR (129). Furthermore, studies on human HaCaT keratinocytes exposed to UVR and FICZ have been forwarded to suggest that UVR mediated expression of CYP1A1 and CYP1B1 through AhR may prime skin tissue to PAH exposures, thereby increasing the risk factor for skin cancers exposed to PAHs (130). Alternatively, UVR mediated activation of AhR may provide resistance to PAH driven malignancies (130).
Nrf2-
The NF-E2-related factor 2 (Nrf2) transcription factor is a primary target of response to oxidative stress and metabolic action aimed at minimizing carcinogenic metabolites (88). Nrf2 is a phylogenetically ubiquitous gene of the basic leucine zipper (bZIP) family, that has invertebrate homologs known as SKN-1 (Caenorhabditis sp.) and CncC 9 (Drosphila sp.) (88, 131, 132). Among vertebrates, the Nrf2 system appears to be highly conserved comparing teleost fishes to mammals. In the presence of thiol reactive ROS and xenobiotic inducers, Nrf2 dissociates from the Kealp-1 suppressor protein in association with the endoplasmic reticulum, and translocates to the nucleus to form a heterodimer with a small Maf protein that then binds with the antioxidant responsive element (ARE) (88, 132). Numerous proteins and enzymes that target ROS and exogenous molecules are transcriptionally regulated by Nrf2-ARE binding including, glutathione S-transferase (GST), NAD(P)H quinone oxidase (NQO), super oxide dismutase (SOD), heme oxigenase 1 (HO1), and UDP-glucuronosytransferase (UGT) (131, 133).
Recent research is steadily characterizing the effects of UVR induced ROS on Nrf2 dependent phase II enzymes among various types of mammalian skin cells. The expression of several Nrf2 dependent phase II enzymes are altered in response to UVR, and among human keratinocytes and melanocytes, both UVA and UVB have been shown to upregulate the expression of NQO and HO-1 in vitro, though the expression of HO-1 was only observed in melanocytes (134). Nrf2 dependent induction of HO-1 has also been observed in an in vitro melanocyte cell line treated with hydrogen peroxide (135). Additionally, in vitro studies of human fibroblasts have positively identified Nrf2 mediated gene expression of HO-1 in the presence of UVA (136). Skin culture studies indicate that exposure to chronic levels of ambient UVA and UVB can cause ROS photoaging in both keratinocytes and fibroblasts, and verify differential expression of Nrf2 dependent phase II enzymes among cell types (94). In this study (94) keratinocytes demonstrated modulation in NQO expression, whereas fibroblasts showed upregulation in multiple Nrf2 regulated genes, including NQO, GST, and HO-1. These studies indicate that Nrf2 activity is most significant in deeper layers of skin tissue.
The differential expression of Nrf2 dependent antioxidant enzymes among layered tissues of the skin is coupled with varying degrees of Nrf2 mediated resistance to apoptosis (137, 138). In addition, the end point of enzyme induction versus cellular apoptosis depends on the type of UVR exposure (i.e. UVA, or UVB). Within fibroblast tissue, studies using Nrf2 knockout mice demonstrate that UVA more often induces the activation of Nrf2 mediated antioxidant enzymes, while exposure to UVB increases the propensity for apoptosis (138). Moreover, using transgenic mice containing constitutively active Nrf2 within keratinocytes (137), a gradient of Nrf2 mediated enzyme induction and apoptosis was noted across cross sections of epidermal tissue exposed to UVB. Essentially, in the deeper regions of the epidermis, UVB is more likely to induce apoptosis, whereas, the reliance of Nrf2 dependent antioxidants occurs more superficially (137).
The significance of differential activation of Nrf2 dependent antioxidant enzymes and apoptosis is relevant in the functional context of skin layering. In addition to the pronounced photoaging via the production of ROS by UVA and UVB (94, 139), UVB has been shown to be genotoxic to the epidermis, as observed by the accumulation of oxidative DNA products (140). While malignant cells can occur in either layer of the skin, the relative reduction of Nrf2 dependent antioxidant genes in epidermal keratinocytes (94, 137) is accounted for by the presence of pigment and the frequent renewal of new cells. Indeed, some protection from malignancy is required in the epidermis, as is evident in the gradient of apoptotic induction within the epidermis (137); however, the dermis receives greater amounts of harmful UVB and requires more robust antioxidant protection and safeguards to fight malignancy.
Cross talk between AhR and NRf2 in UV response
The emerging patterns of UVR mediated Nrf2 activity in mammalian skin is just one part of a complex and interactive response mechanism. Recent reports using in vitro human keratinocytes have implicated AhR –Nrf2 crosstalk as part of an anti-inflammatory response mechanism that can be activated by ROS induction of AhR, followed by subsequent downregulation of pro-inflammatory cytokines and Nrf2 dependent expression of NQO (141). Conversely, studies of Nrf2 knockout mice (142) have demonstrated that Nrf2 functions as part of a pro-inflammatory response in keratinocytes during wound healing. In this model, Nrf2 activity increased in the presence of ROS and growth factors, and induced the expression of HO-1 and GST. Also, the presence of Nrf2 provided positive feedback for the maintenance of pro-inflammatory cytokines, purportedly through a transcription complex with the tumor necrosis factors-α promotor (TNF). The occurrence of positive feedback to pro-inflammatory cytokines via Nrf2 is contrary to notions of Nrf2 as a transcription factor for antioxidants and anti-inflammatory response (141, 143); however, recent studies have demonstrated a positive relationship in the expression of Nrf2 and pro-inflammatory cytokines (144), and a transcription complex between Nrf2 and TNF (145). Although the AhR transcription factor was not investigated in the earlier Nrf2 knockout study (142), the potential for crosstalk exists, given the ROS signaling of AhR in keratinocytes (141).
UVR induced modulation lead to altered carcinogenicity of metabolites, increase oxidative stress, and the disruption of lipid metabolism (9, 104). UVA produced lipid peroxides have been also been shown to signal Nrf2 dependent antioxidants in keratinocytes and fibroblasts (93). Additionally, low density lipoproteins which are sources of oxidized lipids, are verified inducers of AhR in vascular tissue (146). This lipid based pathway of AhR activation is hypothesized to contribute to fatty liver disease and atherosclerosis (146, 147). Modulations of adipogenic processes have been demonstrated in mammalian fibroblasts through bidirectional crosstalk between Nrf2 and AhR (148). This study demonstrated that the Nrf2 pathway in mouse fibroblasts was shown to inhibit adipocyte differentiation in an AhR dependent manner. It is suspected that UVR activation of these two transcription factors may exasperate the photoaging process that reduces the lipid content of skin and contributes to adipogenic disease (i.e. dyslipidemia) (9).
Seasonal and Circadian Dysregulations via AhR and Nrf2-
The relatively extended timeframe of seasonal cycles challenge the assessment of UVR mediated physiological dysregulations across seasons. Nevertheless, given the threats of stratospheric ozone depletion are greatest during the spring season, short term excessive UVR exposures can potentially drive melatonin based hormonal shifts during critical life history stages associated with the spring season (49). Homeostatic shifts from nominal cycles of reproduction, growth and metabolism can potentially alter individual fitness. Furthermore, seasonally correlated effects of UVR exposure are likely to be co-linked with physiological responses to climate change, including abiotic shifts such as temperature and biotic shifts in ecosystem productivity (149, 150). Climate change may have deleterious modulations of immunity and increase the propensity for diseases that can impact physiological condition independent of UVR (150, 151, 152). Moreover, photoperiod plays a normal role in seasonal cycling of immune response genes (153) and may be modulated by UVR exposure through AhR and Nrf2.
Melatonin dependent signaling originating in the central nervous system (CNS) links the immune system across seasons and circadian cycles, suggesting that UVR mediated shifts in circadian homeostasis are likely, over and above seasonal dysregulations (153, 154). Environmental factors including UVR may cause rhythmic modulations of the inflammatory system that naturally cycles diurnally according to melatonin and cortisol levels, with subsequent regulation of pro and anti-inflammatory cytokine levels (154). Conversely, environmental stress factors can modulate expression of circadian genes such as Per (155, 156), potentiating the risk of tumorgeneis (155). Indeed, the propensity for cancer development and immunological modulations resulting from melatonin and circadian rhythm disruption are active areas of basic and clinical research (155, 157, 158). Circadian linked tumorogenesis is complex and not fully characterized; however, alterations of melatonin dependent cytokine expression (154, 158) and glucocorticoid dependent synchronization of peripheral clocks are emerging pathways of circadian linked cancers (157).
In mammals, cancers linked to circadian dysregulations may be attributed to the decoupling of circadian entrainment of cell cycles (157) that are purportedly coordinated for peripheral clock optimization (159). UVR induced modulations of this coordination system may target the linkage between circadian (Per, Timeless (Tim), cryptochrome and cell cycling proteins (Checkpoint proteins; Chk) (160, 161, 162). Additionally, in mammals, UVB can accelerate cellular proliferation by altering the phosphorylation status of cyclin-dependent kinase/cyclin complexes (cdk-4- cyclin D1, E, A) (163). Interestingly, studies indicate that in the absence of exogenous ligands, AhR dependent CYP1A1 maintains an integral proliferative role in the cell cycling of various tissues including keratinocytes (164), in an association with cyclins and retinoblastoma proteins (RB) (164, 165, 166), while TCDD activation of AhR induces cell cycle arrest in the G1 phase and inhibition of cellular apoptosis (165, 166). A tissue specific role of AhR in cell proliferation is apparent such as in the human lung, activated AhR promotes progression from G1 to the S phase following the PAS domain heterodimer AhR/Arnt complex interaction with the E2F1 regulatory domain (167), whereas no interaction was observed in liver cells. Additionally, AhR activation in human keratinocytes exposed to UVB causes cell proliferation by activation of EGFR and TNF (123, 168).
The mammalian expression of AhR dependent CYP (1A1, 1B1) genes in response to FICZ in vivo was first reported by Mukai and Tischkau (113), with concurrent data to suggest an endogenous role of AhR in the regulation of circadian rhythms, and possible pathway leading to an increased risk factor for cancer (113). These initial studies also reported a time dependent repression of CYP1A1, BMAL and Per, and Cry in suprachiasmatic nucleus (SCN) cells, in vitro (113) with the treatment of tryptophan photoproducts. Together these findings further validate light as a cue in the SCN and highlight the coactivation of AhR and circadian PAS genes including CLOCK, BMAL, and Per. Follow-up studies confirming interactions between circadian disruptions and AhR have primarily utilized treatments of TCDD, further implicating atypical time activation of PAS genes as a decoupling mechanism between master and peripheral clocks via modulations of Per gene expression (169, 170). Treatment of Hepa-1c1c7 cells in vitro and extracted mice liver cells treated in vivo with TCDD collectively demonstrate that AhR activation causes a downstream repression of Per1 through competitive heterodimerization of AhR/BMAL (versus CLOCK/BMAL) (170). The AhR/BMAL heterodimer inhibits binding to the Per Ebox promotor, thereby downregulating Per expression (170). Essentially the same observation was noted with TCDD treated mouse ovaries treated in vivo, where AhR interactions with BMAL caused a modulation of Per2 expression (169). Similarly, the circadian driven maturation of blood cells from stem cells was modulated by disruption of Per1 and Per2 after treatment with TCDD (171). Collectively, these studies reveal a pattern of circadian clock disruption, including a consistent repression of Period genes that may lead to various cancers.
Circadian signaling is tightly coupled to the redox state of cells, and studies suggest there is the likelihood for UVR mediated oxidative stress to modulate circadian rhythms and metabolic homeostasis through circadian genes (172, 173, 174) or redox sensitive metabolizing enzymes (68, 71). Mechanically, circadian genes including BMAL, CLOCK, and NPAS2 (homologue of CLOCK, with brain specific expression in mammals), function as redox sensors that respond to the redox state of nicotinamide adenine dinucleotide (NADH, reduced; NAD+, oxidized) (174). The reduced NADH induces the heterodimer complex of Clock or NPAS2 with BMAL, that subsequently upregulates expression of circadian Per and cryptochromes (71, 173). This circadian redox sensoring system is responsive to external inputs such as diet as a signal that providing feedback for circadian oscillators and the entrainment of peripheral clocks that translates to cycling of cellular metabolism (173). Repression of this metabolic/circadian system occurs through the oxidized NAD (173) or through negative feedback via Per and Cry (74). Interestingly, polymorphisms in NPAS2 have been linked to “Seasonal Affective Disorder” (172).
The occurrence of AhR dependent CYP expression among various signaling pathways that cycle in a circadian pattern suggests that UVR mediated oxidative stress may result in oxidative modulations of circadian patterns of fatty acid metabolism (175), cross talk with classical estrogen receptors (176), and development among both invertebrates (177) and vertebrate lineages (178). Lastly, melatonin is a strong antioxidant with potential interactions with Nrf2 (179), over and above its role in photoperiod signaling. Melatonin is UVR responsive in keratinocytes (180), yet the interplay of UVR, oxidative stress, melatonin and Nrf2 are poorly understood.
While food intake secondarily signals peripheral circadian oscillators that entrain circadian expression of metabolic enzyme expression, the metabolism of dietary intake is regulated by circadian rhythms (67, 68, 71, 79). This bidirectional feedback between circadian clocks and metabolism acts on multiple systems, including Nrf2 (131). In addition to the upregulation of the Nrf2 transcription factor in the presence of UVR mediated intracellular ROS and oxidative stress (88), Nrf2 is a major regulator of dietary metabolism (131), including fatty acid metabolism that can be modulated by oxidative stress (104). In fact, Nrf2 functions in an intestinal regulatory capacity during development that is hypothesized as the precursor to the detoxifying action of Nrf2 in response to oxidative stress (181). During the intestinal development of invertebrates, the Nrf2 (Cnc) homologs regulate stem cell proliferation and mesendodermal tissue differentiation through balancing cellular redox potential (181, 182). In larva teleost fishes, the expression of dietary enzymes has been shown to be modulated by exposure to UVB (183), suggesting that the normal circadian expression of Nrf2 may be modulated by altered UVB exposures (68), translating to genomic alterations of normal digestive patterns.
Finally, as an extension of the coupling between cyclic rhythms, metabolism, and their functional roles in the maintenance of metabolic homeostasis, suspected consequences on lifespan are emerging across broad taxonomic scales. The role of invertebrate Nrf2 homologs in regulating oxidative stress has been coupled with the capacity to determine lifespan (182, 184), and similar Nrf2 roles have been identified for vertebrate species (104, 131, 185). In addition to nutritional feedback and entrainment, the coupling between circadian cycles and metabolism are also correlated with feedback loops that rely on glucocorticoid signaling and nuclear receptors including, ROR (activator) and REV-ERB (repressor), which can reset circadian clocks, alter lipid metabolism, and exasperate the inflammatory system (67, 71, 186). Interestingly among humans, polymorphisms in the circadian Per gene system are linked to alterations in glucose metabolism (172), while aging in Drosophila is correlated with stress dependent accumulation of Per mutations (187). The relationships of circadian redox sensors, metabolism and aging are all the more interesting when considering the role of accumulated free radicals in the aging process. It is perhaps fruitful to speculate how UVR exposure may modulate these complex set of conceptual interrelationships.
Conclusions
While the Montreal Protocol has been a great achievement in Environmental Policy a real threat of detrimental exposure to UVR persists. Long term trends and implications of persistent ozone depletion must be considered in the context of global climate change as recent research has shown interactions between these two phenomena (23, 27). Naturally biochemical and hormonal signaling is driven by the sun, and in this context we have highlighted the potential for excessive UVR exposure to induce dysregulations of biological rhythmicity at the scale of seasons and circadian cycles. Current research in signal transduction has revealed newly discovered physiological targets of UVR exposure, including the AhR and Nrf2 transcription factors, which have been discussed herein as UVR targets of dysregulation. This review has attempted to demonstrate the convergence of the current hot topics of ozone depletion, climate change, and biological rhythmicity, with the strong potential for negative consequences for ecological integrity and human health. It is hopeful that further research in any one of these individual topics of study will consider results in a manner that integrates the interaction of different fields of study (i.e. ozone depletion, climate change, ecosystems in context of the former, organismal and human health in the context of the former).
Indeed, biological timing that is driven by solar cues is a ubiquitous trait across the taxonomic landscape and the consequences for anthropogenic mediated alterations in solar radiance reaching the earths are equally broad in scope. The coupling of physiological homeostasis and biological rhythmicity should not be underemphasized. UVR mediated imbalances can translate to changes in overall ecosystem landscape, loss of individual fitness (a marker of natural selection) due to temporal life history shifts, or health consequences such as cancer. Moreover, the AhR and Nrf2 transcription factors highlighted in this review are widely conserved across taxonomic groups, providing further evidence that UVR induced modulation of AhR/Nrf2 signaling may be targeted pathways of dysregulation throughout large portions of the taxonomic tree of life. For humans in particular, the AhR and Nrf2 pathways are ever emerging as routes of UVR-induced skin cancer, which remains a major health concern in this modern era of climate change and increased UVR exposure. In conclusion, these are worthy avenues of continued pursuit in an era of climate change and increased UVR exposure.
Acknowledgements
This project was supported by NOAA-Environmental Cooperative Science Center Grant # NA11SEC4810001-Sub Contract # 003499; NIH RCMI-Center for Environmental Health Grant # 5G12RR013459-15, and NIH NIMHD Grant # 8G12MD007581-15, at Jackson State University.
Contributor Information
Mark A. Dugo, Email: mark.a.dugo@students.jsums.edu.
Fengxiang Han, Email: fengxiang.han@jsums.edu.
Paul B. Tchounwou, Email: paul.b.tchounwou@jsums.edu.
Literature Cited
- 1.Montreal Protocol. United Nations Environment Programme. 1987 Available at: http://ozone.unep.org.
- 2.Manney GL, Santee ML, Rex M, Livesey NJ, Pitts MC, Veefkind P, Nash ER, Wohltmann I, Lehmann R, Froidevaux L, Poole LR, Schoeberl MR, Haffner DP, Davies J, Dorokhov V, Gernandt H, Johnson B, Kivi R, Kyrö E, Larsen N, Levelt PF, Makshtas A, Thomas McElroy C, Nakajima H, Parrondo MC, Tarasick DW, von der Gathen P, Walker KA, Zinoviev NS. Unprecedented Arctic ozone loss in 2011. Nature. 2011;(478):469–475. doi: 10.1038/nature10556. [DOI] [PubMed] [Google Scholar]
- 3.Kyoto Protocol. Framework Convention on Climate Change. 1997 Available at: http://unfccc.int/kyoto_protocol/items/2830.php.
- 4.Hader D-P, Kumar HD, Smith RC, Worrest RC. Effects of solar UV radiation on aquatic ecosystems and interactions with climate change. Photochem Photobiol Sci. 2007;6:267–285. doi: 10.1039/b700020k. [DOI] [PubMed] [Google Scholar]
- 5.Hernando MP, Malanga G, Ferreyra GA. Arntz WE, Lovrich GA, Thatje S, editors. Oxydative stress and antioxidant defences generated by solar UV in a sub-Antarctic marine phytoflagellate. The Magellan-Antarctic Connection : Links and Frontiers at High Southern Latitudes. Scientia Marina. 2005;69(suppl.2):287–295. [Google Scholar]
- 6.Mekkawy IAA, Mahmoud UM, Osman AG, Sayed A, El-Din H. Effects of ultraviolet A on the activity of two metabolic enzymes, DNA damage and lipid peroxidation during early developmental stages of the African catfish, Clarias gariepinus (Burchell, 1822) Fish Physiol Biochem. 2010;36:605–626. doi: 10.1007/s10695-009-9334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkins GI. Signal Transduction in Responses to UV-B Radiation. Annual Review of Plant Biology, Vol. 2009;60:407–431. doi: 10.1146/annurev.arplant.59.032607.092953. [DOI] [PubMed] [Google Scholar]
- 8.Bischof K, Janknegt PL, Buma AGJ, Rijstenbil JW, Peralta G, Breeman AM. Oxidative stress and enzymatic scavenging of superoxide radicals induced by solar UB-B radiation in Ulva canopies from southern Spain. Sci Mar. 2003;67(3):353–359. [Google Scholar]
- 9.Krutmann J, Morita A, Chung JH. Sun exposure: What molecular photodermatology tells us about its good and bad sides. J Investig Dermatol. 2012;132:976–984. doi: 10.1038/jid.2011.394. [DOI] [PubMed] [Google Scholar]
- 10.Willis I, Menter JM. Effect of varying dose of UV radiation on mammalian skin: simulation of decreasing stratospheric ozone. J Invest Dermatol. 1983;80(5):416–419. doi: 10.1111/1523-1747.ep12555445. [DOI] [PubMed] [Google Scholar]
- 11.Morales LO, Tegelberg R, Brosche M, Keinanen M, Lindfors A, Aphalo PJ. Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula pendula leaves. Tree Physiol. 2010;30:923–934. doi: 10.1093/treephys/tpq051. [DOI] [PubMed] [Google Scholar]
- 12.Kaiserli E, Jenkins G. I.UV-B promotes rapid nuclear translocation of the Arabidopsis UV-B specific signaling component UVR8 and activates its function in the nucleus. Plant Cell. 2007;19(8):2662–2673. doi: 10.1105/tpc.107.053330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crump D, Lean D, Trudeau VL. Octylphenol and UV-B radiation alter larval development and hypothalamic gene expression in the leopard frog (Rana pipiens) Environ Health Perspect. 2002;110(3):277–284. doi: 10.1289/ehp.02110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stolarski RS. A hole in Earth’s shield. In: Garwin L, Lincoln T, editors. A Century of Nature. Chicago: Univ. of Chicago Press; 2003. pp. 283–289. [Google Scholar]
- 15.Farman JC, Gardiner BG, Shanklin JD. Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction. Nature. 1985;315(16):207–210. [Google Scholar]
- 16.Molina MJ, Rowland FS. Stratospheric sink for chloroflouromethanes: chlorine atomic-atalysed destruction of ozone. Nature. 1974;(249):810–812. [Google Scholar]
- 17.National Oceanic and Atmospheric Administration (NOAA). Twenty Questions and Answers about the ozone layer: 2010 Update. Scientific Assessment of ozone depletion. 2010:21pp. [Google Scholar]
- 18.World Meteorological Organization (WMO) Global Ozone, Research and Monitoring Project Report 52. Geneva, Switzerland: WMO; 2010. Scientific Assessment of Ozone Depletion: 2010; p. 24pp. [Google Scholar]
- 19.Solomon S, Chanin M-L. The Antarctic ozone hole: A unique example of the science and policy interface. In: Smithsonian I, editor. Science Diplomacy in Science Diplomacy: Antarctica, Science, and the Governance of International Spaces. Smithsonian Institutional Scholarly Press; 2011. pp. 189–197. [Google Scholar]
- 20.Weber M, Dikty S, Burrows JP, Garny H, Dameris M, Kubin A, Abalichin J, Langematz U. The Brewer-Dobson circulation and total ozone from seasonal to decadal time scales. Atmos Chem Phys. 2011;11:11221–11235. [Google Scholar]
- 21.Waugh DW, Polvani LM. Stratospheric polar vortices. The Stratosphere: Dynamics, Transport, and Chemistry. Geophys Monogr Ser. 2010:190. [Google Scholar]
- 22.Tully MB, Klekociuk AR, Alexander SP, Dargaville RJ, Deschamps LL, Fraser PJ, Gies HP, Henderson SI, Javorniczky J, Krummel PB, Petelina SV, Shanklin JD, Siddaway JM, Stone KA. The Antarctic ozone hole during 2008 and 2009. Aust Meteor Ocean J. 2011;61:77–90. [Google Scholar]
- 23.Solomon S, Portmann RW, Thompson DWJ. Contrasts between Antarctic and Arctic ozone depletion. Proc Natl Acad Sci USA. 2007;104(2):445–449. doi: 10.1073/pnas.0604895104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randel WJ, Wu F. Cooling of the Arctic and Antarctic polar stratospheres due to ozone depletion. J Climate. 1999;12:1467–1479. [Google Scholar]
- 25.Waters JW, Froidevaux L, Read WG, Manney GL, Elson LS, Flower DA, Jarnot RF, Harwood RS. Stratospheric ClO and ozone from the microwave Llimb Sounder on the Upper Atmosphere Research Satellite. Nature. 1993;362:597–602. [Google Scholar]
- 26.Austin J, Butchart N, Shine KP. Possibility of an Arctic ozone hole in a doubled-CO2 climate. Nature. 1992;360:221–225. [Google Scholar]
- 27.Velders GJM, Anderson SO, Daniel JS, Fahey DW, McFarland M. The importance of the Montreal Protocol in protecting climate. Proc Natl Acad Sci USA. 2007;104:4814–4819. doi: 10.1073/pnas.0610328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson DWJ, Solomon S, Kushner PJ, England MH, Grise KM, Karoly DJ. Signatures of the Antarctic ozone hole in Southern Hemisphere surface climate change. Nat Geosci. 2011:1–9. [Google Scholar]
- 29.Douville H. Stratospheric polar vortex influence on Northern Hemisphere winter climate variability. Geophys Res Lett. 2009;36:L18703. [Google Scholar]
- 30.Garcia RR, Randel WJ. Acceleration of the Brewer-Dobson circulation due to increases in greenhouse gases. J Atmos Sci. 2008;65:2731–2739. [Google Scholar]
- 31.Winter B, Bourqui MS. The impact of surface temperature variability on the climate change response in the Northern Hemisphere polar vortex. Geophys Res Lett. 2011;38:L08808. [Google Scholar]
- 32.Garfinkel CI, Shaw TA, Hartman DL, Waugh DW. Does the Holton-Tan Mechanism Explain How the Quasi-Biennial Oscillation Modulates the Arctic Polar Vortex? J Atmos Sci. 2012 Electronic ISSN:1520-0469. [Google Scholar]
- 33.Naoe H, Kiyotaka S. Future changes in the influence of the quasi-biennial oscillation on the northern polar vortex simulated with an MRI chemisty climate model. J Geophys Res. 2012;117:D03102. [Google Scholar]
- 34.Espy PJ, Ochoa Fernandez SO, Forkman P, Murtagh D, Stegman J. The role of the QBO in the inter-hemispheric coupling of summer mesospheric temperatures. Atmos Chem Phys. 2011;11:495–502. [Google Scholar]
- 35.Frauenfeld OW, Davis RE. Northern Hemisphere circumpolar vortex trends and climate change implications. J Geophys Res. 2003;108(4423) [Google Scholar]
- 36.Rohli RV, Wrona KM, Mchugh MJ. January Northern Hemisphere circumpolar vortex variability and its relationship with hemispheric temperature and regional teleconnections. Int J Climatol. 2005;25:1421–1436. [Google Scholar]
- 37.Turner J, Comiso JC, Marshall GJ, Lachlan-Cope TA, Bracegirdle T, Maksym T, Meredith MP, Wang Z. Non-annular atmospheric circulation change induced by stratospheric depletion and its role in the recent increase of Antarctic sea ice extent. Geophys Res Lett. 2009;36:1–5. [Google Scholar]
- 38.National Oceanic and Atmospheric Administration (NOAA) Near-Real-Time DMSP SSM/I Daily Polar Gridded Sea Ice Concentrations. 2011 Available at: http://nsidc.org/data/nsidc-0081.html.
- 39.Lefebvre W, Goose H. Influence of the Southern Annular Mode on the sea ice-ocean system: the role of the thermal and mechanical forcing. Ocean Science. 2005;1:145–57. [Google Scholar]
- 40.Sigmond M, Fyfe JC. Has the ozone hole contributed to increased Antarctic sea ice extent? Geophys Res Lett. 2010;37:L18502. [Google Scholar]
- 41.Han FX, King RL, Lindner JS, Yu TZ, Monts DL, Su Y, Luthe JC, Durbha SS, Younan NH, Plodinec MJ. Nutrient fertilizer requirements for sustainable biomass supply to meet bioenergy goal. Biomass Bioenergy. 2011;35:253–262. [Google Scholar]
- 42.Han FX, Plodinec MJ, Su Y, Monts DL, Li Z. Terrestrial carbon pools in southeast and south-central United States. Clim Change. 2007;84:191–202. [Google Scholar]
- 43.Li ZP, Han FX, Su Y, Zhang TL, Sun B, Monts DL, Plodinec MJ. Assessment of soil organic and carbonate carbon storage in China. Geoderma. 2007;138:119–126. [Google Scholar]
- 44.McKenzie ML, Aucampb PJ, Bais AF, Björn LO, Ilyas M. Changes in biologically active ultraviolet radiation reaching the Earth’s surface. Photochem Photobiol Sci. 2007;6:218–231. doi: 10.1039/b700017k. [DOI] [PubMed] [Google Scholar]
- 45.Matzarakis A, Amelung B. Physiological Equivalent Temperature as an indicator for impacts of climate change on thermal comfort of humans. In: Thomson MC, et al., editors. Seasonal Forecasts, Climatic Change and Human Health. Springer; 2008. pp. 161–72. [Google Scholar]
- 46.Way DA, Sage RF. Elevated growth temperatures reduce the carbon gain of black spruce [Picea mariana (Mill.) B.S.P.] Glob Change Biol. 2008;14:624–636. [Google Scholar]
- 47.Salazar-Parra C, Aguirreolea J, Sánchez-Díaz M, José Irigoyen J, Morales F. change FClimate (elevated CO2 temperature elevated and moderate drought) triggers the antioxidant enzymes' response of grapevine cv Tempranillo, avoiding oxidative damage. Physiologia Plantarum. 2011;144(2):99–110. doi: 10.1111/j.1399-3054.2011.01524.x. [DOI] [PubMed] [Google Scholar]
- 48.Southern A Allen, Kellar N. Molecular signature of physiological stress in dolphins based on protein expression profiling of skin. SWFSC Admin Rept. 2002 LJ-02-27. 35p. [Google Scholar]
- 49.Ross AW, Helfer G, Russell L, Darras VM, Morgan PJ. Thyroid hormone signaling genes are regulated by photoperiod in the hypothalamus of F344 rats. PLoS One. 2011;6(6):e21351. doi: 10.1371/journal.pone.0021351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero LM. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocr. 2002;128:1–24. doi: 10.1016/s0016-6480(02)00064-3. [DOI] [PubMed] [Google Scholar]
- 51.Bau F, Parent JP. Seasonal variation of thyroid hormone levels in wild fish. C. R. Acad. Sci. III. 2000;323:365–372. doi: 10.1016/s0764-4469(00)00137-2. [DOI] [PubMed] [Google Scholar]
- 52.Mylonas CC, Scott AP, Zohar Y. Plasma gonadotropin II, sex steroids, and thyroid hormones in wild striped bass (Morone saxatilis) during spermiation and final oocyte maturation. Gen Comp Endocr. 1997;108:223–236. doi: 10.1006/gcen.1997.6967. [DOI] [PubMed] [Google Scholar]
- 53.Dahl GE, Evans NP, Thrun LA, Karsch FJ. Thyroxine is permissive to seasonal transitions in reproductive neuroendocrine activity in the Ewe. Biol Reprod. 1995;5:690–696. doi: 10.1095/biolreprod52.3.690. [DOI] [PubMed] [Google Scholar]
- 54.Chemineau P, Martin GB, Saumande J, Normante E. Seasonal and hormonal control of pulsatile LH secretion in the dairy goat (Capra hircus) J Reprod Fertil. 1988;83:91–98. doi: 10.1530/jrf.0.0830091. [DOI] [PubMed] [Google Scholar]
- 55.Light P, Breitenbach GL, Congdon JD. Seasonal cycles in testicular activity, gonadotropin, and thyroxine in the painted turtle, Chrysemys picta, under natural conditions. Gen Comp Endocr. 1985;59:130–139. doi: 10.1016/0016-6480(85)90427-7. [DOI] [PubMed] [Google Scholar]
- 56.Lagercrantz U. At the end of the day: a common molecular mechanism for photoperiod response in plants. J Exp Bot. 2009;139:1–15. doi: 10.1093/jxb/erp139. [DOI] [PubMed] [Google Scholar]
- 57.Yasuo S, Yoshimura T. Comparative analysis of the molecular basis of photoperiodic signal transduction in vertebrates. Integ Comp Biol. 2009;49(5):507–518. doi: 10.1093/icb/icp011. [DOI] [PubMed] [Google Scholar]
- 58.Christie JM, Arvai AS, Baxter KJ, Heilmann M, Pratt AJ, O'Hara A, Kelly SM, Hothorn M, Smith BO, Hitomi K, Jenkins GI, Getzoff ED. Plant UVR8 Photoreceptor Senses UV-B by Tryptophan-Mediated Disruption of Cross-Dimer Salt Bridges. Science. 2012;335(6075):1492–1496. doi: 10.1126/science.1218091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen JD, Slovin JP, Hendrickson AM. Two genetically discrete pathways convert tryptophan to auxin: more redundancy in auxin biosynthesis. Trends Plant Sci. 2003;8(5):197–199. doi: 10.1016/S1360-1385(03)00058-X. [DOI] [PubMed] [Google Scholar]
- 60.Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 2001;291:306–309. doi: 10.1126/science.291.5502.306. [DOI] [PubMed] [Google Scholar]
- 61.Hull AK, Vij R, Celenza JL. Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc Natl Acad Sci U S A. 2000;97(5):2379–2384. doi: 10.1073/pnas.040569997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R. Melatonin Nature’s most versatile biological signal? FEBS J. 2006;273:2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- 63.Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, Zatz M, Iuvone PM, Rodriquez IR, Begay V, Falcon J, Cahill GM, Cassone VM, Baler R. The Melatonin rhythm-generating enzyme: Molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 1997;52:307–358. [PubMed] [Google Scholar]
- 64.Vivien-Roels B, Pevet P, Beck O, Fevre-Montage M. Identifitcation of melatonin in the compound eye of an insect, the locust (Locusta migratoria), by radioimmuno-assay and gas chromatography-mass spectrometry. Neurosci Lett. 1984;49:153–157. doi: 10.1016/0304-3940(84)90152-6. [DOI] [PubMed] [Google Scholar]
- 65.Gore AC. Neuroendocrine targets of endocrine disruptors. Hormones. 2010;9(1):16–27. doi: 10.14310/horm.2002.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helfman GS, Collette BB, Facey DE. The Diversity of Fishes. Malden, Massachusetts:Blackwell Science. 1999:528. [Google Scholar]
- 67.Yang X. A wheel of time: the circadian clock, nuclear receptors, and physiology. Genes Dev. 2010;24:741–747. doi: 10.1101/gad.1920710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Claudel T, Cretenet G, Saumet A, Gachon F. Crosstalk between xenobiotic metabolism and circadian clock. DEBS Letters. 2007;581:3626–3633. doi: 10.1016/j.febslet.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 69.Schmutz I, Albrecht U, Ripperger JA. The role of clock genes and rhythmicity in the liver. Mol Cell Endocrinol. 2012;349(1):38–44. doi: 10.1016/j.mce.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 70.Zuber A, MCenteno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. PNAS. 2009;106(38):16523–16528. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin JD, Liu C, Li S. Integration of energy metabolism and the mammalian clock. Cell Cycle. 2008;7(4):453–457. doi: 10.4161/cc.7.4.5442. [DOI] [PubMed] [Google Scholar]
- 72.Paranjpe DA, Sharma VK. Evolution of temporal order in living organisms. J Circadian Rhythms. 2005;3(1):7. doi: 10.1186/1740-3391-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma VK. Adaptive significance of circadian clocks. Chronobio Int. 2003;20(6):901–919. doi: 10.1081/cbi-120026099. [DOI] [PubMed] [Google Scholar]
- 74.Young MW, Kay SA. Time Zones: A comparative genetics of circadian clocks. Nat Rev Genet. 2001;2(9):702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- 75.Troncoso-Ponce MA, Mas P. Newly Described Components and Regulatory Mechanisms of Circadian Clock Function in Arabidopsis thaliana . Mol Plant. 2012 doi: 10.1093/mp/ssr117. [DOI] [PubMed] [Google Scholar]
- 76.Fehér B, Kozma-Bognár L, Kevei E, Hajdu A, Binkert M, Jon Davis S, Schäfer E, Ulm R, Nagy F. Functional interaction of the circadian clock and UV Resistence Locus 8-controlled UV-B signaling pathways in Arabidopsis thaliana . Plant J. 2011;67(1):37–48. doi: 10.1111/j.1365-313X.2011.04573.x. [DOI] [PubMed] [Google Scholar]
- 77.Nikaido SS, Johnson CH. Daily and circadian variation in survival from ultraviolet radiation in Chlamydomonas reinhardtii . Photochem Photobiol. 2000;71(6):758–765. doi: 10.1562/0031-8655(2000)071<0758:dacvis>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 78.Yoshida Y, Iigusa H, Wang N. Hasunuma. Cross-talk between the cellular redox state and the circadian system in Neurospora . PLoS One. 2011;6(12):e28277. doi: 10.1371/journal.pone.0028227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zmrzljak UP, Rozman D. Circadian regulation of endobiotic and xenobitoic detoxification pathways: the time matters. Chem Res Toxicol. 2012 doi: 10.1021/tx200538r. [DOI] [PubMed] [Google Scholar]
- 80.Fukushima A, Kusano M, Nakamichi N, Kobayashi M, Hayashi N, Sakakibara H, Mizuno T, Saito K. Impact of clock-associated Arabidopsis pseudo-response regulators in metabolic coordination. Proc Natl Acad Sci U S A. 2009;106(17):7251–7256. doi: 10.1073/pnas.0900952106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harmer SL, Panda S, Kay SA. Molecular bases of circadian rhythms. Annu Rev Cell Dev Biol. 2001;17:215–253. doi: 10.1146/annurev.cellbio.17.1.215. [DOI] [PubMed] [Google Scholar]
- 82.Reitzel AM, Behrendt L, Tarrant AM. Light entrained rhythmic gene expression in the sea anemone Nematostella vectensis: The evolution of the animal circadian clock. PLoS One. 2010;5(9):e12805. doi: 10.1371/journal.pone.0012805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gu Yi-Zhong, Hogenesch JB, Bradfield CA. The PAS Superfamily: Sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 84.Taylor BL, Zhulin IB. PAS Domains: Internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev. 1999;63(2):479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pellequer Jean-Luc, Wager-Smith KA, Kay SA, Getzoff ED. Photoactive yellow protein: A structural prototype for the three-dimensional fold of the PAS domain superfamily. Proc Natl Acad Sci U S A. 1998;95:5884–5890. doi: 10.1073/pnas.95.11.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vreede J, Juraszek J, Bolhuis PG. Predicting the reaction coordinates of millisecond light-induced conformation changes in photoactive yellow protein. Proc Natl Acad Sci USA. 2010;107(6) doi: 10.1073/pnas.0908754107. 2397-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oda A, Fujiwara S, Kamada H, Coupland G, Mizoguchi T. Antisense suppression of the Arabidopsis PIF3 gene does not affect circadian rhythms but causes early flowering and increases FT expression. FEBS Lett. 2004;557:259–264. doi: 10.1016/s0014-5793(03)01470-4. [DOI] [PubMed] [Google Scholar]
- 88.Kasper JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47(9):1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dahms H-U, Lee J-E. UV radiation in marine ectotherms: molecular effects and responses. Aquat Toxicol. 2010;97:3–14. doi: 10.1016/j.aquatox.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 90.Omiecinski CJ, Vanden Heuvel JP, Perdew GH, Peters JM. Zenobiotic metabolism, disposition, and regulation by receptors: from biochemical phenomenon to predictors of major toxicities. Toxicol Sci. 2011;120(S1):S49–S75. doi: 10.1093/toxsci/kfq338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu C, Li CY, Kong AN. Induction of Phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28(3):249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 92.Jux B, Kadow S, Luecke S, Rannug A, Krutmann J, Esser C. The aryl hydrocarbon receptor mediates UVB radiation-induced skin tanning. J Investig Dermatol. 2011;131:203–10. doi: 10.1038/jid.2010.269. [DOI] [PubMed] [Google Scholar]
- 93.Gruber F, Mayer H, Lengauer B, Mlitz V, Sanders JM, kadl A, Bilban M, de Martin R, Wagner O, Kensler TW, Yamamoto M, Leitinger N, Tschachler E. NF-E2-related factor 2 regulates the stress response to UVA-1 oxidated phopholipds in skin cells. FASEB. 2010;24:39–48. doi: 10.1096/fj.09-133520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marionnet C, Pierrard C, Lejeune F, Sok J, Thomas M, Bernerd F. Different Oxidative Stress Response in Keratinocytes and Fibroblasts of Reconstructed Skin Exposed to Non Extreme Daily-Ultraviolet Radiation. PLoS One. 2010;5(8):e12059. doi: 10.1371/journal.pone.0012059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Linderdoth M, Ledesma M, Noaksson E, Widell B, Zebuhr Y, Balk L. Seasonal testosterone UDP-glucuronosyltransferase activity and biliary steroids in Eurasian perch: Response to leachate exposure. Ecotoxicol Environ Saf. 2007;68:49–56. doi: 10.1016/j.ecoenv.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 96.Rewitz KF, Styrishave B, Lobner-Olesen A, Anderson O. Marine invertebrate cytochrome p450: emerging insights from vertebrate and insect analogies. Comp Biochem Physiol C. 2006;143:363–381. doi: 10.1016/j.cbpc.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 97.Kohle C, Bock KW. Coordinate regulation of phase I and II xenobiotic metabolisms by Ah receptor and Nrf2. Biochem Pharm. 2009;73:1853–1862. doi: 10.1016/j.bcp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 98.Shen G, Kong A-N. Nrf2 plays an important role in coordinated regulation of phase II drug metabolism enzymes and phase III drug transporters. Biopharm Drug Dispos. 2009;30:345–355. doi: 10.1002/bdd.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Korashy HM, El-Kadi AOS. Transcriptional regulation of the NAD(P)H:Quinone oxidoreductase 1 and glutathione S- transferase Ya genes by mercury, lead, and copper. Drug Metab Dispos. 2006;34(1):152–165. doi: 10.1124/dmd.105.005397. [DOI] [PubMed] [Google Scholar]
- 100.Rushmore TH, Pickett CB. Transcriptional regulation of the rat gluthathione S- Transferase Ya subunit gene. J Biol Chem. 1990;265(24):14648–14653. [PubMed] [Google Scholar]
- 101.Hayes JD, Dinkova-Kostova AT, McMahon M. Cross-talk between transcription factors AhR and Nrf2: Lessons for cancer chemoprevention from dioxin. Toxicol Sci. 2009;111(2):199–201. doi: 10.1093/toxsci/kfp168. [DOI] [PubMed] [Google Scholar]
- 102.Miao W, Hu L, James Scrivens P, Batist G. Transcriptional regulation of NF-E2 related p45-related factor (Nrf2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway. J Biol Chem. 2005;280(21):20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- 103.Ma Q, Kinneer K, Bi Y, Chan JY, Kan YK. Induction of murine NAD(P)H:quinone oxidoreductase by 2,3,7,8-tetrachlorodibenzo-p-dioxin requires the CNC (cap’n’collar) basic leucine zipper transcription factor Nrf2 (nuclearfactorerythroid2-related) factor2):cross interaction between AhR (aryl hydrocarbon receptor) and Nrf2 signal transduction. Biochem J. 2004;377:205–213. doi: 10.1042/BJ20031123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y-KJ, Yeager RL, Tanaka Y, Klaasen CD. Enhanced expression of Nrf2 in mice attenuates the fatty liver produced by a methionine- and choline-deficient diet. Toxicol Appl Pharmacol. 2010;245:326–334. doi: 10.1016/j.taap.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karchner SI, Powell WH, Hahn ME. Identification receptors functional characterization of two highly divergent aryl hydrocarbon (AHR1, AHR2) in the teleost Fundulus heteroclitus Evidence for a novel class of ligand-binding basis helix-loop-helix Per-ARNT-Sim(bHLH-PAS) factors. J Biol Chem. 1999;274:33814–33824. doi: 10.1074/jbc.274.47.33814. [DOI] [PubMed] [Google Scholar]
- 106.Zhou H, Liao HWC, Diao X, Zhen J, Chen L, Xue Q. Toxicology mechanism of the persistent organic pollutants (POPs) in fish through AhR pathway. Toxicol Mech Methods. 2010;20(6):279–286. doi: 10.3109/15376516.2010.485227. [DOI] [PubMed] [Google Scholar]
- 107.Kazlauskas A, Sundstrom S, Poellinger L, Pongratz I. The hsp90 chaperone complex regulates intracellular localization of the dioxin receptor. Mol Cell Biol. 2001;21(7) doi: 10.1128/MCB.21.7.2594-2607.2001. 2594-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kazlauskas A, Poellinger L, Pongratz I. Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (Aryl hydrocarbon) receptor. J Biol Chem. 1999;274(19):13519–13524. doi: 10.1074/jbc.274.19.13519. [DOI] [PubMed] [Google Scholar]
- 109.Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome p450 genes. Biochem Biophys Res Commun. 2005;338:311–317. doi: 10.1016/j.bbrc.2005.08.162. [DOI] [PubMed] [Google Scholar]
- 110.Bohonowych JE, Denison MS. Persistent binding of ligands to the aryl hydrocarbon receptor. Toxicol Sci. 2007;98(1):99–109. doi: 10.1093/toxsci/kfm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wincent E, Amini N, Luecke S, Glatt H, Bergman J, Crescenzi C, Rannug A, Rannug U. The suggested physiological Aryl hydrocarbon receptor activator and cytochrome p4501 substrate 6-formylindolo (3,2-b] carbazole is present in humans. J Biol Chem. 2009;284(5):2690–2696. doi: 10.1074/jbc.M808321200. [DOI] [PubMed] [Google Scholar]
- 112.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;(21):102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mukai M, Tischkau SA. Effects of tryptophan photoproducts in the circadian timing system: Searching for a physiological role for the aryl hydrocarbon receptor. Toxicol Sci. 2007;95(1):172–181. doi: 10.1093/toxsci/kfl126. [DOI] [PubMed] [Google Scholar]
- 114.Wei YD, Rannug U, Rannug A. UV-mediated CYP1A1 gene expression in human cells is mediated by tryptophan. Chem Biol Interact. 1999;118:127–140. doi: 10.1016/s0009-2797(98)00118-5. [DOI] [PubMed] [Google Scholar]
- 115.Fritsche E, Scafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, Hubenthal U, Cline JE, Hajimiragha H, Schroeder P, Klotz L-O, Rannug A, Furst P, Hanenberg Abel J, Krutmann J. Lighting up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmic target for ultraviolet B radiation. Proc Natl Acad Sci U S A. 2007;104(21):8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Diani-Moore S, Labitzke E, Brown R, Garvin A, Wong L, Rifkind AB. Sunlight generates multiple tryptophan photoproducts eliciting high efficacy CYP1A induction in chick hepatocytes and In Vivo . Toxicol Sci. 2006;90(1):96–110. doi: 10.1093/toxsci/kfj065. [DOI] [PubMed] [Google Scholar]
- 117.Behrendt L, Jonsson ME, Goldstone JV, Stegeman JJ. Induction of cytochrome P450 1 genes and stress response genes in developing zebrafish exposed to ultraviolet radiation. Aquat Toxicol. 2010;98(1):74–82. doi: 10.1016/j.aquatox.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jonsson ME, Franks DG, Woodin BR, Jenny MJ, Garrick RA, Behrendt L, Hahn ME, Stegeman JJ. The tryptophan photoproduct 6-formylindolo [3,2-b] carbazole (FICZ) binds multiple AHRs and induces multiple CYP1 genes via AHR2 in zebrafish. Chem Biol Interact. 2009;181(3):447–454. doi: 10.1016/j.cbi.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ma Q. Influence of light on aryl hydrocarbon receptor signaling and consequences in drug metabolism, physiology and disease. Expert Opin Drug Metab Toxicol. 2011;7(10):1267–1293. doi: 10.1517/17425255.2011.614947. [DOI] [PubMed] [Google Scholar]
- 120.Ahmad N, Mukhtar H. Cytochrome p450: A target for drug development for skin disease. J Investig Dermatol. 2004;123:417–415. doi: 10.1111/j.0022-202X.2004.23307.x. [DOI] [PubMed] [Google Scholar]
- 121.Stockinger B, Hirota K, Duarte J, Veldhoen M. External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin Immunol. 2011;23(2):99–105. doi: 10.1016/j.smim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 122.Esser C, Ranug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30(9):4447–4454. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 123.Haarmann-Stemman T, Bothe H, Abel J. Growth factors, cytokines and their receptors as downstream targets of arylhydrocarbon receptor (AhR) signaling pathways. Biochem Pharmacol. 2009;77:508–520. doi: 10.1016/j.bcp.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 124.Ho PP, Steinman L. The aryl hydrocarbon receptor: a regulator of Th17 and Treg cell development in disease. Cell Res. 2008;18:605–608. doi: 10.1038/cr.2008.63. [DOI] [PubMed] [Google Scholar]
- 125.Clark RA. Skin-resident T Cells: The ups and downs of on site immunity. J Investig Dermatol. 2010;130:362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Davidovici BB, Sattar N, Jorg PC, Puig L, Emery P, Barker JN, Kerkhof P, van de, Stahle M, Nestle FO, Girolomoni G, Krueger JG. Psoriasis and systemic inflammatory diseases: Potential mechanistic links between skin disease and Co-Morbid conditions. J Investig Dermatol. 2010;130:1785–1796. doi: 10.1038/jid.2010.103. [DOI] [PubMed] [Google Scholar]
- 127.Richter-Hintz D, Their R, Steinwachs S, Kronenberg S, Fritsche E, Sachs B, Wulferink M, Tonn T, Esser C. Allelic variants of drug metabolizing enzymes as risk factors in psoriasis. J Invest Dermatol. 2003;120(5):765–770. doi: 10.1046/j.1523-1747.2003.12124.x. [DOI] [PubMed] [Google Scholar]
- 128.Katiyar SK, Matsui MS, Mukhtar H. Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J Invest Dermatol. 2000;114(2):328–333. doi: 10.1046/j.1523-1747.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- 129.Luecke S, Backlund M, Jux B, Esser C, Krutmann J, Rannug A. The aryl hydrocarbon receptor (AHR), a novel regulator of human melanogenesis. Pigm Cell Melanoma R. 2010;23(6):828–833. doi: 10.1111/j.1755-148X.2010.00762.x. [DOI] [PubMed] [Google Scholar]
- 130.Nair S, Kekatpure VD, Judson BL, Rifkind AB, Granstein RD, Boyle JO, Subbaramaiah K, Guttenplan JB, Dannenberg AJ. UVR exposure sensitizes keratinocytes to DNA adduct formation. Cancer Prev Res. 2009;2(10):895–902. doi: 10.1158/1940-6207.CAPR-09-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sykiotis GP, Habeos IG, Samuelson AV, Bohmann D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr Opin Clin Nutr Metab Care. 2011;14(1):41–48. doi: 10.1097/MCO.0b013e32834136f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maher J, Yamamoto M. The rise of antioxidant signaling- The evolution and hermetic actions of Nrf2. Toxicol Appl Pharmacol. 2010;244:4–15. doi: 10.1016/j.taap.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 133.Pi J, Freeman ML, Yamamoto M. Nrf2 in toxicology and pharmacology: the good, the bad, and the ugly? Toxicol Appl Pharmacol. 2010;244(1):1–3. doi: 10.1016/j.taap.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marrot L, Jones C, Perez P, Meunier J-R. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigm Cell Melanoma Res. 2007;21(1):79–88. doi: 10.1111/j.1755-148X.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- 135.Jian Z, Li K, Liu L, Zhang Y, Zhou Z, Li C, Gao T. Heme Oxygenase-1 Protects Human Melanocytes from H2O2-Induced Oxidative Stress via the Nrf2-ARE Pathway. J Investig Dermatol. 2011;131:1420–1427. doi: 10.1038/jid.2011.56. [DOI] [PubMed] [Google Scholar]
- 136.Zhong JL, Edwards GP, Raval C, Li H, Tyrrell RM. The role of Nrf2 in ultraviolet A mediated heme oxygenase 1 induction in human skin fibroblasts. Photochem Photobiol Sci. 2010;(9):18–24. doi: 10.1039/b9pp00068b. [DOI] [PubMed] [Google Scholar]
- 137.Schafer M, Dutsch S, Keller U auf dem, Navid F, Schwarz A, Johnson DA, Johnson JA, Werner S. Nrf2 establishes a glutathione-mediated gradient of UVB cytoprotection in the epidermis. Genes & Dev. 2010;24:1045–1058. doi: 10.1101/gad.568810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hirota A, Kawachi Y, Itoh K, Nakamura Y, Xu X, Banno T, Takahashi T, Yamamoto M, Otsuka F. Ultraviolet A irradiation induces NF-E2-related factor 2 activation in dermal fibroblasts: Protective role in UVA-induced apoptosis. J Invest Dermatol. 2005;124(4):825–832. doi: 10.1111/j.0022-202X.2005.23670.x. [DOI] [PubMed] [Google Scholar]
- 139.Hirota A, Kawachi Y, Yamamoto M, Koga T, Hamada K, Otsuka F. Acceleration of UVB-induced photoageing in nrf2 gene-deficient mice. Exp Dermatol. 2011;20(8):664–668. doi: 10.1111/j.1600-0625.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- 140.Kawachi Y, Xu X, Taguchi S, Sakurai H, Nakamura Y, Ishii Y, Fujisawa Y, Furata J, Takahashi T, Itoh K, Yamamoto M, Yamazaki F, Otsuka F. Attenuation of UVB-induced sunburn reaction and oxidative DNA damage with no alterations in UBV-induced skin carcinogenesis in Nrf2 gene-deficient mice. J Invest Dermatol. 2008;128(7):1773–1779. doi: 10.1038/sj.jid.5701245. [DOI] [PubMed] [Google Scholar]
- 141.Tsuji G, Takahara M, Uchi H, Matsuda T, Chiba T, Takeuchi S, Yasukawa F, Moroi Y, Furue M. Identification of ketoconazole as an AhR-Nrf2 activator in cultured human keratinocytes: the basis of its anti-inflammatory effect. J Investig Dermatol. 2012;132:59–68. doi: 10.1038/jid.2011.194. [DOI] [PubMed] [Google Scholar]
- 142.Braun S, Hanselmann C, Gassmann MG, Keller Ulrich auf dem, Born-Berclaz B, Chan K, Ka YW, Werner S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol. 2002;22(15):5492–5505. doi: 10.1128/MCB.22.15.5492-5505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Luo C, Urgard E, Vooder T, Metspalu A. The role of COX-2 and Nrf2/ARE in anti-inflammation and antioxidative stress: aging and anti-aging. Med Hypotheses. 2011;(2):174–178. doi: 10.1016/j.mehy.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 144.Merchant AA, Singh A, Matsui W, Biswal S. The redox-sensitive transcription factor Nrf2 regulates murine hematopoietic stem cell survival independently of ROS levels. Blood. 2011;118(25):6572–6579. doi: 10.1182/blood-2011-05-355362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rushworth SA, Shah S, MacEwan DJ. TNF mediates the sustained activation of Nrf2 in human monocytes. J Immunol. 2011;187(2):702–707. doi: 10.4049/jimmunol.1004117. [DOI] [PubMed] [Google Scholar]
- 146.McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor s activated by modified low-density lipoprotein. Proc Natl Acad Sci U S A. 2007;104(4):1412–1417. doi: 10.1073/pnas.0607296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.He J, Lee JH, Febbraio M, Xie W. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp Biol Med. 2011;236:1116–1121. doi: 10.1258/ebm.2011.011128. [DOI] [PubMed] [Google Scholar]
- 148.Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler TW. NRF2 modulates aryl hydrocarbon receptor signaling: Influence on adipogenesis. Mol Cell Biol. 2007;27(20):7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Isaac JL. Effects of climate change on life history:implications for extinction risk in mammals. Endang Species Res. 2009;7:115–123. [Google Scholar]
- 150.Noyes PD, McElwee MK, Miller DH, Clark WB, Van Tiem LA, Walcott KC, Erwin KN, Levin ED. The toxicology of climate change: Environmental contaminants in a warming world. Environ Int. 2009;35:971–986. doi: 10.1016/j.envint.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 151.Martin LB, Hawley DM, Ardia DR. An introduction to ecoimmunology. Funct Ecol. 2011;25(1):1–4. [Google Scholar]
- 152.Acecedo-Whitehouse K, Duffus AL. Effects of environmental change on wildlife health. Philos Trans R Soc Lond B Biol Sci. 2009;364(1534):3429–3438. doi: 10.1098/rstb.2009.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nelson RJ, Demas GE. Role of melatonin in mediating seasonal energetic and immunologic adaptations. Brain Res Bull. 1997;44:423–430. doi: 10.1016/s0361-9230(97)00222-0. [DOI] [PubMed] [Google Scholar]
- 154.Scheff JD, Calvano SE, Lowry SF, Androulakis IP. Modeling the influence of circadian rhythms on the acute inflammatory response. J Theor Biol. 2010;264(3):1068–1076. doi: 10.1016/j.jtbi.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 155.Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS One. 2010;5(6):e10995. doi: 10.1371/journal.pone.0010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Takahashi S, Yokota S-I, Hara R, Kobayashi T, Akiyama M, Moriya T, Shibata S. Physical and inflammatory stressors elevate circadian clock gene mPer1 mRNA levels in the paraventricular nucleus of the mouse. Endocrinology. 2001;142(11):4910–4917. doi: 10.1210/endo.142.11.8487. [DOI] [PubMed] [Google Scholar]
- 157.Eismann EA, Lush E, Sephton SE. Circadian effects in cancer-relevant psychoneuroendocrine and immune pathways. Psychoneuroendocrinology. 2010;35:963–976. doi: 10.1016/j.psyneuen.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 158.Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13:257–264. doi: 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 159.Kang B, Li Y-Y, Chang X, Liu L, Li Y-X. Modeling the effects of cell cycle M-phase transcriptional inhibition on circadian oscillation. PLoS Comput Biol. 2008;4(3):e1000019. doi: 10.1371/journal.pcbi.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kondratov RV, Antoch MP. Circadian proteins in the regulation of cell cycle and genotoxic stess responses. Trends Cell Biol. 2007;17(7):311–317. doi: 10.1016/j.tcb.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 161.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene Per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22:375–382. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 162.Unsal-Kacmaz K, Mullen TE, Kaufmann WK, Sancar A. Coupling of human circadian and cell cycles by the Timeless protein. Mol Cell Biol. 2005;25(8):3109–3116. doi: 10.1128/MCB.25.8.3109-3116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Berton TR, Pavone A, Fischer SM. Ultraviolet-B irradiation alters the cell cycle machinery in murine epidermis In Vivo . J Investig Dermatol. 2001;117(5):1171–1178. doi: 10.1046/j.0022-202x.2001.01536.x. [DOI] [PubMed] [Google Scholar]
- 164.Kalmes M, Hennen J, Clemens J, Blomeke B. Impact of aryl hydrocarbon receptor (AhR) knockdown on cell cycle progression in human HaCaT keratinocytes. Biol Chem. 2011;392(7):643–51. doi: 10.1515/BC.2011.067. [DOI] [PubMed] [Google Scholar]
- 165.Barhoover MA, Hall JM, Greenlee WF, Thomas RS. The AhR regulates cell cycle progression in human breast cancer cells via a functional interaction with CDK4. Mol Pharmacol. 2009 doi: 10.1124/mol.109.059675. mol.109.059675. [DOI] [PubMed] [Google Scholar]
- 166.Marlowe JL, Puga A. Aryl hydrocarbon receptor, cell cycle regulation, toxicity, and tumorigenesis. J Cell Biochem. 2005;96:1174–1184. doi: 10.1002/jcb.20656. [DOI] [PubMed] [Google Scholar]
- 167.Watabe Y, Nazuka N, Tezuka M, Shimba S. Aryl Hydrocarbon Receptor Functions as a Potent Coactivator of E2F1Dependent Trascription Activity. Biol Pharm Bull. 2010;33(3):389–397. doi: 10.1248/bpb.33.389. [DOI] [PubMed] [Google Scholar]
- 168.Xu Y, Voorhees JJ, Fisher GJ. Epidermal growth factor receptor is a critical mediator of ultraviolet B irradiation-induced signal transduction in immortalized human keratinocyte HaCaT cell. Am J Pathol Pathology. 2006;169(3):823–830. doi: 10.2353/ajpath.2006.050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tischkau SA, Jaeger CD, Krager SL. Circadian clock disruption in the mouse ovary in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Lett. 2011;201:116–122. doi: 10.1016/j.toxlet.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu C-X, Krager SL, Liao D-L, Tischkau SA. Disruption of CLOCK-BMAL1 transcriptional activity is responsible for aryl hydrocarbon receptor-mediated regulation of Period1 gene. Toxicol Sci. 2010;115(1):98–108. doi: 10.1093/toxsci/kfq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Garrett RW, Gasiewicz TA. The aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin alters the circadian rhythms, quiescence, and expression of clock genes in murine hematopoietic stem and progenitor cells. Mol Pharmacol. 2006;69(6):2076–2083. doi: 10.1124/mol.105.021006. [DOI] [PubMed] [Google Scholar]
- 172.Englund A, Kovanen L, Saarikoski ST, Haukka J, Reunanen A, Aromaa A, Lonnqvist J, Partonen T. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J Circadian Rhythms. 2009;7(5) doi: 10.1186/1740-3391-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu Rev Biochem. 2002;71:307–331. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- 174.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of Clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 175.Lee JH, Wada R, Febbraio M, He J, Matsubara T, Lee MJ, Lee MJ, Gonzalez FJ, Xie W. A Novel Role for the Dioxin Receptor in Fatty Acid Metabolism and Hepatic Steatosis. Gastroenterology. 2010;139(2):653–663. doi: 10.1053/j.gastro.2010.03.033. 2010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Matthews J, Gustafsson Jan-Åke. Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nucl Recept Signal. 2006;(4) doi: 10.1621/nrs.04016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Qin H, Powell-Coffman JA. The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Dev Biol. 2004;270(1):64–75. doi: 10.1016/j.ydbio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 178.Billiard SM, Meyer JN, Wassenberg DM, Hodson PV, Di Giulio RT. Nonadditive effects of PAHs on early vertebrate development: mechanisms and implications for risk assessment. Toxicol Sci. 2008;105(1):5–23. doi: 10.1093/toxsci/kfm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Luchetti F, Canonico B, Curci R, Battistelli M, Mannello F, Papa S, Tarzia G, Falcieri E. Melatonin prevents apoptosis induced by UV-B treatment in U937 cell line. J Pineal Res. 2006;40:158–167. doi: 10.1111/j.1600-079X.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 180.Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski AJ. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006;20(9):897–908. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]
- 181.An JH, Blackwell TK. SKN-1 links C elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;(17):1882–1893. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila . Cell Stem Cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sharma J, Rao YV, Kumar S, Chakrabarti R. Impact of UV-B radiation on the digestive enzymes and immune system of larvae of Indian major carp Catla catla . Int J Radiat Biol. 2010;86(3):181–186. doi: 10.3109/09553000903419312. [DOI] [PubMed] [Google Scholar]
- 184.Wang J, Robida-Stubbs S, Tulet JMA, Rual J-F, Vidal M, Blackwell TK. RNAi screening implicates a SKN-1 dependent transcriptional response in stress resistance and longevity deriving from translation inhibition. PLoS Genet. 2010;6(8):e1001048. doi: 10.1371/journal.pgen.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Leiser SF, Miller RA. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol Cell Biol. 2010;30(3):871–884. doi: 10.1128/MCB.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ramakrishnan SN, Muscat GEO. The orphan Rev-erb nuclear receptors: a link between metabolism, circadian rhythm and inflammation. Nucl Recept Signal. 2006;4 doi: 10.1621/nrs.04009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Krishnan N, Kretzschmar D, Rakshit K, Chow E, Giebultowicz JM. The circadian clock gene period extends healthspan in aging Drosophila melanogaster. Aging. 2009;1(11):937–948. doi: 10.18632/aging.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]