Abstract
Carbon nanotubes (CNTs), the most promising material with unique characteristics, find its application in different fields ranging from composite materials to medicine and from electronics to energy storage. However, little is known about the mechanisms behind the interaction of these particles with cells and their toxicity. The aim of this study was to assess the effects, after intraperitoneal injection, of functionalized multi walled carbon nanotubes (MWCNT) (carboxyl groups) on various hepatotoxicity and oxidative stress biomarkers (ROS, LHP, ALT, AST, ALP and morphology of liver) in the mouse model. The mice were dosed intraperitoneally at 0.25, 0.5 & 0.75 mg/kg/day for 5 days of purified/functionalized MWCNTs and two controls (negative; saline and positive; carbon black 0.75 mg/kg) as appropriate. Samples were collected 24 hours after the fifth day treatment following standard protocols. Exposure to carboxylated functionalized MWCNT; the body-weight gain of the mice decreased, induced reactive oxygen species (ROS), and enhanced the activities of serum amino-transferases (ALT/AST), alkaline phosphatases (ALP) and concentration of lipid hydro peroxide compared to control. Histopathology of exposed liver showed a statistically significant effect in the morphological alterations of the tissue compared to controls. The cellular findings reported here do suggest that purified carboxylated functionalized MWCNT has the potential to induce hepatotoxicity in Swiss-Webster mice through activation of the mechanisms of oxidative stress, which warrant in vivo animal exposure studies. However, more studies of functionalization in the in vivo toxicity of MWCNTs are required and parallel comparison is preferred.
Keywords: multi-walled carbon nanotube, serum amino-transferases, alkaline phosphatases, reactive oxygen species (ROS), hepatotoxicity, Swiss-Webster mice
Introduction
Nanotechnology a rapidly developing industry can have substantial impacts on the economy, society and the environment. Potential health and environmental effects of nanomaterials need to be thoroughly assessed before their widespread commercialization. Carbon nanotubes [CNT’s] are an example of a carbon-based nanomaterial [1], which has won enormous popularity and applications especially in biomedicine [2]. Recently, many groups have functionalized CNTs with polymers, proteins, nucleic acids and lipids for biomedical applications. For example, CNTs have been used as substrates for neural growth [3], bone cement [4], biosensors [5], tumor targeting [6,7] and delivery of drugs, proteins, peptides and nucleic acids [8].
While their applications in biomedical and material science continue and broaden, the toxicity issue of CNTs is emerging as one of the most urgent concerns [9]. With increasing interest in their potential toxicity, the adverse effects of engineered nanotubes are intensively being investigated. In recent years, many in vivo [10-17] and in vitro [18-23] studies have documented the potential adverse health effects associated with it. However, some other studies observed no obvious toxicity of properly functionalized or purified CNTs [24]. These conflicting conclusions show that physical and chemical parameters of CNTs play an important role in the toxicity and biocompatibility of CNTs, including surface functionalization, length, contaminants etc [24]. Previous studies have revealed that the chemical state of the surface of CNTs may strongly influence tissue response [22]. Chemically treating the surface of CNTs can alter their susceptibility to form agglomerates or to disperse in an environment, as well as to evoke an interaction with cells responsible for inflammation [25].
The ability of engineered nanomaterials to interact with biological tissues and generate reactive oxygen species (ROS) has been proposed as possible mechanisms involved in toxicity [26]. Reactive Oxygen Species are well known to play both a deleterious and a beneficial role in biological interactions. Generally, harmful effects of reactive oxygen species on the cell are most often damage of DNA, oxidations of polydesaturated fatty acids in lipids (lipid peroxidation), oxidations of amino acids in proteins and oxidatively inactivate specific enzymes by oxidation of co-factors. The increased generation of ROS caused by exposure to particles has been shown for many different forms of fine, ultrafine, and nanoscale particles, including SWCNTs, to be associated with minimal metal contamination [27].
Lipid peroxidation (LPO), the oxidative catabolism of polyunsaturated fatty acids, is widely accepted as a general mechanism for cellular injury and death [28, 29]. LPO and free radical generation are complex and deleterious processes that are closely related to toxicity [30]. LOP has been implicated in diverse pathological conditions. The extension of the oxidative catabolism of lipid membranes can be evaluated by several endpoints, but the most widely used method is the quantification of lipid hydroperoxide (LHP), one of the stable aldehydic products of lipoperoxidation, present in biological samples [31].
Because most chemicals are metabolized in the liver, the hepatocyte is the cell where a free radical attack results in lipid peroxidation, which can be linked to the electron transport chain of chemical metabolism. The methods normally employed for the detection of hepatotoxicity vary with the circumstances of their use. In vivo studies are essential to demonstrate a toxic agent that has in fact a demonstrable adverse effect on the liver in a setting of physiological significance. Biochemically, serum enzyme analyses have become the standard measure of hepatotoxicity during the past 25 years [32]. Measurement of enzyme activities of serum permit detection of hepatotoxicity with far less labor than that required for other tests. The rationale for the use of serum transaminases and other enzymes is that these enzymes, normally contained in the liver cells, gain entry into the general circulation when liver cells are injured [32].
Recently, many other research groups have successfully indicated that CNTs were trapped by the reticuloendothelial system and retained mainly in the liver of mice for a long time. This study assesses the effects, after intraperitoneal (ip) injection of functionalized MWCNTs (carboxyl groups) on induction of ROS and various hepatotoxicity markers in the mouse model. The question of the health effects of MWCNTs is quite acute and this study brings new data in a field where the largest proportion of publications have been conducted with pulmonary models. The few studies involving ip delivery of CNTs focus on the possible mesothelioma induction, lung granulomas [13, 14] or on pharmacokinetics [36]. Herein, we selected the liver as the target organ to investigate the in vivo biocompatibility of functionalized MWCNTs. Therefore the results presented here are of importance for health risk assessment.
Materials & Methods
Chemicals
Methanol, glacial acetic acid and superfrost microscope slides were purchased from Fischer-Scientific (Houston TX, USA). Xylene, ethyl alcohol, paraffin wax, hematoxylin-eosin stain, Serum Aminotransferases (GOT/GPT) and Alkaline Phosphatases Diagnostic assay kits were obtained from Sigma, (St. Louis MO, USA) and Lipid Peroxidation assay kit (Calbiochem, San Diego, CA, USA) DCFH-DA probe (Cell Biolabs, San Diego, CA, USA).
Multi-walled Carbon Nanotubes Characteristics
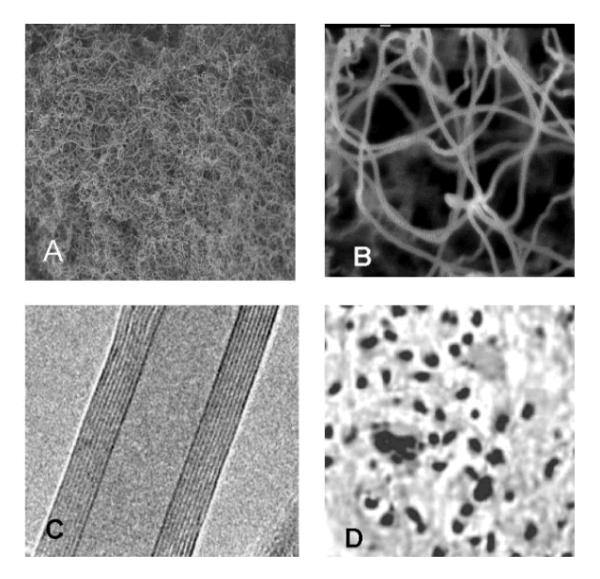
Multi-walled carbon nanotubes (MWCNTs) were synthesized by NanoLab Inc. (Newton MA, USA) by catalytic chemical vapor deposition (outer diameter of 15-30 nm, lengths of 15-20 μm, purity > 95%). After synthesis MWCNT were heated under argon (2L/min) to 2000 °C at the rate of 10 °C/minutes in order to extract catalyst (Fe-impurities). Purified MWCNTs (purity >95% by Thermo Gravimetry Analysis (TGA)) were subjected to a reflux process in sulfuric/nitric acid (3:1) to functionalize their surfaces. This process resulted in a large concentration of carboxyl (COOH) groups on the nanotube surface. After functionalization, these carboxylated nanotubes have 2-7% COOH by weight. Figures 1(A, B, C and D) represents the Transmission electron microscope (TEM) structures of COOH functionalized nanotubes, and dispersed MWCNTs after sonication.
Figure1.
Transmission electron microscope (TEM) photographs of functionalized carbon nanotubes: A. low magnification (2.0 KV × 1,500, 10 um), B: high magnification (2.0 KV × 50,000, 100 nm) C: Inside multiwall nature of the carbon nanotube (10 nm inner diameter, 9 concentric walls, and a clear inner channel), D: Dispersed MWCNT after sonication.
Functionalized MWCNT morphology and size were read by transmission electron microscopy (TEM). Prior to visualizing samples with TEM, MWCNT were directly deposited on a TEM grid and allowed to dry. Surface areas were determined by the isothermal gas adsorption method BET [37] using a Micromeritics Flowsorb 2300 (Norcross, USA).
To characterize our system, we processed TEM observations of the carbon nanotubes (Fig. 1A - C). MWCNT suspension was correctly dispersed in 1% tween 80 + sterile saline as surfactant during sonication. The length of the carbon nanotubes was up to 12 μm for the longer ones (60 minutes of sonication). The diameter was 11.5 nm after functionalization. Specific surface of carbon nanotube was measured by the classical BET method [37]. The specific surfaces of long carbon nanotubes were 41 m2/g and 42m2/g for non-purified and purified form, respectively.
Animal maintenance
Healthy adult male Swiss-Webster mice (5-7 weeks of age, with average body weight (BW) of 30±2g were used in this study. They were obtained from Charles River Laboratories, Inc. (Wilmington, MA, USA) and allowed to acclimate for 10 days before treatment. The animals were randomly selected and housed in polycarbonate cages (five mice per cage) with corn-cob bedding and steel wire tops. They were maintained in a controlled atmosphere with a 12h:12h dark/light cycle, a temperature of 22 ± 2°C and 50-70% relative humidity with free access to pelleted feed and fresh tap water. The animals were supplied with dry food pellets commercially available from PMI Feeds Inc. (St. Louis, MO, USA).
Dosing
MWCNTs were suspended and sonicated in a sterile saline solution containing 1% Tween-80 [38] and were dispersed using an ultrasonic liquid processor (Misonix, Long Island, NY) at 4° C and 30% amplitude to read pulses (1 sec on and 1 sec off) for a during 30 min for long MWCNT. This suspension generated a majority of MWCNT aggregates with a hydrodynamic diameter of ~1 μm. Thirty five mice were randomly divided into seven groups, five for each group. One group was chosen as positive control (Carbon black, CB, 0.75 mg/kg), the other three were used as the tween-saline control groups, and the last three were used as experimental groups. MWCNT suspension was administered intraperitoneally to animals at the doses of 0.25, 0.5, and 0.75 mg/kg/day BW, given for 5 days. Each mouse received a total of five doses at 24 h intervals. Saline, 1% tween-80, a suspension of saline and 1% tween-80 was administered to the five animals each of control groups in the same manner as in the treatment groups.
The local Ethics committee for animal experiments [Institutional Animal Care and Use Committee] at Jackson State University, Jackson, MS, (USA) approved this study. Procedures performed on the animals and care of animals conformed to the institutional guidelines were in compliance with national and international laws and guidelines for the use of animals in biomedical research [39].
Preparation of homogenate
At the end of the 5-day exposure to functionalized MWCNTs, liver was excised under anesthesia. The liver was washed thoroughly in ice-cold physiological saline and weighed. A 10% (10:1 w/v) homogenate of each liver tissue was prepared separately in 0.05 M phosphate buffer (pH 7.4) containing 0.1 mM EDTA using a motor driven Teflon-pestle homogenizer (Fischer), followed by sonication (Branson sonifer), and centrifugation at 500 × g for 10 min at 4° C. The supernatant was aspirated and centrifuged at 2000 × g for 60 min at 4° C. The cellular fraction obtained after centrifugation ‘homogenate’ was used for the assays.
Reactive Oxygen Species (ROS) Detection
DCFH-DA, a redox-sensitive fluorescent probe that emits light in the green spectrum when oxidized, was purchased from Cell Biolabs, Inc (San Diego, CA). DCFH-DA passes through cell membranes where it is cleaved by esterases to DCFH and becomes activated by oxidation. The liver was isolated as described above and loaded one-half of the samples with DCFH-DA (50 μM) in phosphate buffer saline (PBS), and placed the other half of the samples in PBS alone as a control. Samples were then incubated on a shaker at 37° C for 30 min. Following incubation, the liver specimen were homogenized (10:1 w/v) in potassium phosphate buffer (pH=7.4). Total protein was measured using the Bradford technique. Fluorescence of the samples was measured using Fluorescence Plate Reader (Turner Biosystems, Sunnyvale, CA, USA). Peak excitation wavelength for oxidized DCFH was 488 nm and emission was 525 nm. Calibration of the fluorometry procedure was accomplished by running standard curves for serial dilutions of fluorescein. Standards for the samples were conducted using serial dilutions of metal contaminant-free hydrogen peroxide (Merck, Darmstadt, Germany) incubated in DCFH-DA with esterase (20 U/ml) for 30 min at 37° C. DCFH oxidation was then calculated per μM H2O2. Samples loaded with DCFH-DA were subtracted by their respective controls to determine the true DCFH oxidation levels in the liver samples.
Enzyme Analysis
Serum Aminotransferases
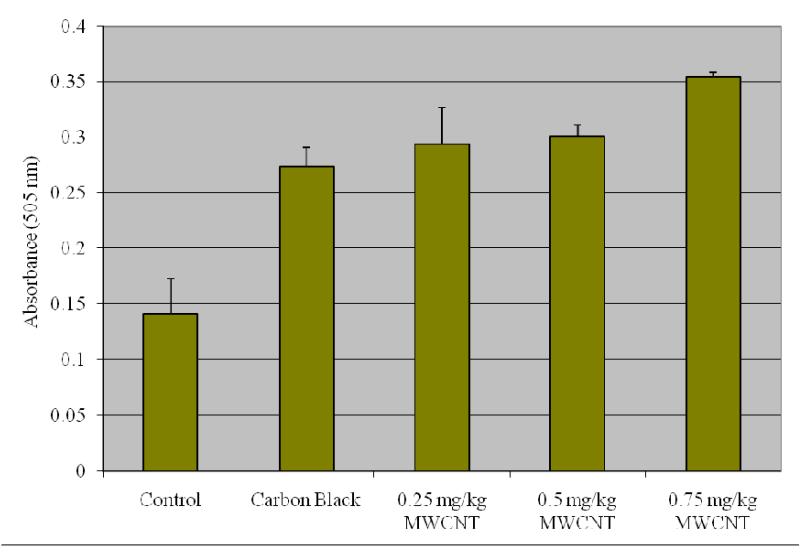
To determine the activities of alanine or glutamate pyruvate transaminase (ALT/GPT) and aspartate or glutamate oxaloacetate transaminase (AST/GOT) in serum a method by Reitman and Frankel [41] was followed. Human serum contains many different transaminases. The two most commonly determined are ALT/GPT and AST/GOT. These enzymes catalyze transfer of alpha amino groups from specific amino acids to alpha-ketoglutaric acid [AKG] to yield glutamic acid and oxaloacetic or pyruvic acid. The keto acids are then determined colorimetrically after their reaction with 2,4-dinitrophenyl hydrazine [DNP]. The absorbance of the resulting color is then measured at wavelength of approximately 505 nm to take advantage in the absorption that exists between the hydrazones of AKG and the hydrazones of oxaloacetic acid or pyruvic acid. The reaction for GOT is as follows:
The reaction for GPT is as follows:
Determination of GPT or Alanine Transaminases
1.0 ml of Sigma-prepared alanine α-KG substrate (Catalog No 505-51) is pipetted out into test tubes for exposed and control samples and placed in 37° C water bath to warm. Next, 0.2 ml serum is added and gently shaken to mix and left in the water bath. Exactly 30 minutes after adding serum, 1.0 ml Sigma color reagent (Catalog No. 505-2) is added, gently agitated, and left at room temperature for 20 minutes. 10 ml of 0.40 N (Normal) sodium hydroxide solution is added to the reaction mixture after 20 minutes, mixed by inversion, and left at room temperature for an additional 5 minutes. The absorbance was read and recorded at the same wavelength (505 nm) as used in preparing the calibration curve using water as a reference. The GPT activity is determined in Sigma Frankel (SF) units/ml from corresponding readings on the calibration curve.
Determination of GOT or Aspartate Transaminases
1.0 ml of Sigma-prepared aspartate substrate (Catalog No 505-1) is pipetted out into test tubes for exposed and control samples and placed in 37° C water bath to warm. Next, 0.2 ml serum is added, gently shaken to mix, and left in the water bath. Exactly 60 minutes after adding serum, 1.0 ml Sigma color reagent (Catalog No. 505-2) is added, gently agitated, and left at room temperature for 20 minutes. 10 ml of 0.40 N (Normal) sodium hydroxide solution is added to the reaction mixture after 20 minutes, mixed by inversion, and left it at room temperature for additional 5 minutes. The absorbance was read and recorded at the same wavelength (505 nm) as used in preparing the calibration curve, using water as a reference. The GOT activity is determined in Sigma Frankel (SF) units/ml from corresponding readings on the calibration curve.
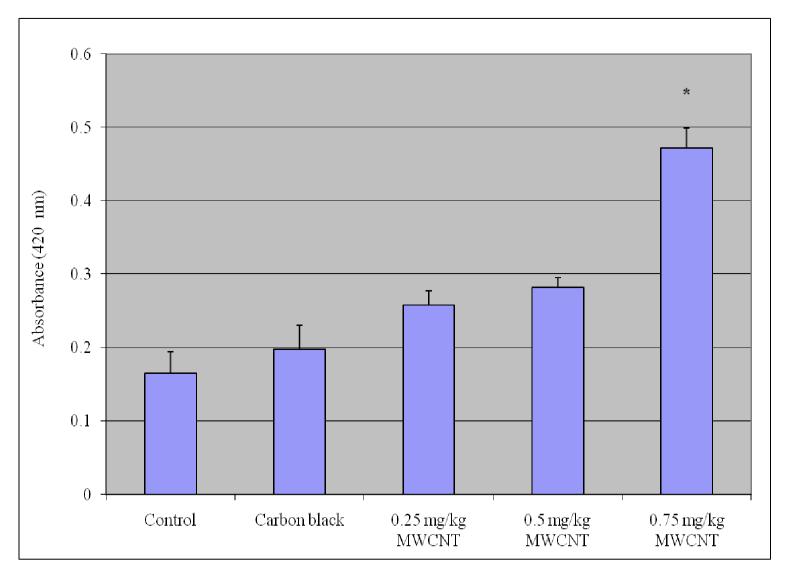
Alkaline Phosphatases
To determine the activity of alkaline phosphatase in serum, a method by Kay [42] was followed, it was measured using an Alkaline Phosphatase Diagnostic kit from Sigma (St. Louis, MO, USA). Alkaline phosphatase is also known as orthhophosphoric monoester phosphohydrolase, ALP. It is a prototype of those enzymes that reflect pathological reductions in bile flow. This enzyme has been extensively employed in experimentally induced hepatic dysfunction. Alkaline phosphatase refers not to a single enzyme but to a family of enzymes with different physico-chemical properties and broad overlapping substrate specificities.
The procedure for alkaline phosphatase depends upon the hydrolysis of p-nitrophenyl phosphate by the enzyme, yielding p-nitrophenol and inorganic phosphate. When made alkaline, p-nitrophenol is converted to a yellow complex readily measured at 400- 420 nm. The intensity of color formed is proportional to phosphatase activity. The reaction for ALP is as follows:
Determination of serum alkaline phosphatases
In order to determine the activity of serum alkaline phosphatases, 15 ml test tubes are taken with one labeled as “Blank” and another set as “TEST” which contains the exposed and control samples. Into each of the test tubes, 0.5 ml alkaline buffer solution (Sigma catalog # 221) and 0.5 ml of stock substrate solution is pipetted out and placed in 37° C water bath to equilibrate. Into the test tube labeled “Blank”, 0.1 ml water is added and 0.1ml serum of exposed group into respective tubes labeled “Test.” Time is recorded, and samples are mixed gently and promptly replaced in 37° C water bath. After 15 minutes, 10.0 ml of 0.05 N (Normal) sodium hydroxide (NaOH) is added to all of the test tubes and mixed by inversion. The absorbance of “Test” versus “Blank” is read as a reference at a wavelength of 420 nm in a visible spectrophotometer (BIO-RAD). The first absorbance of the reaction mixture is the initial readings of the activity of alkaline phosphatase in serum. The units of alkaline phosphatases are determined from the corresponding calibration curve. To each of the test tubes four drops of (approximately 0.2 ml) concentrated hydrochloric acid (HCl) is added and mixed. The absorbance of the “Test” versus “Blank” is read again. These recorded absorbances are the final readings of the reaction mixture. The alkaline phosphatase activity is obtained by subtracting the final absorbance reading of the corresponding group from the initial absorbance reading.
Lipid hydro peroxides (LHP) assay
The tissues were homogenized (1:8, w/v) in cold HPLC-grade water. Five hundred microliter of each tissue homogenates were taken in a glass test tube and to read equal volume of Extract R saturated methanol (Calbiochem) was added. The mixture was vortexed for few minutes and 1 ml of cold deoxygenated chloroform was added to the sample mixture and vortexed thoroughly. The mixture was centrifuged at 1500 × g for 5 min at 4°C (Beckman XL-100K, USA) and bottom chloroform layer was collected. Five hundred μl of the bottom chloroform was mixed with 450μl of chloroform:methanol (2:1) mixture and 50μl of chromogen (thiocyanate ion)(Calbiochem). Then the mixture was incubated for 5 min and the absorbance of each sample was recorded at 500 nm wavelength using spectrophotometer (2800 Unico spectrophotometer USA). This method directly measures the lipid hydro-peroxides utilizing redox reactions with ferrous ions. The produced hydroperoxides are highly unstable and react readily with ferrous ions to produce ferric ions. The produced ferric ions were detected using thiocyanate ion as chromogen.
Histopathology Evaluation of tissue
Portions of liver samples were cut into small pieces and fixed immediately in 10 percent phosphate-buffered formalin for 48 hrs. The tissues were then transferred to 70% ethyl alcohol and stored until processed. The liver specimens were processed and embedded in paraffin, sectioned at 0.1 μm using microtome (Olympus CUT 4055E, USA) and stained with hematoxylin and eosin (H & E). The stained tissues were visualized under the microscope and evaluated for the changes in morphology of MWCNTs exposed liver compared with the control. According to the method described by [43] the morphology of liver was evaluated at 1000× magnification using Axiovert S 100, inverted light microscope (Carl Zeiss Micro Imaging Inc, Thornwood, NY, USA).
The extent of tissue injury was estimated semi-quantitatively and lesions scored as multi-focal fibrosis/necrosis. At least 10 slides (blinded, one scorer) of each sample were scored for liver histology. The liver morphology scored as follows: 0= normal, 1 = mild cellular disruption in less than 25% of field area, 2 = moderate cellular disruption and hepato cellular vacuolation greater than 50% of field area, 3 = extensive cell disruption, hepato cellular vacuolation and condensed nuclei (pycknotic) of hepatocytes in greater than 50% of field area, 4 = extensive cell disruption, hepato cellular vacuolation, pycknotic and occasional central vein injury and 5= extensive cell disruption, multi central vein necrosis and degenerating of liver in more than 50% of field area.
Statistical Analysis
Data was analyzed with one-way ANOVAs using SAS 9.1 software for Windows XP. Where appropriate, Dunnett T- Test was used for post hoc comparisons. All values were reported as means ± SDs. for all the experiments. The significance level was set at p<0.05.
Results
ROS Detection
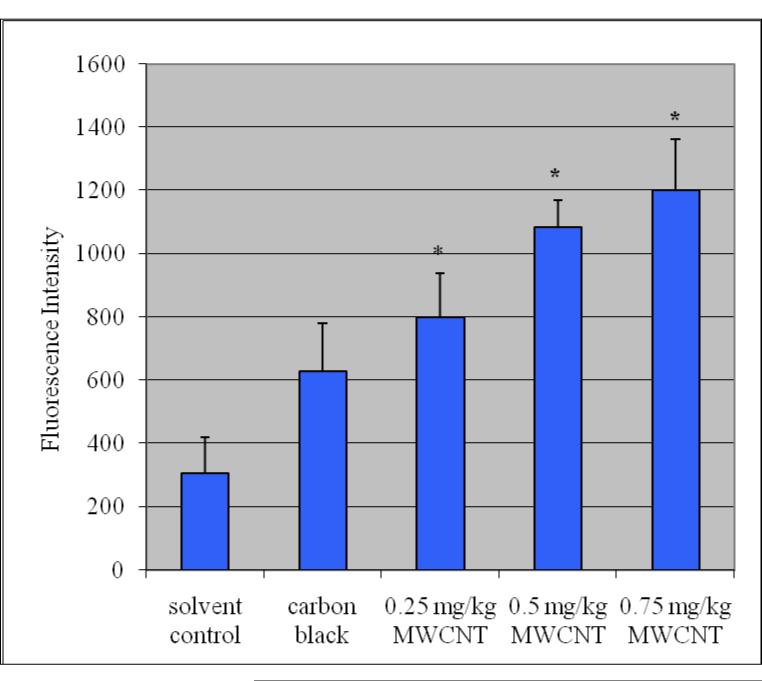
The administration of purified/functionalized MWCNT to mice showed an increase in the ROS level in the exposed groups as compared to the controls and the increase was statistically significant (p≤0.05). Figure 2 summarizes the detection of intracellular production of ROS in Swiss-Webster mice exposed to purified/functionalized MWCNT compared with controls. Total protein levels in liver were 125.23 + 1.32, 113.87±4.95, 109.28±1.95 107.08±3.29 and 105.68±2.9 mg/ G tissue for control, 0.25mg/kg/day, 0.5 mg/kg/day and 0.75 mg/kg/day respectively.
Figure2.
ROS induction in liver homogenate exposed to purified/functionalized MWCNT. Each experiment was done in triplicate. Data represents mean + SD. Statistical significance (p<0.05) is depicted as (*).
Lipid Hydro Peroxides (LHP)
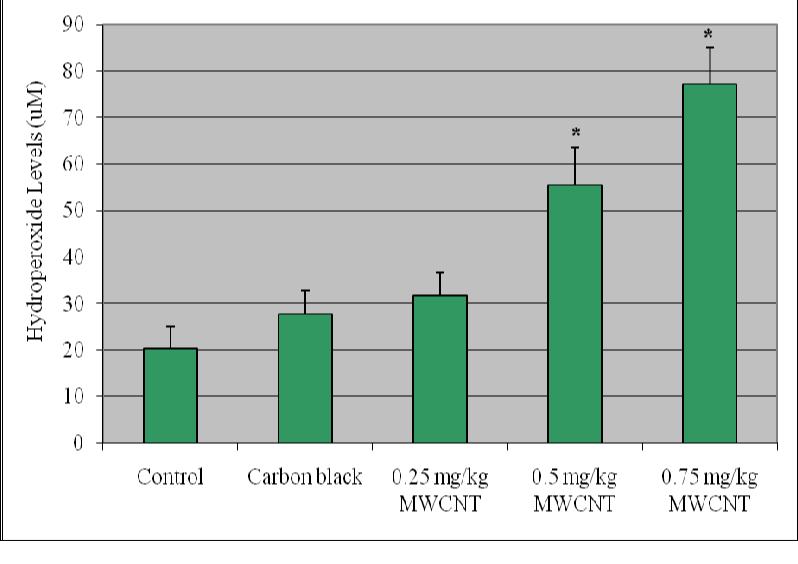
Lipid hydro peroxides assay was performed to determine the hydroperoxides levels in liver homogenates of mice exposed to purified/functionalized MWCNT compared with controls. The LHP levels showed an increase in the exposed groups compared with controls and the increase was statistically significant (p ≤ 0.05). Figure 3 represents the experimental data of LHP.
Figure 3.
Effect of purified/functionalized MWCNT on the level of lipid hydroperoxides in liver homogenate of Swiss-Webster Mice Each experiment was done in triplicate. Data represents mean ± SD. Statistical significance (p<0.05) is depicted as (*).
Alanine aminotransferase
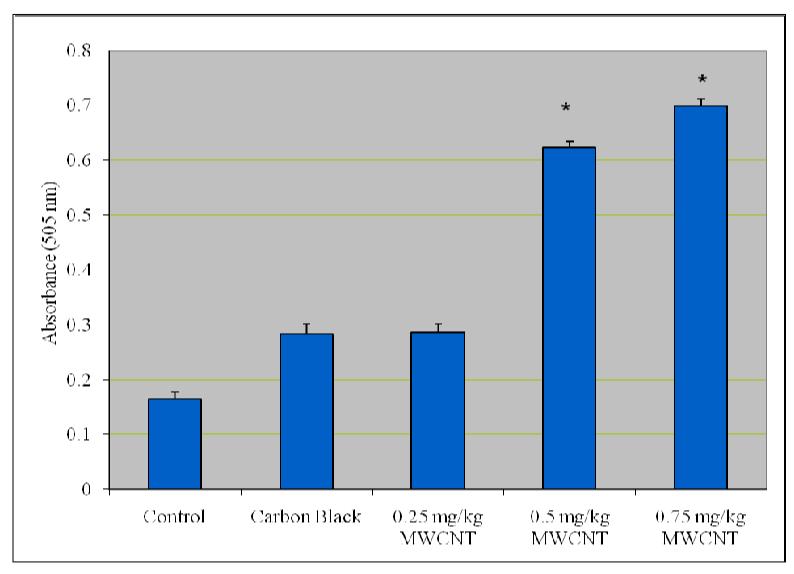
Figure 4 presents the experimental data obtained from the analysis of alanine aminotansferases (ALT/GPT). As shown in this figure there was a statistically significant (p ≤ 0.05) increase in the activity of alanine (ALT/GPT) in the serum of Swiss-Webster mice compared with control.
Figure 4.
Effect of purified/functionalized MWCNT on the activity of Alanine Transferases (ALT) in serum of Swiss-Webster Mice. Each experiment was done in triplicate. Data represents mean ± SD. Statistical significance (p<0.05) is depicted as (*).
Aspartate Aminotransferase
Figure 5 presents the experimental data obtained from the analysis of aspartate aminotransferases. Functionalized MWCNT exposure resulted in elevating the activity of AST/GOT in dose-dependent manner. However, the increase was not statistically significant (p ≤ 0.05 when compared with control.
Figure 5.
Effect of purified/functionalized MWCNT on the activity of Aspartate Transferases (AST) in serum of Swiss-Webster Mice. Each experiment was done in triplicate. Data represents mean ± SD. Statistical significance (p<0.05) is depicted as (*).
Alkaline Phosphatases
The activity of alkaline phosphatase exposed to purified/functionalized MWCNT is represented in Figure 6. As shown in the figure there was an increase in the activity of alkaline phosphatases in mice treated with purified/functionalized MWCNT’s compared with control and the increase was statistically significant at (p ≤ 0.05.
Figure 6.
Effect of purified/functionalized MWCNT on the activity of Alkaline phosphatases (ALP) in serum of Swiss-Webster Mice. Each experiment was done in triplicate. Data represents mean ± SD. Statistical significance (p<0.05) is depicted as (*).
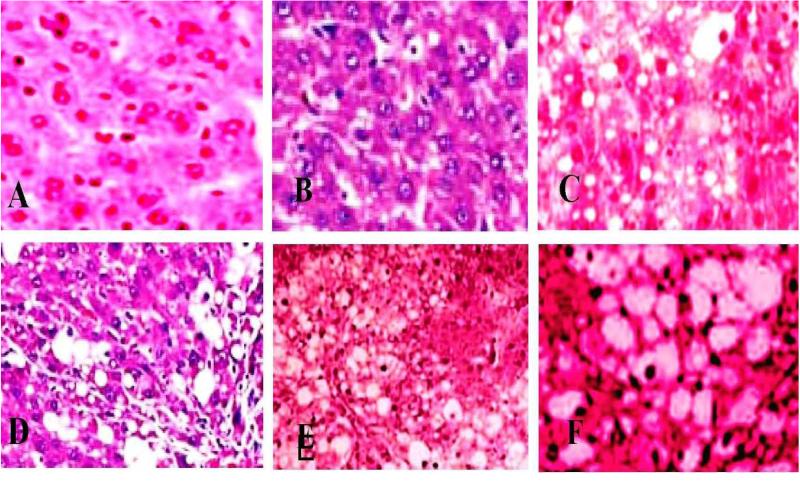
Histopathological Analysis
Figure 7 summarizes the histological score for each group. Indices of liver (liver wet weight/body weight) were increased in mice after intraperitoneal injection of purified functionalized MWCNT compared with normal animals. The liver from control showed normal structure and compactly arranged hepatocytes under microscopic examination. Sinusoids were scattered randomly all over the hepatocytes and had uniform morphology along with central vein. However, the mice exposed to 0.25, 0.5 and 0.75 mg/kg/day bwt. of purified functionalized MWCNT had remarkable morphological alterations. Hepatocytes disruption and hepatocellular vacuolation was observed in microscopic examination of 0.25 mg/kg/day MWCNT exposed mice liver. In addition to the 0.25 mg/kg/day MWCNT alterations, pycknotic or karyomegaly (condensed nuclei) of hepatocytes and partial disruption of central vein was observed in 0.5 mg/kg/day MWCNT exposed mice liver. In addition to the above alterations, degeneration of liver (atrophy) and central vein injury was observed in 0.75 mg/kg/day exposed mice liver. The results indicated that hepatic injury was successfully induced in mice treated with purified functionalized MWCNT.
Figure 7.
Histopathological Characterization (H & E Staining 1000 ×) of liver in Swiss-Webster mice exposed to purified/functionalized MWCNT. A= Negative Control (Saline), B= saline+tween control, Control C= Positive Control (carbon black), D= 0.25 mg/kg/day, E= 0.5 mg/kg/day and F= 0.75 mg/kg/day.
Discussion
There are increasing possibilities for human to contact CNTs because of their extensive applications in the field of biomedical and material science. Accidental or involuntary contact during production or use is most likely to happen via the lungs from where a rapid translocation through the blood stream is possible to other vital organs and can be taken up by liver, spleen, bone marrow, heart and other organs. In particular, the behavior of CNTs inside the cells is still an enigma, and no cellular responses induced by these CNTs or particles are properly understood [44]. Therefore, it is essential to thoroughly investigate the toxicity of CNTs. As one of the most important kinds of CNTs in the CNT family, functionalized MWCNT are easily obtained and readily dispersed in aqueous solutions and therefore are widely employed as drug carriers to deliver cargoes such as Rhodamine123, DNA and protein into cells [24]. These functionalized MWCNTs will ultimately be administered, metabolized and excreted by animals. Unfortunately, there are very few published reports on the fate and biological consequences of these functionalized CNTs in vivo [45-47].
In the present study oxidative stress and hepatotoxicity biomarkers were investigated using ROS induction, measurement of lipid hydroperoxide, activities of certain liver enzymes such as ALT/GPT, AST/GOT, ALP and histopathological characterization of liver in mice, exposed to purified/functionalized MWCNT. There was a significant increase in the level of ROS in liver homogenate of mice exposed to functionalized MWCNT compared to controls. ROS has been implicated in the toxicity of carbon nanotubes by several authors [23, 27, 48]. Their formation with subsequent cellular damage is considered as the molecular mechanism of carbon nanotube-induced toxicity. Living organisms have developed elaborate systems to defend themselves against toxic agents. Metabolism, distribution, and excretion are linked aspects that are essential in predicting the adverse effects of an agent and thus determining the risk of exposure to it. Although, most cells in the body are capable of metabolism, the primary organ for detoxification is the liver. The liver has a variety of specialized cells that produce enzymes to aid in the metabolism of toxic agents. Liver is often important in tests of oxidative stress because of LPO is a major cause of liver lesions. According to our results, the dominant toxicological mechanism of the intraperitoneally exposed MWCNTs would be oxidative stress. It is a broadly existent phenomenon when cells are exposed to CNTs [49]. Although the cytotoxcity of CNTs is not always observed in the cell culture studies, oxidative stress is regarded as the cause of the cytotoxicity [50]. In fact, oxidative stress is taken as an important pathway of toxicity of CNTs and other nanomaterials [9].
The data obtained for the hepatotoxicity biomarker study clearly show that highest dose 0.75 mg/kg/day of purified/functionalized MWCNT has significantly increased the activity of serum alkaline phosphatases compared to control. Alkaline phosphatase of the liver is produced by the cells lining the small bile ducts (ductoles) in the liver. If the liver disease is primarily of an obstructive nature (cholestatic) i.e., involving the biliary drainage system, the alkaline phosphatase will be the first and foremost enzyme that is found to increase. Serum activity of the enzyme has been reported to increase and is indicative of an impaired hepatic clearance (cholestasis). Liver injury or metabolism dysfunctions are reflected by changes of liver enzymes in serum [51]. The vast majority of ALT/GPT in liver exists in the cytoplasm, and only a small amount resides in the mitochondria. The enzymes will penetrate into the blood when the liver cell membrane is damaged. However, the majority of AST exists in the mitochondria in liver cells. When the damage involves hepatocyte mitochondria, the increase of AST will surpass that of ALT. An increase in the activity of ALT/GPT and AST/GOT with increasing concentration of purified functionalized MWCNT was observed in our study, however only the highest doses 0.5 mg/kg and 0.75 mg/kg/day were found to show a statistically significant increase in the activity of ALT/GPT compared to control. Aspartate transferases (AST/GOT) did not show any statistically significant effect in elevating the activity of the enzyme. Our results are in agreement with the studies of Lacerda et al [52] and Ji et al [15] with serum suspended multiwalled carbon nanotubes showing higher acute toxicity in mice and in elevating the levels of serum aminotranferases in serum of mice. Since the toxicity of CNTs is dependent on their functionalization degree [22], such hepatic toxicity might be reduced when proper chemical functionalization is adopted to obtain a high functionalization degree of CNTs with higher in vivo stability. To clarify the role of functionalization in the in vivo toxicity of CNTs, more efforts are required and parallel comparison is preferred.
To establish the role of oxidative stress as a decisive factor in MWCNT-induced toxicity, the level of lipid hydroperoxides in liver homogenates was performed. Lipid hydroperoxides (LOOHs) are prominent non-radical intermediates of lipid peroxidation whose identification can often provide valuable mechanistic information, e.g., whether a primary reaction is mediated by singlet oxygen or oxyradicals. The results in the present investigation demonstrated that a dose-dependent increase in the level of lipid hydroperoxides was observed. Our data is also in accordance with the report of Reddy et al [53] indicating the influence of nanotubes in implicating lipid hydroperoxides in CNT toxicity. Recently, in our laboratory we demonstrated that exposure to carboxy-functionalized single-walled carbon nanotube induced lipid peroxidation in the liver of mice implicating it in CNT toxicity [16]
Kupffer cells are resident macrophages of the liver and play an important role in its normal physiology and homeostasis as well as participating in the acute and chronic responses of the liver to toxic compounds. Activation of Kupffer cells directly or indirectly by toxic agents results in the release of an array of inflammatory mediators, growth factors, and reactive oxygen species. This activation appears to modulate acute hepatocyte injury as well as chronic liver responses including hepatic cancer. Understanding the role Kupffer cells play in these diverse responses is a key in understanding mechanisms of liver injury [54]. In our study histopathological evaluation of liver exposed to purified/functionalized MWCNT showed remarkable morphological alterations such as hepatocytes disruption, hepatocellular vacuolation, pycknotic or karyomegaly of hepatocytes and atrophy when compared to control. Although it is most likely that this impairment in hepatotoxicity biomarkers is associated with MWCNT toxicity, further experiments are needed to elucidate the biochemical mechanisms involved.
Elimination of metal residues from MWCNT samples is very hard, even though most of the metals can be removed by refluxing MWCNTs sample in H2O2 and acid. Majority of metals loaded on the outside and internal surface of tubes can be removed by traditional purification; however this method is in vain for metals packed inside the surrounding carbon fragments. A few studies reported that metal contents in CNTs were partly responsible for the serious oxidative stress [50, 55-57]. Recently, a report by Liu et al [56] focused on the bioavailibilty of metals in CNTs suggests that the encapsulated metals are non-bioavailable for at least two months [57]. Our purification procedure is almost similar to Liu et al [57]. The metal impurities left in our CNTs samples should be inside the CNTs or encapsulated in carbon fragments, therefore they are hardly attributed to the toxicity of CNTs. In otherwords, the oxidative stress is mediated completely from CNTs per se.
In summary, short-term and high toxicity in mice exposed to functionalized MWCNTs are reported. ROS induction, increase in the level of LHP, serum biochemical changes and damage to the liver tissue were observed. The results indicated that functionalized MWCNT induce hepatotoxicity. The main proposed toxicological mechanism is oxidative stress aroused in liver. The high toxicity of functionalized MWCNTs does not implicate that they should be banned for biomedical applications, however improving the dispersion and excretion of MWCNTs by further chemical functionalization is required. Therefore, further toxicological studies in vivo have to be developed for evaluating hazards of occupational or environmental exposure to nanomaterials.
Acknowledgement
This research was supported in part by a grant from the Air Forces Research Laboratory/Wright Patterson AFB (Grant No. FA8650-07-1-6851) and in part by a grant from National Institutes of Health-RCMI Center for Environmental Health (Grant No. 2G12RR01349-12) at Jackson State University.
References
- 1.Iijima S. “Helical microtubules of graphitic carbon”. Nature. 1991;354:56–59. [Google Scholar]
- 2.Dresselhans MS, Dresselhaus G, Avouris P, editors. “Carbon nanotubes: Snthesis, structure, properties and applications”. Springer; Berlin: 2001. [Google Scholar]
- 3.Hu H, Ni C, Mandal SK, Montana V, Zhao B, Haddon RC, Parpura V. Polethleneimine functionalized single walled carbon nanotubes as a substrate for neuronal growth. J Phs Chem B. 2005;109(10):4285–4289. doi: 10.1021/jp0441137. [DOI] [PubMed] [Google Scholar]
- 4.Shokuhfar T, Makradi A, Cabral G, Ahzi S, Sousa AC, Belouettar S, Gracio J, Titus E. Prediction of the mechanical properties of hdroxapatite/polmethl methacrlate/carbon nanotubes nanocomposite. J Nanosci Nanotechnol. 2008;8:4279–4284. doi: 10.1166/jnn.2008.an26. [DOI] [PubMed] [Google Scholar]
- 5.Gruner G. Carbon nanotube transistors for biosensing applications. Anal Bioanal Chem. 2006;384(2):322–335. doi: 10.1007/s00216-005-3400-4. [DOI] [PubMed] [Google Scholar]
- 6.McDevitt MR, Chattopadha D, Kappel BJ, Jaggi JS, Schiffman SR, Antczak C, Njardarson JT, Brentjens R, Scheinberg DA. Tumor targeting with antibod-functionalized radiolabeled carbon nanotubes. J Nucl Med. 2007;48(7):1181–1189. doi: 10.2967/jnumed.106.039131. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Chen K, Davis C, Sherlock S, Cao QZ, Chen Z, Dai HJ. Drug-deliver with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68(16):6652–6660. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu FS, Gu LR, Meziani MJ, Wang X, Luo PG, Veca LM, Cao L, Sun P. Advances in bioapplications of carbon nanotubes. Adv Mater. 2009;21(2):139–152. [Google Scholar]
- 9.Stern ST, McNeil SE. Nanotechnolog safet concerns revisited. Toxicol Sci. 2008;101:4–21. doi: 10.1093/toxsci/kfm169. [DOI] [PubMed] [Google Scholar]
- 10.Lam CW, James JT, McCluske R, Hunter RL. Pulmonar toxicit of single-wall carbon nanotubes in mice 7 and 90 das after intratracheal instillation. Toxicol Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 11.Shvedova AA, Castranova V, Kisin ER, Schwegler-Berr D, Murra AR, Gandelsman VZ, Manard A, Baron P. Exposure to carbon nanotube material: assessment of nanotube ctotoxicit using human keratinocte cells. J Toxicol Environ Health. 2003;A66(20):1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 12.Warheit DB. What is currentl known about the health risks related to carbon nanotube exposures? Carbon. 2006;44:1064–1069. [Google Scholar]
- 13.Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, Ohashi N, Kitajima S, Kanno J. Induction of mesothelioma in p53± mouse b intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008;33(1):105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- 14.Poland CA, Duffin R, Kinloch I, Manard A, Wallace WA, Seaton A, Stone V, Brown S, Macnee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavit of mice show asbestos-like pathogenicit in a pilot stud. Nat Nanotechnol. 2008;3(7):423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 15.Ji Z, Zhang D, Li L, Shen X, Deng X, Dong L, Wu M, Liu Y. The hepatotoxicit of multi-walled carbon nanotubes in mice. Nanotech. 2009;20:445101–445109. doi: 10.1088/0957-4484/20/44/445101. [DOI] [PubMed] [Google Scholar]
- 16.Patlolla AK, Mcginnis B, Tchounwou PB. Biochemical and histo-pathological evaluation of functionalized single-walled carbon nanotube in Swiss-Webster mice. J Appl Toxicol. 2011;31(1):75–83. doi: 10.1002/jat.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patlolla AK, Hussain SM, Schlager J, Patlolla S, Tchounwou PB. Comparative clastogenic stud of functionalized and non-functionalized multi-walled carbon nanotube in bone marrow cells of Swiss-Webster mice. Environ Toxiciol. 2010;25(6):608–621. doi: 10.1002/tox.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui D, Tian F, Ozkan CS, Wang M, Gao H. Effect of single-wall carbon nanotubes on human HEK293 cells. Toxicol Lett. 2005;155:73–85. doi: 10.1016/j.toxlet.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, Zhao Y, Guo X. Ctotoxicit of carbon nanomaterials: Single-wall nanotube, multiwall nanotube, and fullerene. Environ Sci Technol. 2005;39:1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- 20.Monteiro-Riviere NA, Nemanich RJ, Inman AO, Wang YY, Riviere JE. Multi-walled carbon nanotube interactions with human epidermal keratinoctes. Toxicol Lett. 2005;155:377–384. doi: 10.1016/j.toxlet.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Tian F, Cui D, Sehwarz H, Estrada GG, Kobaashi Ctotoxicit of single-wall carbon nanotube on human fibroblasts. Toxicolog In Vitro. 2006;20:1202–1212. doi: 10.1016/j.tiv.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Saes CM, Liang F, Hudson JL, Mendez J, Guo W, Beach JM, Moore VC, Dole CD, West JL, Billups WE, Ausman KD, Colvin VL. Functionalization densit dependence of single-walled carbon nanotubes ctotoxicit in vitro. Toxicol Lett. 2006;161(2):135–142. doi: 10.1016/j.toxlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Muller J, Decordier I, Hoet PH, Lombaert N, Thomassen L, Huaux F, Lison D, Kirsch-Volders M. Clastogenic and aneugenic effects of multiwalled carbon nanotube in epithelial cells. Carcinogenesis. 2008;29:427–433. doi: 10.1093/carcin/bgm243. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Tabakman S, Welsher K, Dai HJ. Carbon Nanotubes in Biolog and Medicine: In vitro and in vivo Detection, Imaging and Drug Deliver. Nano Res. 2009;2(2):85–120. doi: 10.1007/s12274-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wick P, Manser P, Limbach LK, Weglikowska U, Krumeich F, Roth S, Stark WJ, Bruinink A. The degree and kind of agglomeration affect carbon nanotube ctotoxicit. Toxicol Lett. 2007;168(2):121–131. doi: 10.1016/j.toxlet.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nano level. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 27.Shvedova AA, Kisin E, Murra AR, Johnson VJ, Gorelik O, Arepalli S, Hubbs AF, Mercer RR, Keohavong P, Sussman N, Jin J, in J, Stone S, Chen BT, Dee G, Manard A, Castranova V, Baron PA, Kagan VE. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Phsiol Lung Cell Mol Phsiol. 2008 Oct;295(4):L552–565. doi: 10.1152/ajplung.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutteridge JMC, Quinlan GJ. Malondialdehde formation from lipid peroxides in thiobarbituric acid test. The role of lipid radicals, iron salts and metal chelator. J Appl Biochem. 1983;5:293–299. [PubMed] [Google Scholar]
- 29.Halliwell B. Oxgen radicals: A common sense look at their nature and medical importance. Med Biol. 1984;62:71–77. [PubMed] [Google Scholar]
- 30.Murra RK, Granner DK, Maes PA, Rodwell V. Harper’s Biochemistr. 21st ed Prentice Hall; Englewood Cliffs, NJ: 1988. pp. 138–139. [Google Scholar]
- 31.De Zwart LL, Meerman JH, Commandeur JN, Vermeulen NP. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radical Biol Med. 1999 Jan;26(1-2):202–226. doi: 10.1016/s0891-5849(98)00196-8. Review. [DOI] [PubMed] [Google Scholar]
- 32.Zimmerman, Seeff . Enzmes in hepatic disease. In: Goodl EL, editor. Diagnostic Enzmolog. Lea & Febiger; Philadelphia, USA: 1970. pp. 1–38. [Google Scholar]
- 33.Deng XY, Yang ST, Nie H, Wang HF, Liu YF. A generall adoptable radiotracing method for tracking carbon nanotubes in animals. Nanotechnolog. 2008;19(7):075101. doi: 10.1088/0957-4484/19/7/075101. [DOI] [PubMed] [Google Scholar]
- 34.Deng XY, Jia G, Wang HF, Sun HF, Wang X, Yang ST, Wang TC, Liu YF. Translocation and fate of multiwalled carbon nanotubes in vivo. Carbon. 2007;45(7):1419–1424. [Google Scholar]
- 35.Liu Z, Cai WB, He LN, Nakaama N, Chen K, Sun XM, Chen X, Dai HJ. In vivo biodistribution and highl efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 36.Cherukuri P, Gannon CJ, Leeuw TK, Schmidt HK, Smalle RE, Curle SA, Weisman RB. Mammalian pharmacokinetics of carbon nanotubes using intrinsic near-infrared fluorescence. Proc Natl Acad Sci USA. 2006 Dec 12;103(50):18882–18886. doi: 10.1073/pnas.0609265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunauer S, Emmett PH, Teller E. Adsorption of gases in multi-molecular laers. J Am Chem Soc. 1938;60(2):309–319. [Google Scholar]
- 38.Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, Arras M, Fonseca A, Nag JB, Lison D. Respirator toxicit of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207:221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Giles AR. Guidelines for the use of animals in biomedical research. Thromb Haemost. 1987;58(4):1078–1084. [PubMed] [Google Scholar]
- 40.Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupt antioxidant capacit in skeletal muscle. Free Radical Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 41.Reitman, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pruvic transaminases. Am J Clin Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 42.Ka HD. Plasma phosphatase. I Method of determination. Some properties of the enzme. J Biol Chem. 1930;89:235–cit. [Google Scholar]
- 43.Kerem M, Bendirli N, Gurbuz N, Ekinci O, Bedirli A, Akkaa T, Sakrak O, Pasaoglu H. Effects of acute fenthione toxicit on liver and kidne function and histolog in rats. Turk J Med Sci. 2007;37(5):281–288. [Google Scholar]
- 44.Oberdörster G, Manard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D, Yang H, ILSI Research Foundation/Risk Science Institute Nanomaterial Toxicit Screening Working Group Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strateg. Part Fibre Toxicol. 2005;6:2–8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z, Cai WB, He LN, Nakaama N, Chen K, Sun XM, Chen X, Dai HJ. In vivo biodistribution and highl efficient tumour targeting of carbon nanotubes in mice. Nat. Nanotechnol. 2007;2(1):47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 46.Villa CH, McDevitt MR, Escorcia FE, Re DA, Bergkvist M, Batt CA, Scheinberg DA. Snthesis and biodistribution of oligonucleotide-functionalized tumor-targetable carbon nanotubes. Nano lett. 2008;8(12):4221–4228. doi: 10.1021/nl801878d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Wang H. Nanomedicine: Nanotechnolog Tackles tumours. Nat. Nanotechnol. 2007;2(1):20–1. doi: 10.1038/nnano.2006.188. [DOI] [PubMed] [Google Scholar]
- 48.Inoue K, Yanagisawa R, Koike E, Nishikawa M, Takano H. Repeated pulmonar exposure to single-walled carbon nanotubes exacerbates allergic inflammation of the airwa: Possible role of oxidative stress. Free Radic Biol Med. 2010;48(7):924–934. doi: 10.1016/j.freeradbiomed.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Lewinski N, Colvin V, Drezek R. Ctotoxicit of nanoparticles. Small. 2008;4(1):26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 50.Pulskamp K, Diabate S, Krug HF. Carbon nanotubes show no sign of acute toxicit but induce intracellular reactive oxgen species in dependence on contaminants. Toxicol Lett. 2007;168(1):58–74. doi: 10.1016/j.toxlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Murakami S, Okubo K, Tsuji Y, Sakata H, Takahashi T, Kikuchi M, Hiraama R. Changes in liver enzmes after surger in anti-hepatitis C virus-positive Patients. World J. Surg. 2004;28(7):671–674. doi: 10.1007/s00268-004-7377-5. [DOI] [PubMed] [Google Scholar]
- 52.Lacerda L, Ali-Boucetta H, Herrero MA, Pastorin G, Bianco A, Prato M, Kostarelos K. Tissue histolog and phsiolog following intravenous administration of different tpes of functionalized multiwalled carbon nanotubes. Nanomedicine (Lond) 2008 Apr;3(2):149–161. doi: 10.2217/17435889.3.2.149. [DOI] [PubMed] [Google Scholar]
- 53.Redd AR, Rao MV, Krishna DR, Himabindu V, Redd N. Evaluation of oxidative stress and anti oxidant status in rat serum following exposure of carbon nanotubes. Regul Toxicol Pharmacol. 2011;59(2):251–257. doi: 10.1016/j.yrtph.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 54.Roberts RA, Gane PE, Ju C, Kamendulis LM, Rusn I, Klaunig JE. Role of kupffer cell in mediating hepatic toxicit and carcinogenesis. Toxicol Sci. 2007;96(1):2–15. doi: 10.1093/toxsci/kfl173. [DOI] [PubMed] [Google Scholar]
- 55.Guo L, Morris DG, Liu X, Vaslet C, Hurt RH, Kane AB. Iron bioavailabilit and redox activit in diverse carbon nanotube samples. Chem Mater. 2007;19:3472–3478. [Google Scholar]
- 56.Liu X, Gurel V, Morris D, Murra DW, Zhitkovich A, Kane AB, Hurt RH. Bioavailabilit of nickel in single-wall carbon nanotubes. Adv Mater. 2007;19:2790–2796. [Google Scholar]
- 57.Liu X, Guo L, Morris D, Kane AB, Hurt RH. Targeted removal of bioavailable metal as a detoxification strateg for carbon nanotubes. Carbon. 2008;46:489–500. doi: 10.1016/j.carbon.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]