Abstract
Methyl parathion (C8H10NO5PS) and parathion (C10H14NO5PS) are both organophosphate insecticides (OPI) widely used for household and agricultural applications. They are known for their ability to irreversibly inhibit acetylcholinesterase which often leads to a profound effect on the nervous system of exposed organisms. Many recently published studies have indicated that human exposure to OPI may be associated with neurologic, hematopoietic, cardiovascular, and reproductive adverse effects. Studies have also linked OPI exposure to a number of degenerative diseases including Parkinson's, Alzheimer's, and amyotrophic lateral sclerosis. Also, oxidative stress (OS) has been reported as a possible mechanism of OPI toxicity in humans. Hence, the aim of the present investigation was to use human liver carcinoma (HepG2) cells as a test model to evaluate the role of OS in methyl parathion- and parathion-induced toxicity. To achieve this goal, we performed the MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay for cell viability, lipid peroxidation assay for malondialdehyde (MDA) production, and Comet assay for DNA damage, respectively. Results from MTT assay indicated that methyl parathion and parathion gradually reduce the viability of HepG2 cells in a dose-dependent manner, showing 48 h-LD50 values of 26.20 mM and 23.58 mM, respectively. Lipid peroxidation assay resulted in a significant increase (p<0.05) of MDA level in methyl parathion- and parathion-treated HepG2 cells compared to controls, suggesting that OS plays a key role in OPI-induced toxicity. Comet assay indicated a significant increase in genotoxicity at higher concentrations of OPI exposure. Overall, we found that methyl-parathion is slightly less toxic than parathion to HepG2 cells. The cytotoxic effect of these OPI was found to be associated, at least in part, with oxidative cell/tissue damage.
Keywords: methyl parathion, parathion, cytotoxicity, oxidative stress, DNA damage, HepG2 cells
INTRODUCTION
Organophosphate compounds such as malathion, parathion, methyl parathion, diazanon, and chlorpyrifos are widely used in agriculture as insecticides and acaricides. Residual amounts of organophosphate insecticides (OPI) have been detected in the soil, water bodies, vegetables, grains and other food products (IARC 1993; John et al., 2001; Poet et al., 2004, Choudhary and Sharma, 2008). They are also the most frequently used class of insecticides in household applications (Racke 1992). However, the increased use of these compounds has caused concern for not only the environment but also the health of the public (Bhanti et al., 2007). Acute or chronic exposure to these compounds may result in impaired memory and concentration, disorientation, severe depression, irritability, confusion, headache, speech difficulties, delayed reaction time, nightmare, sleepwalking, and drowsiness (Hayes and Wayland 1982; USEPA 1992, 2000). A recent study from our laboratory has demonstrated that malathion (an OPI) induces toxicity and DNA damage in HepG2 cells through oxidative stress by increasing malondialdehyde (MDA) level, a byproduct of lipid peroxidation (Moore et al., 2009).
Methyl parathion is an OPI that was initially registered in 1954 in the United States (U.S. EPA 2000). Its use was restricted in 1978 as a result of detrimental effects to humans (Garcia et al., 2003). Although methyl parathion was restricted specifically to outdoor use and application by a licensed applicator, there have been several incidences of illegal applications of methyl parathion across the United States. These illegal applications have occurred in the homes of families living in Alabama, Arkansas, Illinois, Louisiana, Michigan, Mississippi, Ohio, Tennessee, and Texas (U.S. EPA, 2000) as well as in collected soil samples from 11 Atlanta homes (Riederer et al., 2010). Parathion is also an OPI with a weak cholinesterase inhibitor activity; it is responsible for tissue injury through an unknown mechanism, although some studies have reported that such pesticides generate reactive oxygen species (Bagchi et al., 1995). Several studies have shown that parathion is one of the most toxic insecticides studied.
Published research has indicated that OPI exert their toxicity through inhibition of acetylcholinesterase (AChE); an enzyme responsible for the degradation of the cholinergic neurotransmitter acetylcholine (Chambers et al., 1994). The involvement of oxidative stress leading to the generation of free radicals or reactive oxygen species (ROS) formation has been implicated in the toxicology of OPI (Bagchi et al., 1992; Yang and Dettbarn 1996). Recent investigations have pointed out that oxidative stress and DNA damage are possibly linked to pesticides-induced adverse health effects in agricultural workers (Muniz, 2008). However, the literature is scarce regarding the involvement of oxidative stress in methyl parathion- and parathion-induced toxicity. Therefore, the aim of the present investigation was to use HepG2 cells as a test model to determine whether their cytotoxicity and genotoxicity are mediated through oxidative stress.
MATERIALS AND METHODS
Chemicals and Media
Test chemicals, methyl parathion (CAS No. 298-00-0, Lot No. 332-52B) and parathion (CAS No. 56-38-2, Lot No. 341-35A) were purchased from ChemService, Inc. (West Chester, Pennsylvania).Growth medium, Dulbecco’s Modified Eagle’s Medium/Ham’s Nutrient Mixture F-12 (Lot No. 3000427) and supplements, G418 Sulfate and fetal bovine serum (FBS) were purchased from American Type Culture Collection -ATCC (Manassas, VA)
Cell Culture
Human liver carcinoma (HepG2) cells were obtained from ATCC, and stored in liquid nitrogen until use. They were thawed by gentle agitation of their containers (vials) for 2 min in a water bath at 37°C. After thawing, the content of each vial was transferred to a 75cm2 tissue culture flask, diluted with Dubelcco’s Modified Eagle’s Minimum Essential Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% streptomycin and penicillin, and incubated for 24 hr at 37°C in a 5% CO2 incubator to allow the cells to grow, and form a monolayer in the flask. The growth medium was changed two times per week. Cells grown to 75–85% confluence were washed with phosphate buffer saline (PBS), trypsinized with 3mL of 0.25% (v) trypsin-0.0.3% /v) EDTA, diluted with fresh medium, and counted using a hemocytometer.
Cell Viability/Cytotoxicity Assay
For this experiment, 1 × 104 cells plated in each well of 96-well plates were placed in the humidified 5% CO2 incubator at 37°C and allowed to attach to the substrate for 24h period. Cells grown to 70–85% confluence were exposed to various concentrations (0 mM, 6 mM, 12 mM, 18 mM, 24 mM, and 30 mM) of parathion and methyl parathion, respectively, and subsequently incubated for 48 hr. The cells incubated in culture medium alone served as a control for cell viability (untreated wells). After incubation the cell viability was determined using the MTT [3-(4,5- Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay according to the standard test protocol commonly used in our laboratory (Mosmann 1983; Tchounwou et al. 2003; Brown et al. 2008).
Lipid Peroxidation Assay
Briefly, 2 × 106 HepG2 cells/mL were placed in a total volume of 10ml growth medium and treated with different concentrations (0–30mM) of methyl parathion and parathion, respectively. Cells were incubated for 48 h in the humidified 5% CO2 incubator at 37°C. After the incubation period, cells were collected in test tubes and centrifuged. The cell pellets were re-suspended in 0.5 ml of Tris-HCl, pH 7.4, and lysed using a sonicator (W-220; Ultrasonic, Farmingdale, NY) under the conditions of duty cycle 25% and output control 40% for 5 sec on ice. All samples were centrifuged at 15,000 rpm and 200 µl aliquot of the sample or 2 mg of cell lysate protein was assayed for malondialdehyde (MDA) according to the lipid peroxidation assay kit protocol (Calbiochem-Novabiochem, San Diego, CA). The absorbance of the sample was monitored at 586 nm, and the concentration of MDA was determined from a standard curve (Halliwell 1985, Moore et al., 2009).
Genotoxicity Assay
The Comet Assay was performed as recently described in our laboratory (Stevens et al., 2010; Trivergen, Inc., 2010). Briefly, 1 × 106 HepG2 cells were cultured in 6-well tissue culture plates and exposed to various concentrations (0 – 30mM) of parathion and methyl parathion for 48 hr and stored in a 37°C, 5% CO2 incubator. Following chemical treatment, the medium was removed, and the cells were washed three times with cold PBS, trypsinized with 1 mL of 0.25% trypsin-EDTA, harvested, and counted. The cells were spun down at 3000 rpm for 5 min. The pellet was re-suspended in PBS at a cell density of 1 × 105. The cells were combined with molten LMAgarose (at 37°C) at a ratio of 1:10 (v/v), and 75 µL was immediately pipetted onto CometSlide™. The slides were placed flat in a refrigerator at 4 °C for 30 min, and then immersed in prechilled lysis solution on ice for 1 h. Excess buffer from slides was removed, and the slides were immersed in freshly prepared alkaline solution, pH > 13 (0.6 g of NaOH pellets, 250 µL of 200 mM EDTA and 49.75 mL of dH2O) for 1 h. Slides were washed twice for 5 min with 1X TBE electrophoresis buffer (Tris base, boric acid, and EDTA) and electrophoresed in a horizontal gel apparatus at 1 V/cm (22 V) for 10 min. Slides were placed in 70% ethanol for 5 min, removed, and tapped to remove excess ethanol. Slides were air dried overnight, stained with SYBR Green, and allowed to set for 12 h. Photographs were taken to illustrate the changes in DNA morphology associated with chemical exposure. A total of 150 comets were scored per chemical dose, and 75 comets randomly selected from three replicated slides, examined with an Olympus Epifluorescence Microscope, and analyzed using the LAI’s Automated Comet Assay Analysis System (Loates Associates, Inc. Westminster, MD). The experiment was repeated at least three times.
Statistical Analysis
Experiments were performed in triplicates. Data were presented as means ± SDs. Where appropriate, one-way ANOVA or Student paired t-test was performed using SAS Software available in the Biostatistics Core Laboratory at Jackson State University. P-values less than 0.05 were considered statistically significant. Cell viability, DNA damage, and MDA levels were presented graphically in the form of histograms, using Microsoft Excel computer program.
RESULTS
Measurements of Cell Viability
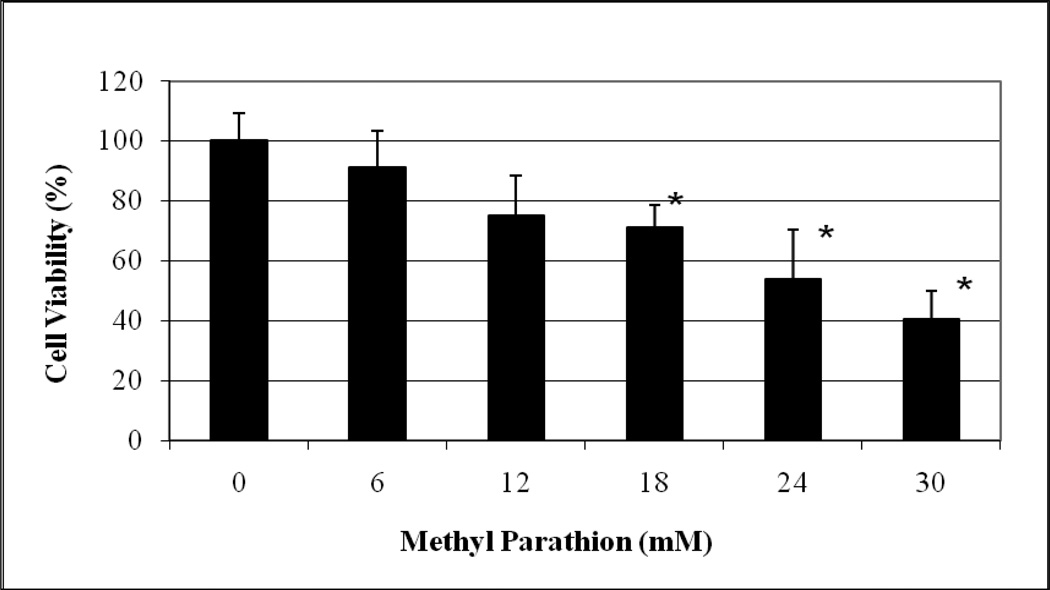
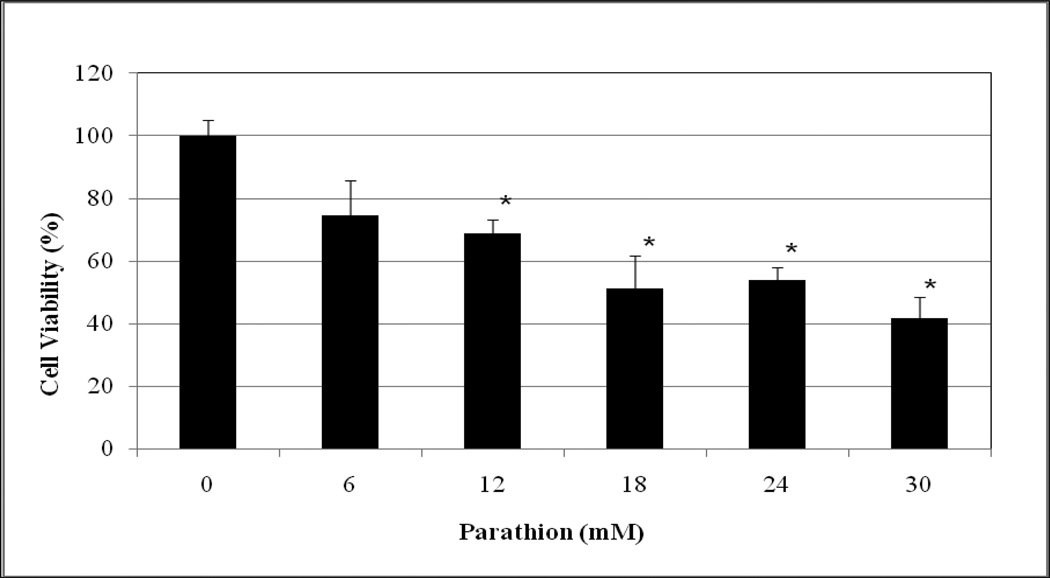
The cytotoxic effects of methyl parathion and parathion to HepG2 cells are represented in Figures 1 and 2, respectively. As shown in these figures, methyl parathion and parathion gradually decrease the viability of HepG2 cells in dose-dependent fashion, showing 48h-LD50 values of 26.20 mM and 22.11 mM, respectively. The percentages of cell viability were 100.00 ± 9.00, 91.25 ± 12.00, 75.11 ± 13.00, 71.25 ± 7.00, 61 ± 12.00, and 43 ± 9.00% for 0, 6, 12, 18, 24 and 30 mM methyl parathion (Figure 1), 100 ± 3.00, 74.53 ± 11.00, 68.82 ± 4.00, 51.31 ± 8.00, 53.96 ± 3.00, and 41.75 ± 7.00 % for 0, 6, 12, 18, 24, and 30 mM parathion (Figure 2). Based on the cell viability measurement, methyl-parathion appears to be slightly less toxic to HepG2 cells than parathion. This reduction of toxicity may be associated with the presence of methyl group in methyl parathion.
Fig. 1.
Cytotoxic effect of methyl parathion to human liver carcinoma (HepG2) cells. HepG2 cells were exposed to different doses (0–30 mM) of methyl parathion as indicated in Materials and Methods. Cell viability was determined based on the MTT assay. Each point represents a mean value and standard deviation of 3 experiments. *Significantly different from the control by ANOVA Dunnett’s test; p < 0.05.
Fig. 2.
Cytotoxic effect of parathion to human liver carcinoma (HepG2) cells. HepG2 cells were exposed to different doses (0–30 mM) of parathion as indicated in Materials and Methods. Cell viability was determined based on the MTT assay. Each point represents a mean value and standard deviation of 3 experiments. *Significantly different from the control by ANOVA Dunnett’s test; p < 0.05.
Measurements of Malondialdehyde (MDA)
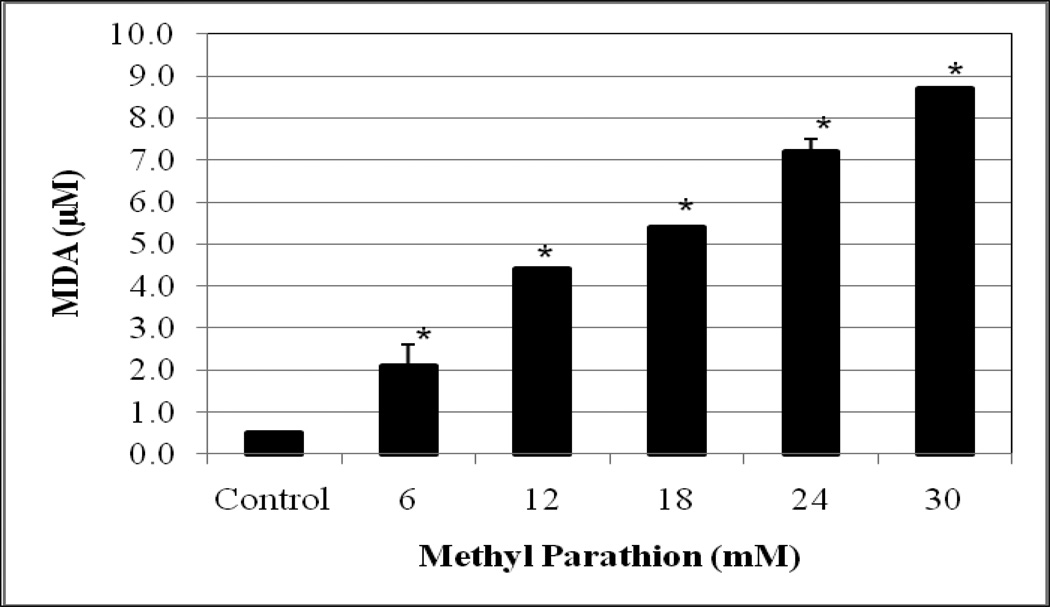
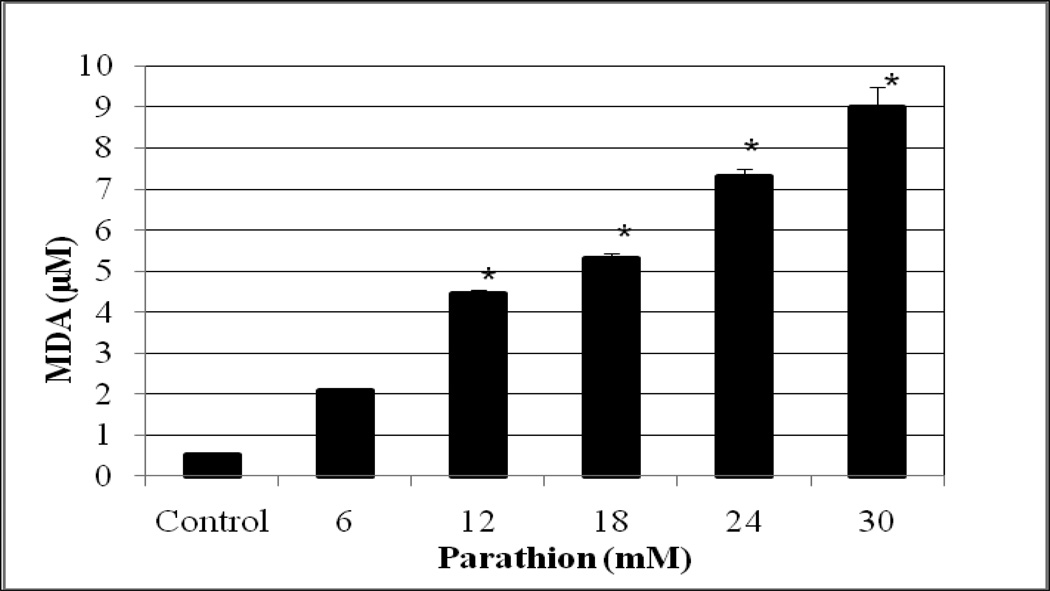
Lipid peroxidation assay resulted in a significant increase (p<0.05) of MDA level in both methyl parathion and parathion treated-HepG2 cells compared to the controls. The amounts of MDA produced were 0.5 ± 0.05, 2.1 ± 0.5, 4.4 ± 0.01, 5.4 ± 0.01, 7.2 ± 0.3 µM, and 8.7 ± 0 for 0, 6, 12, 18, 24 and 30 mM methyl parathion (Figure 3), and 0.52 ± 0, 2.1 ± 0.1, 4.5 ± 0.1, 5.3 ± 0.1, 7.3 ± 0.2, and 9.0 ± 0.5 µM for 0, 6, 12, 18, 24 and 30 mM parathion (Figure 4). We observed a gradual increase of MDA level (an end product of lipid peroxidation) in HepG2 cells with increasing concentrations of both methyl parathion and parathion.
Fig. 3.
Effects of methyl parathion on MDA generation in HepG2 cells. Cells were exposed to different doses (0–30 mM) of methyl parathion, and malondialdehyde formation was determined as described in Materials and Methods. *Significantly different from the control by ANOVA Dunnett’s test; p < 0.05.
Fig. 4.
Effect of parathion on MDA generation in HepG2 cells. Cells were exposed to different doses (0–30 mM) of parathion, and malondialdehyde formation was determined as described in Materials and Methods. *Significantly different from the control by ANOVA Dunnett’s test; p < 0.05.
Measurements of DNA Damage
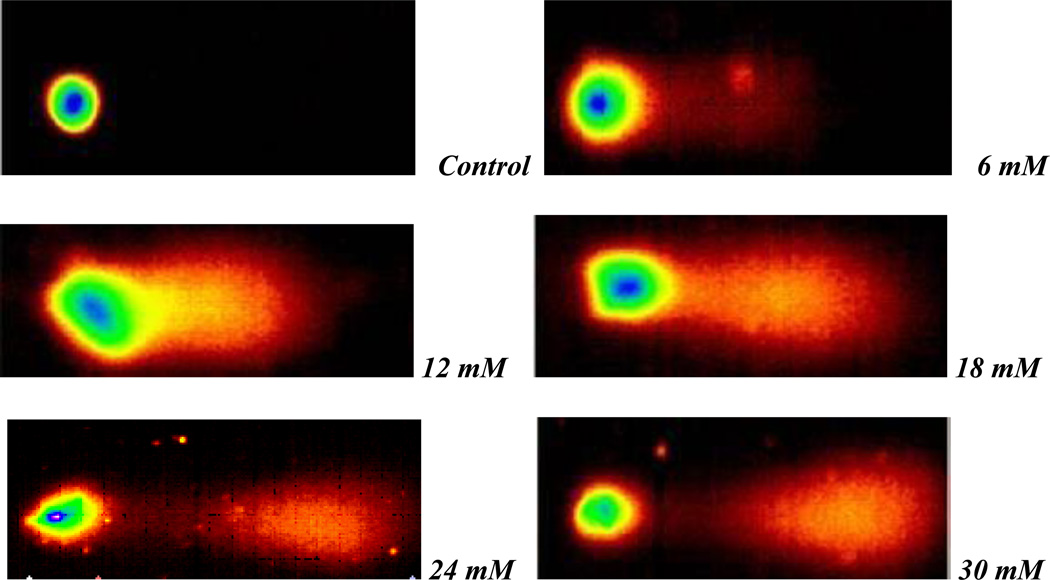
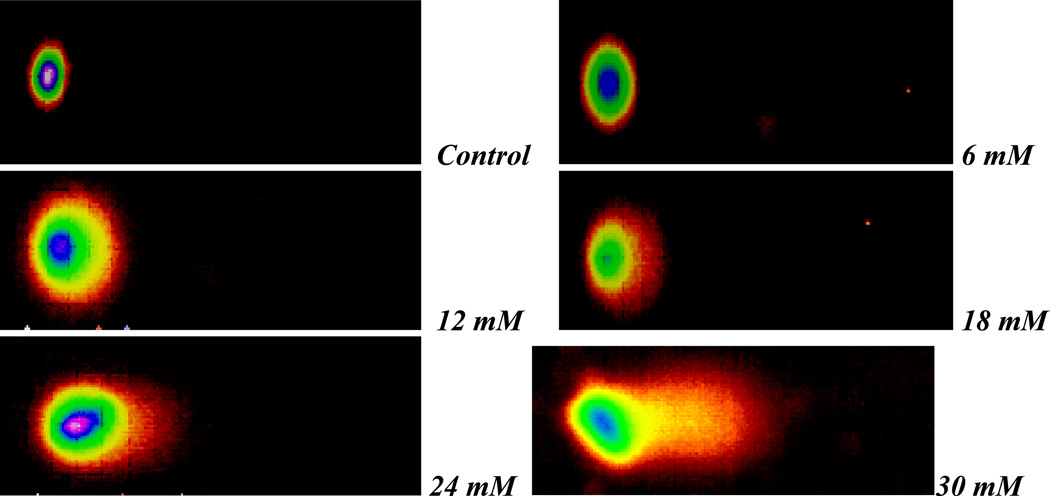
Selected comet assay images of methyl parathion- and parathion-treated HepG2 cells are presented in Figures 5 and 6, respectively. Our data shows that exposure to both compounds significantly increases the degree of genotoxicity in HepG2 cells, as evidenced by the graduate increase in the percentage of DNA damage and the length of comet tail.
Fig. 5.
Representative Comet assay SYBR Green images of HepG2 cells exposed to different doses (0–30 mM) of methyl parathion. Cells were exposed to different doses of methyl parathion, and single cell gel electrophoresis (Comet) assay was performed as described in Materials and Methods.
Fig. 6.
Representative Comet assay SYBR Green images of HepG2 cells exposed to different doses (0–30 mM) of parathion. Cells were exposed to various doses of parathion, and single cell gel electrophoresis (Comet) assay was performed as described in Materials and Methods.
DISCUSSION
Measurement of Cell Viability
Cytotoxicity, the degree to which a chemical can cause cell damage, was assessed in the present study by the means of MTT assay. As shown in Figure 3, the MTT assay results reveal that methyl-parathion and parathion are cytotoxic to human liver carcinoma (HepG2) cells, showing 48 h-LD50 values of 26.20 mM and 22.11 mM for methyl parathion and parathion, respectively. Hence, methyl-parathion is slightly less toxic to HepG2 cells than parathion. Overall, both organophosphate insecticides significantly decrease (p<0.05) the viability of HepG2 cells in dose-dependent fashion. Consistent with our finding, Budreau and Singh (1973) reported that organophosphate pesticides are embryotoxic and can induce fetal anomalies in experimental animals. Several studies have indicated that methyl parathion exposure has toxic effects to mammals, fishes, birds, and non-target invertebrates (Solecki et al. 1996; Fanta et al. 2003; Roque et al., 2005).
Published studies have also demonstrated that organophosphate pesticides/insecticides exposure can affect many organ systems including the nervous, hematopoietic, cardiovascular, and reproductive systems (Handy et al., 2002; Neishabouri et al., 2004; Gokalp et al., 2005, Kalender et al., 2005). Both methyl parathion and parathion primarily affect the nervous system through the inhibition of cholinesterase (an enzyme required for proper nerve functioning), and the reproductive system by crossing the placenta to induce toxicity to the fetus (Kearney and Kaufman 1975; Occupational Health Services 1991, Hayes and Laws 1990). Laboratory reports have indicated that organophosphate pesticides exert their toxicity by inhibiting acetylcholinesterase (AChE), enzyme responsible for the degradation of the cholinergic neurotransmitter acetylcholine (Chambers et al. 1994; John et al., 2001; Yavuz et al., 2005). Due to parathion high toxicity and risks of exposure to agricultural workers and to birds, EPA announced the cancellation of all uses on fruits, nuts, and vegetable crops (U.S. EPA 1992). Although the present study reveals that methyl parathion exposure is slightly less toxic than parathion to HepG2 cells, both organophosphate insecticides are highly cytotoxic and may cause severe damage to the liver cells/tissues. A recent study of rats treated with a single dose (6.25, 12.5, or 50 mg/kg) of methyl parathion resulted in severe signs of acute toxicity within 24 hr of dosing and died within 72 hr (Zhu et al., 2001).
Measurement of Malondialdehyde (MDA)
To investigate the hypothesis that methyl parathion and parathion exposure induces oxidative stress in humans, HepG2 cells were treated with different doses of methyl parathion and parathion for 48 hr and assessed for the degree of MDA production. Our results indicate that methyl parathion and parathion exposure caused significant increase (p < 0.05) in the concentration of MDA, an end product of lipid peroxidation. The lipid peroxidation is an autocatalytic process which is caused by free radicals. The increase of MDA level in this study is an indicator of oxidative stress or free radical formation caused by methyl parathion and parathion in liver cells. Consistent with our finding, recent studies have indicated that organophosphorus compounds cause an increase of lipid peroxidation level in rats (Kalender et al., 2004; Sulak et al., 2005).
A previously published study has reported that pesticides induce the generation of reactive oxygen species, DNA damage, and lactate dehydrogenase leakage in cell and animal models (Bagchi et al. 1995). Ranjbar and his collaborators have also reported an association between induction of oxidative stress and acetylcholinesterase inhibition in organophosphate pesticide manufacturing workers with a minimal exposure of one year (Ranjbar et al., 2003). Research has also pointed out that the mechanism by which organophosphate insecticides exert their toxic effects is through the accumulation of acetylcholine resulting from the inhibition of acetylcholinesterase (AChE). This accumulation often leads to the onset of side effects depending on the interaction of the substrate with cholinergic receptors. Interaction with these receptors often leads to the onset of lipid peroxidation (Hazarika et al., 2003). The release of phospholipids has therefore been reported to be very important in the action of acetylcholinesterase (AChE) (Datta et al., 1994).
Measurements of DNA Damage
The single cell gel electrophoresis (SCGE) or Comet assay, measures DNA damage from individual cells based on the migration of DNA towards the negative anode. This migration takes place through an electrophoretic field. Damaged DNA containing strand breaks migrate farther in the gel than intact DNA thus giving the appearance of a comet. DNA is stained with a fluorescent dye and viewed utilizing an epifluorescent microscope. The extent of the damage is determined by the length of the comet tail and percentage of migrated DNA.
A review of the Comet assay outlines a number of factors effecting DNA damage (Møller et. al, 2000). Several studies assessing DNA damage as a consequence of pesticide exposure have been conducted using the Comet assay. These studies have involved exposure to various pesticides. The Comet assay has been used to assess DNA damage in blood leukocytes of twenty nine Pakistani manufacturing workers exposed to various mixtures of OPIs, carbamates, and pyrethroids. The mean comet tail lengths and DNA damage were found to be significantly higher in exposed workers (Bhalli, et. al 2006). In a more recent study of immigrant farm-workers in Oregon exposed to various pesticides, significantly higher percentages of damaged DNA and comet tail moment were recorded in oral leukocytes of agricultural workers compared to nonagricultural workers (McCauley et. al., 2008). Urine analysis showed the presence of tetrahydrophthalimide, a Captan metabolite, dialhylphosphate metabolites, and Ops. Additionally, in vitro studies with lymphocyte cultures treated with OPs also resulted in a significant induction of DNA damage; validating and supporting the in vivo studies. Also, a recent study conducted in our laboratory has shown that malathion exposure induces DNA damage in HepG2 cells (Moore et al., 2009).
CONCLUSIONS
The present in vitro study demonstrated that both methyl parathion and parathion exposure decreases cell viability, increases MDA levels resulting from reactive oxygen species formation, and induces DNA damage in human liver carcinoma (HepG2) cells. These findings suggest that oxidative stress plays an important role in organophosphate insecticides (OPI)-induced toxicity and genotoxicity in HepG2 cells. Overall, the present research demonstrated that OPI exposure is associated with cytotoxic and genotoxic effects on human liver carcinoma (HepG2) cells. Further investigations are needed to identify the intracellular processes and mechanisms of oxidative stress resulting from methyl parathion and/or parathion exposure.
ACKNOWLEDGEMENTS
This research was financially supported in part by a grant from the National Institutes of Health (Grant No. 5G12RR013459-13), through the RCMI Center for Environmental Health, and in part by a grant from the Department of the Army Cooperative Agreement No. W912HZ-04-2-0002, at Jackson State University.
REFERENCES
- Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995;104:129–140. doi: 10.1016/0300-483x(95)03156-a. [DOI] [PubMed] [Google Scholar]
- Bagchi M, Hassoun EA, Bagchi D, Stohs SJ. Endrin induced increase in hepatic lipid peroxidation, membrane microviscosity and DNA damage in rats. Archives of Environmental Contamination and Toxicology. 1992;23:1–5. doi: 10.1007/BF00225988. [DOI] [PubMed] [Google Scholar]
- Bhalli JA, Khan QM, Nasim A. DNA damage in Pakistani-pesticide manufacturing workers assayed using the Comet assay. Environmental and Molecular Mutagenesis. 2006;47(8):587–593. doi: 10.1002/em.20232. [DOI] [PubMed] [Google Scholar]
- Bhanti M, Taneja A. Contamination of vegetables of different seasons with organophosphorus pesticides and related health risk assessment in northern India. Chemosphere. 2007;69(1):63–68. doi: 10.1016/j.chemosphere.2007.04.071. [DOI] [PubMed] [Google Scholar]
- Brown E, Yedjou CG, Tchounwou PB. Oxidative stress in human liver carcinoma cells exposed to arsenic trioxide (HepG2) Metal Ions in Biology and Medicine. 2008;10:583–587. [PMC free article] [PubMed] [Google Scholar]
- Budreau CH, Singh RP. Effect of fenthion and dimethoate on reproduction in the mouse. Toxicology and Applied Pharmacology. 1973;26:29–38. doi: 10.1016/0041-008x(73)90082-3. [DOI] [PubMed] [Google Scholar]
- Chambers JE, Ma T, Boone JS, Chambers HW. Role of detoxication pathways in acute toxicity levels of phosphorothionate insecticides in the rat. Life Sciences. 1994;54:1357–1364. doi: 10.1016/0024-3205(94)00515-x. [DOI] [PubMed] [Google Scholar]
- Choudhary A, Sharma DC. Pesticide residues in honey samples from Himachal Pradesh (India) Bulletin of Environmental Contamination and Toxicology. 2008;80(5):417–422. doi: 10.1007/s00128-008-9426-5. [DOI] [PubMed] [Google Scholar]
- Datta C, Gupta J, Sengupta D. Interaction of organophosphorus insecticides phosphamidon and malathion on lipid profile and acetylcholinesterase activity in human erythrocyte membrane. Indian Journal of Medical Research. 1994;100:87–89. [PubMed] [Google Scholar]
- Fanta E, Rios FSA, Romao S, Vianna ACC, Freiberger S. Histopathology of the fish Corydoras paleatus contaminated with sublethal levels of organophosphorus in water and food. Ecotoxicology and Environmental Safety. 2003;54:119–130. doi: 10.1016/s0147-6513(02)00044-1. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Abu-Qare AW, Meeker-O’Connel WA, Borton AJ, Abou-Donia MB. Methyl parathion: A review of health effects. Journal of Toxicology and Environmental Health, Part B. 2003;6:285–210. doi: 10.1080/10937400306471. [DOI] [PubMed] [Google Scholar]
- Gokalp O, Buyukvanlı B, Cicek E, Ozer K, Koyu A, Altuntas I, Koylu H. The effects of diazinon on pancreatic damage and ameliorating role of vitamin E and vitamin C. Pesticide Biochemistry and Physiology. 2005;81:123–128. [Google Scholar]
- Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. London: Clarendon Press; 1985. pp. 50–57. [Google Scholar]
- Handy RD, Abd-El Samei HA, Bayomy MFF, Mahran AM, Abdeen AM, El-Elaimy EA. Chronic diazinon exposure: pathologies of spleen, thymus, blood cells, and lymph nodes are modulated by dietary protein or lipid in the mouse. Toxicology. 2002;172:13–34. doi: 10.1016/s0300-483x(01)00575-3. [DOI] [PubMed] [Google Scholar]
- Hayes WJ, Laws ER. Handbook of Pesticide Toxicology. NY: Academic Press, Inc.; 1990. Classes of Pesticides. [Google Scholar]
- Hayes WJ, Wayland Jr. Pesticides studied in man. Baltimore, MD: Williams & Wilkins; 1982. [Google Scholar]
- Hazarika A, Sarkar SN, Hajare S, Kataria M, Malik JK. Influence of malathion pretreatment on the toxicity of anilifos in male rats: a biochemical interaction study. Toxicology. 2003;185(1–2):1–8. doi: 10.1016/s0300-483x(02)00574-7. [DOI] [PubMed] [Google Scholar]
- IARC. Miscellaneous pesticides, International Agency for Research on Cancer. Lyon France: 1983. Monograph on the evaluation of carcinogenic risk of chemicals to man; p. 30. [Google Scholar]
- John S, Kale M, Rathore N, Bhatnagar D. Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. Journal of Nutritional Biochemistry. 2001;12:500–504. doi: 10.1016/s0955-2863(01)00160-7. [DOI] [PubMed] [Google Scholar]
- Kalender S, Ogutcu A, Uzunhisarcikli M, Acikgoz F, Ulusoy D, Kalender Y. Diazinon-induced hepatotoxicity and protective effect of vitamin E on some biochemical indices and ultrastructural changes. Toxicology. 2005;211:197–206. doi: 10.1016/j.tox.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Kearney PC, Kaufman DD. Herbicides: chemistry, degradation, and mode of action. –2. 2nd Ed New York: M. Dekker; 1975. [Google Scholar]
- McCauley LA, Lasarev M, Muniz J, Nazar-Stewart V, Kisby G. Analysis of pesticide exposure and DNA damage in immigrant farmworkers. Journal of Agromedicine. 2008;13(4):237–246. doi: 10.1080/10599240802473817. [DOI] [PubMed] [Google Scholar]
- Møller P, Knudsen LE, Loft S, Wallin H. The comet assay as a rapid test in biomonitoring occupational exposure to DNA-damaging and effect of confounding factors. Cancer Epidemiology, Biomarkers & Prevention. 2009;9:1005–1015. [PubMed] [Google Scholar]
- Moore P, Yedjou CG, Tchounwou PB. Malathion-induced oxidative stress, cytotoxicity and genotoxicity in human liver carcinoma (HepG2) cells. Environmental Toxicology. 2009;25(3):221–226. doi: 10.1002/tox.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman T. Rapid colorimetric assay for cellular growth and survival: applications to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Muniz JF, McCauley L, Scherer J, Lasarev M, Koshy M, Kow YW, Nazar-Stewart V, Kisby GE. Biomarkers of oxidative stress and DNA damage in agricultural workers: A pilot study. Toxicology and Applied Pharmacology. 2008;227:97–107. doi: 10.1016/j.taap.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Neishabouri EZ, Hassan ZM, Azizi E, Ostad SN. Evaluation of immunotoxicity induced by diazinon in C57bl/6 mice. Toxicology. 2004;196:173–179. doi: 10.1016/j.tox.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Occupational Health Services, Inc. Secaucus, NJ: OHS Inc.; 1991. MSDS for Parathion. [Google Scholar]
- Poet TS, Kousba AA, Dennison SL, Timchalk C. Physiologically based pharmacokinetic/pharmacodynamic model for the organophosphorus pesticide diazinon. Neurotoxicology. 2004;25:1013–1030. doi: 10.1016/j.neuro.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Racke KD. Degradation of organophosphorus insecticides in environmental matrices. In: Chambers JE, Levi PE, editors. Organophosphates: Chemistry, Fate, and Effects. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Ranjbar A, Pasalar P, Abdollahi M. Induction of oxidative stress and acetylcholinesterase inhibition in organophosphorus pesticide manufacturing workers. Human and Experimental Toxicology. 2002;21(4):179–182. doi: 10.1191/0960327102ht238oa. [DOI] [PubMed] [Google Scholar]
- Riederer AM, Smith KD, Barr DB, Hayde SW, Hunter RE, Jr, Ryan PB. Current and historically used pesticide in residential soil from 11 homes in Atlanta, Georgia, USA. Archives of Environmental Contamination and Toxicology. 2010;58(4):908–917. doi: 10.1007/s00244-009-9439-z. [DOI] [PubMed] [Google Scholar]
- Roque A, Abad S, Betancourt-Lozano M, Garcia de la Parra LM, Baird D, Guerra-Flores AL, Gomez-Gil B. Evaluation of the susceptibility of the cultured shrimp Litopenaeus vannamei to vibriosis when orally exposed to the insecticide methyl parathion. Chemosphere. 2005;60:126–134. doi: 10.1016/j.chemosphere.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Solecki R, Fagi AS, Pfeil R, Hilbig V. Effects of methyl parathion on reproduction in the Japanese quail. Bulletin of Environmental Contamination and Toxicology. 1996;57:902–908. doi: 10.1007/s001289900275. [DOI] [PubMed] [Google Scholar]
- Stevens JJ, Graham B, Walker AM, Tchounwou PB, Rogers C. The effects of arsenic trioxide on DNA synthesis and genotoxicity in human colon cancer cells. International Journal of Environmental Research and Public Health. 2010;7(5):2018–2032. doi: 10.3390/ijerph7052018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulak I, Altuntaş, Karahan N, Yildirim B, Akturk O, Yilmaz HR, Delibas N. Nephrotoxicity in rats induced by organophosphate insecticide methidathion and ameliorating effects of vitamins E and C. Pesticide Biochemistry and Physiology. 2005;83:21–28. [Google Scholar]
- Tchounwou PB, Yedjou CG, Dorsey WC. Arsenic Trioxide-Induced Transcriptional Activation and Expression of Stress Genes in Human Liver Carcinoma Cells (HepG2) Cellular and Molecular Biology. 2003;49:1071–1079. [PubMed] [Google Scholar]
- Trevigen, Incorporated. Gaithersburg, MD: 2010. CometAssay® Reagent kit for single cell gel electrophoresis assay. [Google Scholar]
- United States Environmental Protection Agency. Washington DC: OPP, USEPA; 1992. Ethyl Parathion, Correction to the Amended Cancellation Order. [Google Scholar]
- United States Environmental Protections Agency. Illegal indoor use of methyl parathion. 2000
- United States Environmental Protection Agency. Organophosphate pesticide information: Overview of the ethyl parathion revised risk assessment. 2000
- Yang ZP, Dettbarn WD. Diisopropylphosphorofluoridate induced cholinergic hyperactivity and lipid peroxidation. Toxicology and Applied Pharmacology. 1996;138:48. doi: 10.1006/taap.1996.0096. [DOI] [PubMed] [Google Scholar]
- Yavuz T, Delibas N, Yildirim B, Altuntas I, Candir O, Cora A, Karahan N, Ibrisim E, Kutsal A. Vascular wall damage in rats induced by organophosphorus insecticide methidathion. Toxicology Letters. 2005;155:59–64. doi: 10.1016/j.toxlet.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Zhu H, Rockhold RW, Baker RE, Kramer RE. Effects of single or repeateddermal exposure to methyl parathion on behavior and blood cholinesterase activity in rats. Journal of Biomedical Science. 2001;8:467–474. doi: 10.1007/BF02256609. [DOI] [PubMed] [Google Scholar]