Abstract
The nickel-catalyzed Suzuki–Miyaura coupling of aryl halides and phenol-derived substrates with aryl boronic acids using green solvents, such as 2-Me-THF and t-amyl alcohol, is reported. This methodology employs the commercially available and air-stable pre-catalyst NiCl2(PCy3)2 and gives biaryl products in synthetically useful to excellent yields. Using this protocol, bis(heterocyclic) frameworks can be assembled efficiently.
Transition metal-catalyzed cross-coupling reactions are widely used in the pharmaceutical industry in both medicinal chemistry and drug manufacturing.1 Although the use of Pd catalysis is most common, complementary approaches are highly sought after. Specifically, cost effective catalyst systems that allow for unconventional couplings to take place smoothly are of great value. Additionally, the ability to efficiently carry out cross-coupling reactions in more environmentally friendly solvents2,3 remains an important goal of green chemistry research.4 It should be noted that organic solvents comprise up to 85% of the waste produced from a drug synthesis.5
Recently, the field of nickel-catalyzed cross-coupling reactions has gained considerable attention. The low cost and high reactivity of nickel is attractive, and a range of substrates has been shown to undergo nickel-catalyzed carbon–carbon and carbon–heteroatom bond forming reactions, including halides6 and a variety of oxygen-based electrophiles (e.g., aryl-esters,7 -ethers,8 -carbamates,9 -sulfamates10,11).12 Considering the promise of nickel-catalyzed couplings and the need to make industrial processes more environmentally friendly, we explored coupling reactions in green solvents. Herein, we demonstrate that a range of substrates, including heterocycles, participate in the nickel-catalyzed Suzuki–Miyaura coupling in solvents that are attractive for industrial applications (Figure 1).
Figure 1.
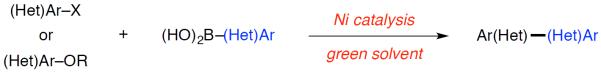
Suzuki–Miyaura cross-coupling of aryl halides and phenol derivatives in green solvents.
We initiated our efforts by examining the cross-coupling of naphthyl sulfamate 1 and phenylboronic acid (2) using the commercially available NiCl2(PCy3)2 precatalyst (Table 1). Although solvents such as 1,4-dioxane and N-methyl-2-pyrrolidone (NMP), which have been deemed as enviromentally unfriendly solvents,2 are commonly used in nickel-catalyzed cross-couplings, we were delighted to find that many other solvents may be employed in the coupling to give biaryl 3. Of the >30 solvents that were surveyed, more than half gave quantititative yields of 3, while many others also showed promise.13 A subset of our findings are summarized in Table 1. The solvent used in our previous studies,7,9 toluene, gave biaryl 3 in quantitative yield (entry 1). Acetone, ethyl acetate, and isopropyl acetate (entries 2–4, respectively) also gave product in comparable yields. Alcohol solvents were also examined. Whereas the use of n-BuOH proved ineffective (entry 5), t-amyl alcohol was found to be an excellent solvent for the cross-coupling (entry 6). Ethereal solvents also provided biaryl 3 in quantitative yield (entries 7–8). Mixed results were observed for highly coordinating solvents; for example, the use of DMSO was unsuccessful (entry 9), but the use of acetonitrile led to the desired coupling. Although many solvents could be employed, we opted to pursue t-amyl alcohol and 2-Me-THF (entries 6 and 8, respectively) for further studies.2,14
Table 1.
Survey of solvents in the Suzuki–Miyaura coupling.a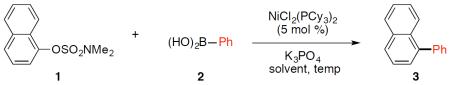
| entry | solvent, temp | yieldb | entry | solvent, temp | yieldb |
|---|---|---|---|---|---|
| 1 | toluene, 110 °C | 100% | 6 | t-amyl alcohol, 100 °C | 100% |
| 2 | acetone, 75 °C | 96% | 7 | MTBE, 80 °C | 100% |
| 3 | EtOAc, 100 °C | 100% | 8 | 2-Me-THF, 80 °C | 100% |
| 4 | i-PrOAc, 110 °C | 100% | 9 | DMSO, 110 °C | 0% |
| 5 | n-BuOH, 110 °C | 0% | 10 | acetonitrile, 100 °C | 99% |
Conditions: NiCl2(PCy3)2 complex (5 mol %), sulfamate substrate 1 (1.00 equiv), 2 (2.50 equiv), K3PO4 (4.50 equiv), hexamethylbenzene (0.10 equiv), 12 h.
Yield of 3 determined by 1H NMR analysis of crude reaction mixtures using hexamethylbenzene as an internal standard.
With promising results in hand, we tested the analogous cross-coupling of several other electrophilic partners (Table 2). In addition to the naphthyl sulfamate (entry 1), the corresponding carbamate15 and pivalate ester were deemed competent substrates (entries 2–3). Furthermore, sulfonate derivatives of 1-naphthol also gave high yields of coupled product (entries 4–6). Moreover, the use of 1-naphthyl chloride, bromide, and iodide each delivered the desired product under our optimized conditions (entries 7–9, respectively).
Table 2.
Survey of cross-coupling partners.a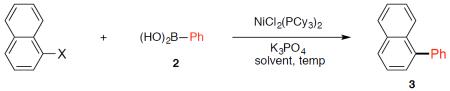
| entry | X | yield (t-amyl alcohol)b | yield (2-Me-THF)b,c |
|---|---|---|---|
| 1 | OSO2NMe2 | 100 | 100 |
| 2 | OCONEt2 | 57 | 50 |
| 3 | OPiv | 94 | 100 |
| 4 | OMs | 97 | 95 |
| 5 | OTs | 100 | 98 |
| 6 | OTf | 100 | 100 |
| 7 | Cl | 100 | 94 |
| 8 | Br | 97 | 92 |
| 9 | I | 100 | 97 |
Conditions: NiCl2(PCy3)2 complex (5 mol %), substrate (1.00 equiv), 2 (2.50 equiv), K3PO4 (4.50 equiv), hexamethylbenzene (0.10 equiv), 100 °C, 12 h.
Yield of 3 determined by 1H NMR analysis of the crude reaction mixtures using hexamethylbenzene as an internal standard.
66 °C
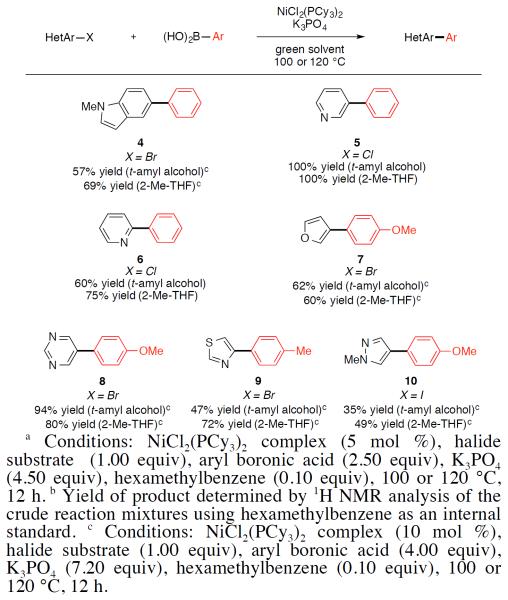
An array of heterocyclic aryl halide substrates underwent the desired cross-coupling with aryl boronic acids in t-amyl alcohol and 2-Me-THF (Figure 2). Nitrogen-containing heterocycles such as indole and pyridine were tolerated to give products 4–6, respectively. 3-Bromofuran also underwent the desired coupling to give cross-coupled product 7. In addition, the methodology was found to be tolerant of substrates that contain multiple heteroatoms, as demonstrated by the formation of products 8–10.
Figure 2.
Coupling of heterocyclic halides with aryl boronic acids.a,b
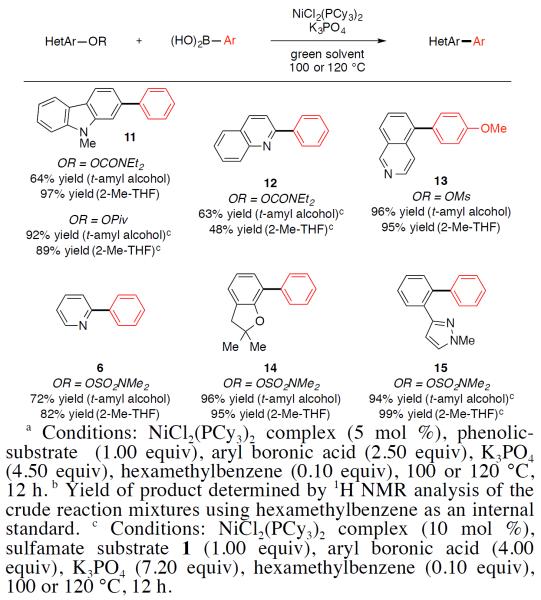
As shown in Figure 3, heterocyclic phenol-derived electrophiles participate in the Suzuki–Miyaura coupling in green solvents.16 Both the carbamate and ester derivatives of 2-hydroxy-N-Me-carbazole coupled smoothly with phenyl boronic acid to give 11 in good yields. Quinoline, isoquinoline, and pyridine derivatives were also tolerated, as demonstrated by the formation of 12, 13, and 6, respectively. Dihydrobenzofuran- and pyrazole-based sulfamate substrates gave excellent yields of products 14 and 15, respectively.
Figure 3.
Coupling of heterocyclic phenolic derivatives with aryl boronic acids.a,b
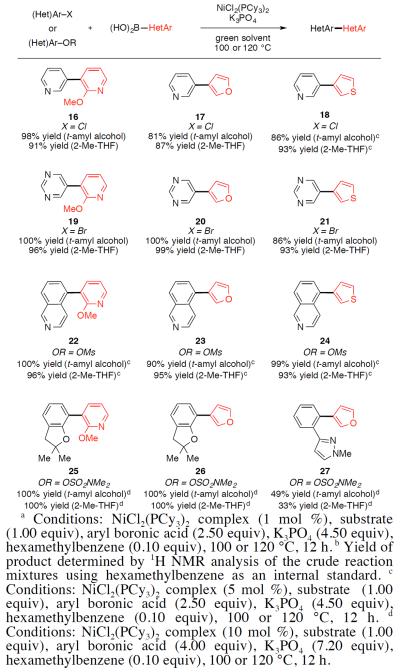
We also tested our cross-coupling procedure for the assembly of bis(heterocyclic) scaffolds (Figure 4), which are prevalent in numerous drugs and natural products, but are sometimes difficult to access using Pd-catalyzed methods.17 3-Cl-Pyridine readily underwent coupling with pyridyl-,18 furyl-, and thiophenyl-boronic acid derivatives to provide bis(heterocyclic) compounds 16–18. Likewise, 5-Br-pyrimidine was coupled to deliver compounds 19–21.19 The mesylate derived from 5-hydroxyisoquinoline also underwent facile coupling, thus affording 22–24 in excellent yields. Additionally, the coupling of a benzofuranyl sulfamate was explored to give bis(heterocycles) 25 and 26.20 We also tested the coupling of a pyrazole derived sulfamate with 3-furanyl boronic acid, which afforded 27 in moderate yield. Our methodology complements the recently disclosed Nicatalyzed cross-couplings to form bis(heterocycles) reported by Hartwig.6e
Figure 4.
Coupling of heterocyclic substrates with heterocyclic aryl boronic acids.a,b
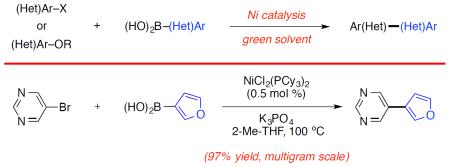
The nickel-catalyzed Suzuki–Miyaura coupling shows promise for the assembly of bis(heterocyclic) frameworks on preparative scale (Figure 5).21 Using 1 mol% Ni catalyst, isoquinoline 28 was coupled with pyridylboronic acid 29 to provide adduct 22 in quantitive yield on gram scale. Additionally, bromopyrimidine 30 underwent Nicatalyzed cross-coupling with furanylboronic acid 31 using 0.5 mol% catalyst. This transformation, which was performed on 5 g scale, delivered 20 in 97% yield.
Figure 5.
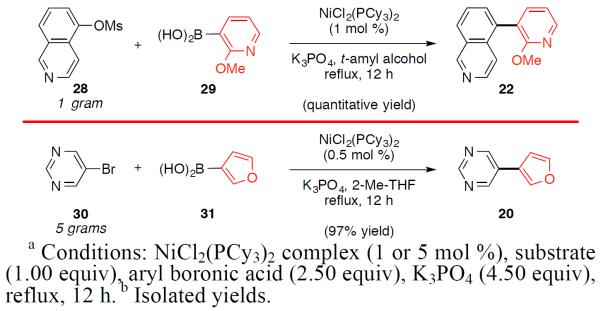
Gram scale couplings.a,b
In summary, we have demonstrated the efficient Nicatalyzed Suzuki–Miyaura cross-coupling of aryl halides and phenolic derivatives in green solvents. The scope of these reactions is broad with respect to both coupling partners and heterocycles are well-tolerated. Additionally, the potential for these couplings to be performed on preparative scale has been demonstrated by the gram scale assembly of bis(heterocycles) using low catalyst loadings (i.e., 0.5–1 mol% Ni). Given the appeal of Ni catalysis and the favorable green solvents that may be employed, we expect the methodology presented will find utility in academic and industrial applications.
Supplementary Material
Acknowledgment
We thank Daniel Richter, Paul Richardson, Helen Sneddon, and the ACS Green Chemistry Institute Pharmaceutical Roundtable for helpful discussions. The authors are grateful to Boehringer Ingelheim, DuPont, Eli Lilly, Amgen, AstraZeneca, Roche, Bristol-Myers Squibb, the Camille and Henry Dreyfus Foundation, the A. P. Sloan Foundation, the ACS GCI, the S. T. Li Foundation, the Foote and Pauley Families (fellowships to S.D.R and L.H., respectively), and the University of California, Los Angeles for financial support. These studies were supported by shared instrumentation grants from the NSF (CHE-1048804) and the National Center for Research Resources (S10RR025631).
Footnotes
Supporting Information Available: Experimental details and compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Diederich F, Meijere A, editors. Metal-Catalyzed Cross-Coupling Reactions. Wiley-VCH; Weinheim: 2004. [Google Scholar]; (b) Hassan J, Sevignon M, Gozzi C, Schulz E, Lemaire M. Chem. Rev. 2002;102:1359–1469. doi: 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]; (c) Miyaura N, editor. Topics in Current Chemistr. Vol. 219. Springer-Verlag; New York: 2002. [Google Scholar]; (d) Corbet J, Mignani G. Chem. Rev. 2006;106:2651–2710. doi: 10.1021/cr0505268. [DOI] [PubMed] [Google Scholar]; (e) Negishi E. Bull. Chem. Soc. Jpn. 2007;80:233–257. [Google Scholar]; (f) Shen HC, Crawley ML, Trost BM, editors. Application of Transition Metal Catalysis in Drug Discovery and Development: An Industrial Perspective. John Wiley & Sons, Inc.; Hoboken: 2012. [Google Scholar]
- 2.For solvent selection guide in medicinal chemistry, see: Alfonsi K, Colberg J, Dunn PJ, Fevig T, Jennings S, Johnson TA, Kleine HP, Knight C, Nagy MA, Perry DA, Stefaniak M. Green Chem. 2008;10:31–36.. Henderson RK, Jiménez-Gonález C, Constable DJC, Alston SR, Inglis GGA, Fisher G, Sherwood J, Binks SP, Curzons AD. Green Chem. 2011;13:854–862.
- 3.For examples of cross-coupling reactions in aqueous media, see Bussolari JC, Rehborn DC. Org. Lett. 1999;1:965–967.. Hesse S, Kirsch G. Synthesis. 2001:755–759.. Anderson KW, Buchwald SL. Angew. Chem. Int. Ed. 2005;44:6173–6177. doi: 10.1002/anie.200502017.. Lipshutz BH, Ghorai S. Aldrichim. Acta. 2008;41:59–72.. Alacid E, Nájera C. J. Org. Chem. 2009;74:2321–2327. doi: 10.1021/jo802356n.. Han J, Liu Y, Guo R. J. Am. Chem. Soc. 2009;131:2060–2061. doi: 10.1021/ja808935n.. Chalker JM, Wood CS, Davis BG. J. Am. Chem. Soc. 2009;131:16346–16347. doi: 10.1021/ja907150m..
- 4.(a) Anastas PT, Warner JC, editors. Green Chemistry: Theory and Practice. Oxford University Press; New York: 1998. [Google Scholar]; (b) Anastas PT, Kirchhoff MM. Acc. Chem. Res. 2002;35:686–694. doi: 10.1021/ar010065m. [DOI] [PubMed] [Google Scholar]
- 5.Fortunak JM. Future Med. Chem. 2009;1:571–575. doi: 10.4155/fmc.09.60. [DOI] [PubMed] [Google Scholar]
- 6.For selected examples examples involving aryl halides, see: Powell DA, Maki T, Fu GC. J. Am. Chem. Soc. 2005;127:510–511. doi: 10.1021/ja0436300.. Yoshikai N, Matsuda H, Nakamura E. J. Am. Chem. Soc. 2009;131:9590–9599. doi: 10.1021/ja903091g.. Everson DA, Shrestha R, Weix DJ. J. Am. Chem. Soc. 2010;132:920–921. doi: 10.1021/ja9093956.. Fan X-H, Yang L-M. Eur. J. Org. Chem. 2011:1467–1471.. Ge S, Hartwig JF. Angew. Chem. Int. Ed. 2012;51:12837–12841. doi: 10.1002/anie.201207428.. Zultanski SL, Fu GC. J. Am. Chem. Soc. 2013;135:624–627. doi: 10.1021/ja311669p..
- 7.For selected examples examples involving aryl esters, see: Guan B-T, Wang Y, Li B-J, Yu D-G, Shi Z-J. J. Am. Chem. Soc. 2008;130:14468–14470. doi: 10.1021/ja8056503.. Li B-J, Li Y-Z, Lu X-Y, Liu J, Guan B-T, Shi Z-J. Angew. Chem. Int. Ed. 2008;47:10124–10127. doi: 10.1002/anie.200803814.. Quasdorf KW, Riener M, Petrova KV, Garg NK. J. Am. Chem. Soc. 2009;131:17748–17749. doi: 10.1021/ja906477r.. Zhou Q, Srinivas HD, Dasgupta S, Watson MP. J. Am. Chem. Soc. 2013;135:3307–3310. doi: 10.1021/ja312087x..
- 8.For selected examples involving aryl ethers, see: Tobisu M, Shimasaki T, Chatani N. Angew. Chem. Int. Ed. 2008;47:4866–4869. doi: 10.1002/anie.200801447.. Shimasaki T, Konno Y, Tobisu M, Chatani N. Org. Lett. 2009;11:4890–4892. doi: 10.1021/ol901978e.. Shimasaki T, Tobisu M, Chatani N. Angew. Chem. Int. Ed. 2010;49:2929–2932. doi: 10.1002/anie.200907287.. Li X-J, Zhang J-L, Geng Y, Jin Z. J. Org. Chem. 2013;78:5078–5084. doi: 10.1021/jo4005537..
- 9.For selected examples involving aryl carbamates, see: Sengupta S, Leite M, Raslan DS, Quesnelle C, Snieckus V. J. Org. Chem. 1992;57:4066–4068.. Quasdorf KW, Tian X, Garg NK. J. Am. Chem. Soc. 2008;130:14422–14423. doi: 10.1021/ja806244b.. Antoft-Finch A, Blackburn T, Snieckus V. J. Am. Chem. Soc. 2009;131:17750–17752. doi: 10.1021/ja907700e.. Quasdorf KW, Antoft-Finch A, Liu P, Silberstein AL, Komaromi A, Blackburn T, Ramgren SD, Houk KN, Snieckus V, Garg NK. J. Am. Chem. Soc. 2011;133:6352–6363. doi: 10.1021/ja200398c.. Mesganaw T, Silberstein AL, Ramgren SD, Fine Nathel NF, Hong X, Garg NK. Chem. Sci. 2011;2:1766–1771. doi: 10.1039/c1sc00230a.. Hie L, Ramgren SD, Mesganaw T, Garg NK. Org. Lett. 2012;14:4182–4185. doi: 10.1021/ol301847m..
- 10.For selected examples involving aryl sulfamates, see: Macklin TK, Snieckus V. Org. Lett. 2005;7:2519–2522. doi: 10.1021/ol050393c.. Baghbanzadeh M, Pilger C, Kappe CO. J. Org. Chem. 2011;76:1507–1510. doi: 10.1021/jo1024464.. Ramgren SD, Silberstein AL, Yang Y, Garg NK. Angew. Chem. Int. Ed. 2011;50:2171–2173. doi: 10.1002/anie.201007325.. Ackerman L, Sandmann R, Song W. Org. Lett. 2011;13:1784–1786. doi: 10.1021/ol200267b.. Zhang N, Hoffman DJ, Gutsche N, Gupta J, Percec V. J. Org. Chem. 2012;77:5956–5964. doi: 10.1021/jo300547v.. Chen G-J, Han F-S. Eur. J. Org. Chem. 2012:3575–3579..
- 11.For selected examples involving aryl sulfonates, see: Bhayana B, Fors BP, Buchwald SL. Org. Lett. 2009;11:3954–3957. doi: 10.1021/ol9015892.. Limmert ME, Roy AH, Hartwig JF. J. Org. Chem. 2005;70:9364–9370. doi: 10.1021/jo051394l.. Naber JR, Fors BP, Wu X, Gunn JT, Buchwald SL. Heterocycles. 2010;80:1215–1226. doi: 10.3987/COM-09-S(S)105.. Chow WK, So CM, Lau CP, Kwong FY. J. Org. Chem. 2010;75:5109–5112. doi: 10.1021/jo100846t..
- 12.For recent reviews, see: Rosen BM, Quasdorf KW, Wilson DA, Zhang N, Resmerita A-M, Garg NK, Percec V. Chem. Rev. 2011;111:1346–1416. doi: 10.1021/cr100259t.. Li B-J, Yu D-G, Sun C-L, Shi Z-J. Chem. Eur. J. 2011;17:1728–1759. doi: 10.1002/chem.201002273.. Mesganaw T, Garg NK. Org. Process Res. Dev. 2013;17:29–39.. Yamaguchi J, Muto K, Itami K. Eur. J. Org. Chem. 2013:19–30..
- 13.Solvents were selected from the ACS Green Chemistry Institute Roundtable Solvent Selection Guide. See Supporting Information for details.
- 14.t-Amyl alcohol and 2-Me-THF were selected in consultation with the ACS Green Chemistry Institute. t-Amyl alcohol is attractive due to its safety profile, low freezing point (in comparison to t-BuOH), and ability to solubilize polar compounds. 2-Me-THF is advantageous because it is obtained from renewable feedstocks and possesses many process advantages over THF. For a discussion of 2-Me-THF, see: Aycock DF. Org. Process Res. Dev. 2007;11:156–159..
- 15.Under standard conditions using 5% Ni, 50–60% yields of 3 were obtained, with the remaining mass being unreacted carbamate substrate.
- 16.The specific combination of heterocyclic framework and leaving group were chosen at random or based on substrate availability to span a broad range of coupling partners for this Letter. As such, the absence of a specific combination should not imply that such a combination would not lead to a successful Ni-catalyzed cross coupling.
- 17.For examples of Pd-catalyzed cross-coupling to access bisheterocycles, see: Zhao D, You J, Hu C. Chem. Eur. J. 2011;17:5466–5492. doi: 10.1002/chem.201003039.. Molander GA, Shin I. Org. Lett. 2013;15:2534–2537. doi: 10.1021/ol401021x..
- 18.The corresponding coupling between 2-chloropyridine and 2-methoxy-3-pyridinylboronic acid gave the desired bis(heteroaryl) in 60% yield (t-amyl alcohol) and 71% yield (2-Me-THF).
- 19.Microwave conditions were also tested for comparison. Cross-coupling of bromopyrimidine 30 with 3-furylboronic acid (31) using microwave conditions gave compound 20 in quantitative yield (t-amyl alcohol) and 100% yield (2-Me-THF). See the Supporting Information for details. For Suzuki–Miyaura couplings of aryl carbamates and sulfamates under microwave conditions, see: ref. 10b.
- 20.Interestingly, the corresponding cross-coupling with thiophene-3-boronic acid resulted in no product formation.
- 21.The choice of solvents for these transformations was arbitrary to showcase that either t-amyl alcohol or 2-Me-THF can be employed on preparative scale. As shown in Figure 4, the couplings to prepare 22 and 20 readily proceed in either solvent.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.