Abstract
Long-term protection against Toxoplasma gondii is dependent on robust CD8+ T cell immunity. In the absence of this response, the host is unable to maintain chronicity, which results in recrudescence of infection and possible death. Factors needed for the persistence of protective CD8+ T cells against the parasite need to be evaluated. Previous studies from our laboratory have reported that synergism between γ chain cytokines like IL-7 and IL-15 is critical for the generation of CD8+ T cell response needed for protection during acute infection. In this study we report that the situation is different during the recall response where CD8+ T cell response is almost entirely dependent on IL-15, with IL-7 at best playing a minor role. In the absence of IL-15, CD8+ T cells fail to respond optimally to parasitic re-challenge and hosts are unable to control their replication, which leads to their death. Thus T. gondii infection may represent a unique situation where CD8+ T cell response during secondary challenge is primarily dependent on IL-15 with other γ chain cytokines having nominal effect. These findings provide important information regarding factors involved in the generation of protective immunity against T.gondii with strong implications in developing immunotherapeutic agents against the pathogen.
Keywords: Toxoplasma gondii, CD8+ T cells, IL-15, IL-7
1. Introduction
Toxoplasma gondii is an obligate intracellular parasite, which poses a serious risk to immunocompromised individuals including those afflicted with AIDS [1, 2]. The parasitic infection elicits robust induction of strong innate and adaptive immune response [3, 4]. Although innate immunity plays an important role during initial infection, long-term protection is dependent on the induction of robust adaptive immunity, with CD8+ T cells having a dominant role [5, 6]. Studies from various laboratories, including ours have established that IFNγ producing CD8+ T cells are critical for long-term protection and keeping the chronic infection under control [7-9]. Moreover, CD8+ T cells from T. gondii infected hosts have the ability to exhibit ex vivo cytotoxic activity against parasite-infected targets [7, 10, 11] and lytic ability of these cells also contributes in preventing the reactivation of chronic infection [12, 13]. Depletion of either IFNγ or CD8+ T cells abrogates protective immunity against T. gondii infection leading to morbidity or mortality of infected host [14, 15]. Based on these facts, it is important to determine the factor(s), which are responsible for the elicitation and maintenance of robust long-term CD8+ T cell immunity against the pathogen. Previous studies from our laboratory have demonstrated that the treatment of infected host exogenously with γ chain cytokines like IL-7 or IL-15 augments CD8+ T cell immunity against Toxoplasma and animals are able to withstand the infection [16, 17]. In later studies, the role of these cytokines, especially IL-15, in the development of CD8+ T cell immunity during natural infection was determined [18]. In a very interesting study, we recently reported that while lack of IL-7 or IL-15 alone had minimal effect on the development of CD8+ T cell immunity during the acute phase of infection, the absence of both cytokines nearly ablated this response against the pathogen [19]. While the aforementioned study demonstrates a synergistic role of the two cytokines in the induction of CD8+ T cell response during primary infection, the data presented in this manuscript reports that the situation is different during recall response. Depletion of IL-7 had minimal effect on the CD8+ T cell response in wild-type (WT) or IL-15 deficient animals (IL15KO) during rechallenge and ultimate survival of these (both IL15KO and WT) mice irrespective of IL-7 depletion was dependent on IL-15.
2. Materials and Methods
2.1 Mice, infections and antibody treatment
Animal studies were carried out in agreement with Institutional Animal Care and Use Committee approved guidelines at George Washington University Medical Center. 6 to 8 week old female IL-15−/− mice (Taconic Farms) and C57BL/6 mice (NCI) were infected per-orally with 10 cysts of ME49 strain of T. gondii. After 28 days post primary infection, secondary challenge with 30 cysts of same strain was performed via per-oral route. CD8+ T cell responses were evaluated at day 14 post secondary challenge. Anti IL-7 antibody (M25) was a kind gift from Amgen. The antibody was injected into wild-type or IL-15−/− mice intraperitoneally (i.p.) at 0.5 mg per mouse at 3-day intervals. The treatment was initiated one day prior secondary challenge infection and continued till termination of experiment. Control mice were injected with equal volume of saline.
2.2 Toxoplasma Lysate Antigen (TLA) preparation
TLA was extracted from RH strain of parasite and preparation was made as previously described [17].
2.3 Lymphocyte isolation, cell surface staining and intracellular staining
Lymphocytes were isolated from spleen or liver as reported earlier [20]. Cells were stained with Lived/Dead Aqua stain (Invitrogen), followed by surface staining and intracellular staining (Cytofix/Cytoperm Kit, BD Biosciences) where needed [21] [22]. Antibodies for detection of IL-7Rα (CD127; A7R34), CD8β (H35-17.2), CD44 (IM7), CD62L (MEL-14) KLRG1 (2F1) and IFNγ (XMG1.2) were purchased from eBIoscience. Antibody for detection of GranzymeB (GB11) was purchased from Invitrogen. For IFNγ and GranzymeB detection, restimulation was carried out for 16h with 30μg/ml of Toxoplasma lysate antigen (TLA) in supplemented DMEM at 37° C in 5% CO2. 0.65 μl/ml of monensin (BD Biosciences) and 0.65 μl/ml of brefeldin A (BD Biosciences) were added during the final 9h of stimulation..Cell fluorescence was measured with a Cytek upgraded 8 color FACSCalibur (BD Biosciences) cytometer or FACSAria and data was analyzed using FlowJo (TreeStar, Inc.) software.
2.4 Quantification of Parasite Load
Quantification of parasite load in spleen, lung, gut and liver was performed at day 14 p.i. as described earlier [18] .DNA was isolated from tissues with the Qiamp tissue kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. Parasite DNA was amplified with primers specific for a 35-fold repetitive sequence of the B1 gene (5′-TCTTTAAAGCGTTCGTGGTC-3′ and 5′-GGAACTGCATCCGTTCATGAG-3′), which is found in all known parasite strains [23]. A 134-bp competitive internal standard containing the same primer template sequences as the 194-bp B1 PCR fragment was synthesized, and amplified along with parasite DNA. Amplification was performed using a 50 μl reaction mixture containing 1.24 U Amplitaq DNA polymerase, 1X PCR buffer (Promega, Madison, WI), 0.2 mM each of dGTP, dATP, dTTP, and dCTP, and 0.4 mM of each B1 primer. For each reaction, a known amount of DNA from the tissues was amplified with varying amounts of the internal standard. The levels of parasite load were estimated by comparison to the internal controls.
2.5 Cytotoxic T lymphocyte (CTL) assay
CTL assay was performed as previously described [16]. Briefly CD8+ T cells stimulated TLA were incubated with infected 51Cr labeled macrophages at various effector-target ratio in 96 well U-bottomed plates. After 4 hr incubation the supernatants were measured for radioactive release and percentage of cytotoxic response calculated.
2.6 Statistical analysis
Differences in percentage, absolute number, MFI and parasite burden were evaluated using Student′s t test with P<0.05 taken as statistically significant. Unless otherwise mentioned, all correlations mentioned in the text is significantly different. Error bars in graphs represent standard deviation of values of individual mice in the group from one experiment. Horizontal bars over bar graphs designate statistically significant population. Comparison of survival curves was performed using Log-rank (Mantel-Cox) Test. For statistical analysis of CTL data, 2-way ANOVA was used. All computations were performed using GraphPad Prism Software.
3. Results
3.1 Anti IL-7 treatment minimally affects CD8+ T cell response in lymphoid tissue during T. gondii rechallenge
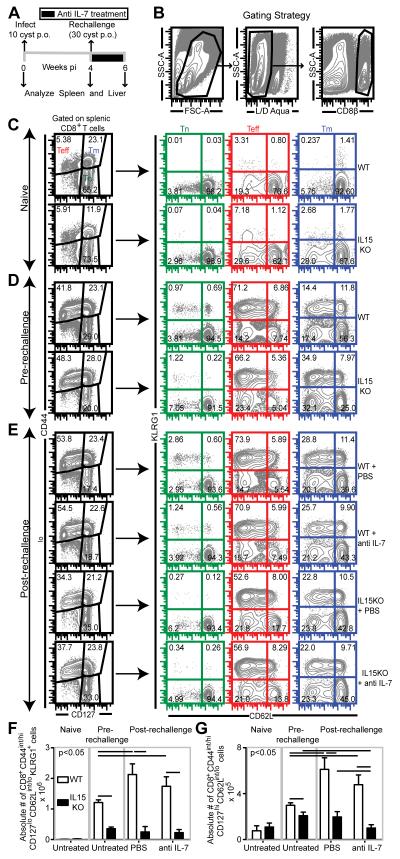
As stated above, since a previous study from our laboratory demonstrated a synergetic role of IL-7 and IL-15 in the induction of CD8+ T cell immunity in primary infection [19], we next wanted to determine if these two cytokines played a similar role during recall response. For this purpose, chronically infected WT and IL15KO mice were rechallenged at 4 weeks post primary infection via peroral (p.o.) route and treated with anti IL-7 antibody or PBS as shown in Figure 1A. To address the role of IL-15 and IL-7 on effector/memory CD8+ T cell development during recall response, splenic and hepatic CD8+ T cells (Figure 1B) were assessed for naïve (Tn)/effector (Teff)/memory (Tm) markers (CD44, CD62L, CD127 and KLRG1) via polychromatic flow cytometry. As shown in the figure 1C and 1D, a robust effector (CD8+CD44int/hiCD62LloKLRG1+) CD8 T cell subset develops after primary infection in both WT and IL15KO mice. However, significant difference in terms of frequency was not noted. Upon rechallenge, independent of anti IL-7 treatment, effector response frequency was augmented in WT animals (Figure 1D and E). In contrast, rechallenged IL15KO mice, irrespective of anti IL-7 treatment, exhibited severely diminished effector CD8 response (Figure 1D and E). This potentially suggests that during recall response against this pathogen IL-15 but not IL-7 is important specifically for effector CD8 development. Similarly, in terms of absolute number, absence of IL-15 but not IL-7 (in WT or IL15KO mice) reduced effector response in the pre-rechallenge phase and nearly ablated effector T cell expansion in rechallenged IL15KO mice receiving either PBS or anti IL-7 antibody (Figure 1F). Since memory CD8+ T cells are an important component of protective immunity against intracellular pathogens and they can give rise to effectors, we next investigated the role of these cytokines on CD8 memory development in spleen during recall response [24]. Although no major difference in memory CD8+ T cell (CD44int/hiCD127hi) percentage was noted between pre-rechallenge and post-rechallenge phase in WT infected animals, the latter exhibited elevated effector memory (CD44int/hiCD127hiCD62Llo) subset frequency, irrespective of anti IL-7 treatment (Figure 1C, D and E). While IL-15 is known to be important for memory CD8 T cell maintenance and proliferation [25], confoundingly week 4 infected IL-15KO mice exhibited higher frequency of effector memory CD8 T cells than the WT counterpart (Figure 1C and D). It is possible that lower inflammation (i.e. lower IL-12) [19, 26] in IL15KO mice promotes increased effector memory differentiation [27]. Alternatively, absence of IL-15 may inhibit migration of effector memory CD8 T cells from lymphoid to effector sites, thereby sequestering them in spleen[28]. In contrast to primary infection, during recall response IL15KO animals, irrespective of IL-7 depletion, exhibited decreased effector memory T cell frequency (Figure 1D and E) vis-à-vis week 4 infected IL15KO mice (pre-rechallenge). Similarly, in terms of absolute number, absence of IL-15 but not IL-7 alone, prevented significant expansion of effector memory subset in rechallenged animals (Figure 1G). Although anti IL-7 treatment of IL15KO mice further reduced the number of effector memory CD8+ T cells, this effect was modest at best (Figure 1G). Combined our data suggests that IL-15 is the dominant cytokine during CD8 recall response in spleen.
Fig. 1.
Absence of IL-15 but not IL-7 severely affects splenic CD8+ T cell response during rechallenge. Splenocytes were harvested from WT and IL15KO treated either with PBS or anti IL-7 antibody at day 14 post-rechallenge. A and B depict the rechallenge and gating strategy respectively. C, D and E: CD8 subsets were analyzed in the spleens of naïve (C) and infected mice, both prior to rechallenge (D) and post rechallenge (E). F and G: Absolute number of splenic effector (F) and effector memory (G) CD8+ T cells was computed in the above groups. The data is representative of two experiments with 3-4 mice per group. Horizontal bars over bar graphs designate statistically significant changes.
3.2 Anti IL-7 treatment has a more pronounced effect on CD8+ T cell response in non-lymphoid tissue during T. gondii rechallenge
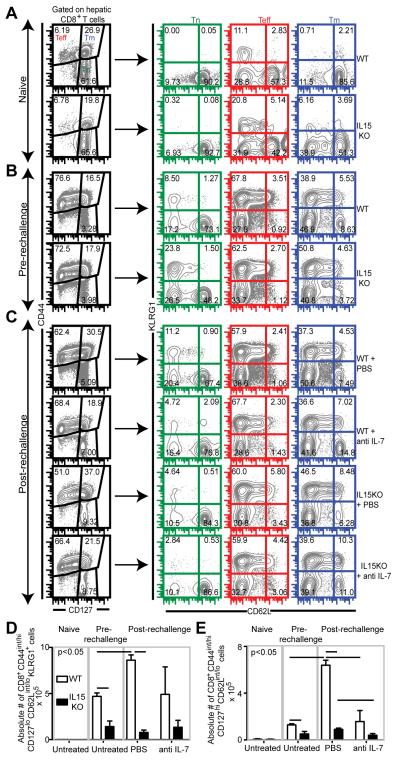
We next investigated if IL-7 and IL-15 had a similar effect on CD8+ T cells response in a non-lymphoid tissue. Since a previous study from our group has shown that in absence of an extended rechallenge period, robust CD8 expansion occurs only in spleen and liver but not in brain [20] (data not shown), we examined CD8+ T cell response in liver (a non-lymphoid tissue). As expected, effector CD8 percentage was higher in liver than in spleen in all groups of infected mice (Figure 2A, B and C). Akin to splenic CD8 effectors, no difference in hepatic effector frequency was noted between WT and IL15KO mice, immediately prior to rechallenge (Figure 2A and B). In contrast to spleen, all 4 groups of rechallenged mice exhibited modest (WT, anti IL-7 treated WT and anti IL7 treated IL15KO) to severe reduction (IL15KO) in hepatic effector CD8 frequency versus the pre-rechallenge levels (Figure 2B and C). Surprisingly, anti IL-7 treated IL15KO mice had higher effector CD8+ T cell frequency than mutant animals treated with PBS (Figure 2C). Although the effector subset exhibited lower CD127 expression vis-a-vis memory subset, the effector CD8+ T cells in anti IL-7 treated IL15KO mice had higher CD127 mean fluorescence intensity than the corresponding subset in PBS treated (IL15KO) mice. Based on this, it is tempting to speculate whether absence of both these cytokines promotes conversion of at least a part of the memory subset to effector subset. Investigation of hepatic effector memory percentage revealed that this subset increased in frequency in both WT and IL15KO mice upon rechallenge (Figure 2B and C). In contrast to spleen, anti IL-7 treated WT and (anti IL-7 treated) IL15KO mice exhibited minimal expansion of the effector memory subset in liver (Figure 2B and C). Taken together, our data highlights a differential CD8 dependence on IL-7 signaling based on anatomical site. Hence it is hardly surprising that anti IL-7 treatment of WT mice severely downregulated absolute number of hepatic effector memory CD8+ T cells but not effector CD8+ T cells (Figure 2D and E). Similar to splenic data, absence of IL-15 or both IL-7 and IL15 nearly ablated effector and effector memory CD8 expansion in terms of absolute numbers in rechallenged animals (Figure 2D and E). Cumulatively our data suggests that in a recall model both IL-7 and IL-15 play a role in hepatic CD8 response development with IL-7 independent of IL-15 presence having a major impact on effector memory CD8 development. However, in contrast to the scenario during acute toxoplasmosis, IL-7 and IL-15 do not synergize during CD8 recall response.
Fig. 2.
IL-7 and IL-15 do not synergize in mediating hepatic effector and effector memory CD8+ T cell response during rechallenge infection. WT, IL15KO mice treated either with PBS or anti IL-7 antibody were sacrificed at day 14 post rechallenge. A, B and C depicts CD8 subsets in the livers of naïve (A) and infected mice both prior to rechallenge (B) and post-rechallenge (C). Absolute number of hepatic effector (D) and effector memory (E) CD8+ T cells present in the above groups is shown. The data depicted here represents at least 2 experiments with 3-4 mice per group. Horizontal bars over bar graphs designate statistically significant changes.
3.3 Neutralization of IL-7 has minimal effect on CD8+ T cell function during T. gondii rechallenge
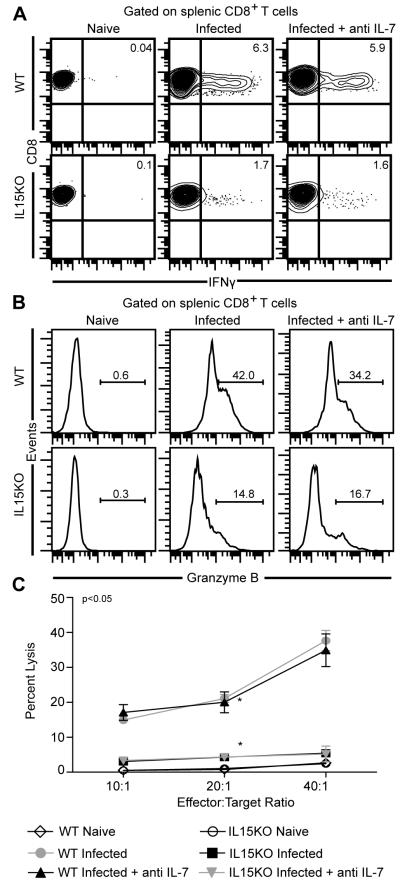
As mentioned above CD8+ T cells generated in response to T. gondii infection are important producers of IFNγ [8] and have been reported to exhibit cytotoxic activity against infected targets [10]. A recent study has reported that cytotoxic function of these cells is critical for controlling chronic toxoplasmosis [13]. Hence, we evaluated antigen-specific IFNγ and cytotoxic response of CD8+ T cell population during secondary T. gondii infection. For this purpose, splenocytes isolated from infected mice were collected at 2 weeks post secondary infection and stimulated in vitro with TLA and evaluated for IFNγ production and Granzyme B (a marker of cytotoxic activity) expression as described in Materials and Methods. As shown in figure 3A, absence of IL-15 severely downregulated IFNγ production by CD8+ T cells and depletion of IL-7 in WT or IL15KO animals did not exacerbate IFNγ production. Overall, similar results were noted in terms of Granzyme B expression (Figure 3B). Although anti IL-7 treatment resulted in a modest decline of Granzyme B expression in WT animals, this difference was not statistically significant (Figure 3B). The data was further confirmed by in vitro cytotoxic assay where only absence of IL-15, irrespective of anti IL-7 treatment resulted in downregulated target cell lysis. Taken together our data suggests that IL-15 alone plays a critical role in mediating CD8 functionality during recall response.
Fig. 3.
Absence of IL-15 but not IL-7 dramatically affects CD8+ T cell function during recall response. (A) For IFNγ production, splenocytes were stimulated in vitro with TLA and after overnight incubation stained for CD8 and IFNγ as described in Materials and Methods. Data are presented as contour plots. (B) depicts GranzymeB production by restimulated splenic CD8 T cells. (C) Cytotoxicity was assessed evaluated using purified splenic CD8+ T cells cultured in presence of TLA and irradiated feeder cells. Radioisotope release was assayed following incubation with 51Cr labeled targets at various effector:target ratio. The data depicted here represents 2 experiments with 3-4 mice per group.
3.4 IL-7 depletion does not affect survival of wild type or IL-15 deficient mice against secondary T. gondii infection
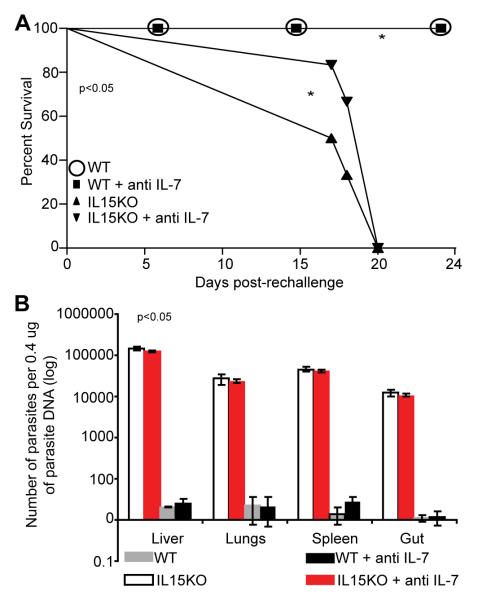
The above studies demonstrated that depletion of IL-7 in both WT and IL15KO mice did not have a dramatic effect on CD8+ T cell immunity against re-challenge infection with T. gondii. Also we observed that unlike primary infection, CD8+T cell response in untreated IL15KO mice was reduced as compared to WT animals. Next we determined how this translates into ultimate survival of these animals during secondary T. gondii challenge. For this purpose we monitored the survival of both IL15KO and WT mice receiving secondary Toxoplasma challenge. A group of animals from each strain was administered anti IL-7 antibody. As shown in figure 4A, neutralization of IL-7 in WT mice did not affect their survival against secondary infection and all the animals were able to live till the termination of the experiment. Conversely, IL-15 deficient animals were unable to survive the challenge and treatment with anti IL-7 antibody made no difference in their mortality. All the IL15KO mice irrespective of anti IL-7 treatment failed to survive beyond 20 post secondary challenge. This is in contrast to our previously published data showing that during primary infection (10 ME49 cyst, p.o. route) there is no significant difference in long-term survival of WT and IL15KO mice [19] .To confirm that mortality in IL15KO mice in response to secondary infection is due to uncontrolled replication of pathogen, some of the infected animals were sacrificed at day 14 post re-challenge and tissues (lung, liver, spleen and gut ) assayed for parasite burden by PCR. As shown in figure 4B, WT animals were able to control parasite infection and very low number of parasites were observed in all the tissues tested. Similar to the mortality data, no increase in the parasite burden was observed when IL-7 was depleted during Toxoplasma re-challenge. Conversely IL-15 deficient mice treated with saline had significantly higher parasite load in all tissues which corelated well with their mortality. Depletion of IL-7 in these mice did not make any difference in terms of parasite burden in the tissues (Fig. 4B).
Fig. 4.
Depletion of IL-7 in IL-15 deficient animals does not accelerate mortality. Rechallenged WT and IL15KO mice were treated with anti IL-7 antibody till termination of experiment. (A) Survival was monitored on daily basis and statistical significance was calculated by Mantel-Cox Test. (B) Parasite burden was evaluated in liver, lung, spleen and gut by quantitative PCR at day 14 post rechallenge. Absence of IL-15 but not IL-7 resulted in statistically significant elevation of parasite burden. The experiment is representative of one of two experiments with 4-6 mice per group.
4. Discussion
The data presented in the current study demonstrates that unlike primary infection, where IL-7 and IL-15 synergize to induce CD8+ T cell response against p.o. T. gondii infection [19] the situation is different during secondary challenge. During secondary infection mice lacking IL-15 gene have a severely down-regulated CD8+ T cell immunity and presence of IL-7 apparently plays at best a minor role especially in the lymphoid tissue..
Overall our data strongly suggests that IL-15 plays a critical role in mediating effector CD8+ T cell development during recall response in both spleen and liver. Since effector CD8+ T cells do not express high levels of CD127 (IL-7Rα), the receptor of IL-7, it is hardly surprising that IL-7 depletion in WT or IL15KO mice had minimal effect on effector development. Considering that IL-15 has been shown to be important for effector CD8 T cell survival [27],it is paradoxical that chronically infected IL15KO animals, immediately prior to rechallenge, did not show a sharp reduction in effector CD8 frequency. It needs to be investigated whether altered de novo effector generation as a result of differential recruitment of naïve T cells into the antigen-specific pool [29] “masks” the potential apoptosis of CD8 effectors in IL15KO mice during primary infection. Similar to CD8 effector population, absence of IL-15 downregulated the expansion of splenic effector memory CD8+ T cell population in the rechallenged animals, in terms of both frequency and absolute numbers. Although effector memory CD8+ T cells expressed high levels of IL-7Rα, anti IL-7 treatment of WT or IL15KO mice had minimal effect on their frequency or absolute number in spleen. In contrast in liver, as evident from frequency of effector memory T cells, absence of IL-7 but not IL-15 preferentially downregulated effector memory development. However, in terms of absolute number both IL-7 and IL-15 were important for hepatic effector memory development during recall response. Taken together this suggests that while IL-7 specifically targets effector memory population in liver, IL-15 targets hepatic CD8+ T cell response development in a subset independent manner. What causes this altered dependence on IL-7 based on anatomical location? A previous study from our laboratory has demonstrated that that during chronic Toxoplasma infection hepatic CD8+ T cells exhibit lower PD-1 (an inhibitory molecule responsible for exhaustion), [20] than splenic CD8+ T cells. In the viral models of infection, it has been demonstrated that exhausted CD8+ T cells lose their responsiveness to IL-7 and IL-15 with progressive exhaustion [30] . Thus it is possible that due to differential PD-1 expression, while splenic cells have lost IL-7 responsiveness, hepatic CD8 T cells are dependent on both IL-15 and IL-7 during secondary challenge. In future studies it will be also important to determine the contribution of other factors such as differential cytokine, chemokine, chemokine receptor and antigen presenting cell milieu toward this altered IL-7 dependence based on anatomical site.
Overall the outcome of secondary Toxoplasma challenge is almost entirely dependent on IL-15 which is further established by the fact that CD8+ T cells from IL15KO mice showed poor functional response as measured by their ability to produce IFNγ and lyse parasite infected targets. Depletion of IL-7 in WT or IL15KO mice did not farther compromise CD8+ T cell functionality. All this leads to inability of IL-15 KO mice to control parasite replication, as a result of which they succumb to infection. Neutralization of IL-7 does not affect the ability of WT or IL15KO mice to control parasitic replication and there was no difference in mortality between treated versus untreated animals. Our data demonstrates that synergism between IL-7 and IL-15 which is essential for the development of CD8+ T cell immunity and survival during primary Toxoplasma infection plays no role during secondary challenge. This is indeed surprising, considering the important role played by IL-7 in mediating homeostasis of naïve and memory CD8+ T cells in other model systems [31, 32].
Role of IL-7 and IL-15 in the development of CD8+ T cell immunity, which plays a critical role in protection against T. gondii infection[33] has been studied our laboratory. An earlier report from our group has pointed towards the importance of exogenous IL-7 treatment in the generation of primary CTL vivo, however the extent of involvement of endogenous levels of this cytokine was not described for recall response [16]. In contrast, the role of related γc cytokine IL-15, in the generation and maintenance of long-term CD8+ T cell immunity during natural T. gondii infection is well established by several studies conducted in our laboratory [18, 34, 35]. In a recent study we demonstrated that while lack of IL-15 or IL-7 alone has minimal or no impact on CD8+ T cell development during primary T. gondii infection, the absence of both cytokines severely impairs this response [19]. In these studies it was demonstrated that depletion of IL-7 alone did not impede the induction of primary CD8+ T cell response in the WT animals. Similarly, lack of IL-15 did not cause significant deficits in CD8+ T cell maturation, IFNγ production and cytolytic function of infected animals. Based on these observations it appeared that IL-7 and IL-15 together, are the key mediators of maturation and effector function development of CD8+ T cells during acute T. gondii infection. However, during secondary challenge IL-15 appears to bear the major brunt in the generation of CD8+ T cell immunity and presence or absence of IL-7 was irrelevant for this response and ultimate survival of the host.
Our current findings are supported by earlier reports from our laboratory that neutralization of IL-15 by sIL-15Rα treatment in the immune animals fails to protect them during subsequent Toxoplasma challenge. Administration of sIL-15Rα to WT mice immunized with Toxoplasma abrogated CD8+ T cell mediated protective immunity against the re-challenge suggesting very important role for this cytokine [18] .The present findings demonstrate that IL-15 deficient animals lack the ability to develop protective CD8+ T cell immunity during secondary challenge and they go a step further in demonstrating that unlike primary infection, synergetic role of IL-7 and IL-15 is absent during recall infection. The data obtained from these studies strongly suggest that IL-15 is a pivotal cytokine which protects the animals against Toxoplasma rechallenge Moreover, the data shows there is no redundant mechanism responsible for the generation of robust CD8+ T cell immunity in rechallenged IL-15 deficient animals. This is surprising, since apart from IL-7 and IL-15 other γc family members like IL-2 [36, 37], IL-4 [38, 39], IL-12 [40, 41] and IL-21 [42] have been implicated in CD8 memory generation in other infectious disease models. Unlike other pathogens, T. gondii infection does not induce a potent IL-2 response even during the acute phase of infection [11, 43-45], thus discounting its role in the induction of CD8+ T cell response against T.gondii. IL-12 is another important cytokine known to regulate the development of memory CD8+ T cell response [40, 41]. In a recent report it has been demonstrated that high IL-12 levels induced during Listeria monocytogenes infection down-regulates CD8+ T cell memory differentiation [46]. However, studies from our laboratory have shown that administration of anti IL-7 to IL15KO mice did not alter their IL-12 production [19]. Nevertheless, not withstanding these observations, role of other γc family members like IL-4 and IL-21 both during primary and secondary T. gondii infection needs to be considered. Cumulatively, the current study shows that IL-15 but not IL-7 is the pivotal cytokine which protects the animals against Toxoplasma rechallenge.
The findings presented in the current manuscript go a long way in understanding development of CD8+ T cell immunity against T. gondii infection. These studies further emphasize the non-redundant role of IL-15 and apparent lack of synergism with other cytokines like IL-7 during secondary infection. An intriguing question that needs to be addressed in future studies: Why are CD8 T cells during acute phase[19] and recall phase differentially dependent on IL-7 and IL-15. A seminal study by Wirth et al [47] showing how repeated antigenic challenge diversifies the CD8 T cell transcriptome while preserving a core signature, bears the implication that transcriptome change during recall response can potentially result in modified cytokine dependence. As such, it will be important to address whether a similar mechanism is involved in our model. A very recent study from our laboratory has reported that chronic Toxoplasma infection over a period results in CD8+ T cell dysfunction which most likely disables the memory response [20]. In future studies it will be very interesting and important to determine if this happens due to decreased IL-15 responsiveness and if exogenous treatment with the cytokine can correct the defect. The findings made in the current manuscript provides important information regarding the role of these cytokines in the development of CD8+ T cell immunity at different stages of Toxoplasma infection and lay a basis for the use of IL-7 and IL-15 as adjuvant for therapeutic vaccination.
Acknowledgements
We thank Amgen for generously providing us the anti IL-7 (M25) antibody and Teresa Hawley of George Washington University Flow Cytometry Core Facility for technical assistance. This work was supported by NIH grant AI33325 awarded to IAK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS, Clin. Infect. Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- [2].Vidal JE, Hernandez AV, de Oliveira AC, Dauar RF, Barbosa SP, Jr., Focaccia R. Cerebral toxoplasmosis in HIV-positive patients in Brazil: clinical features and predictors of treatment response in the HAART era. AIDS Patient Care STDS. 2005;19:626–634. doi: 10.1089/apc.2005.19.626. [DOI] [PubMed] [Google Scholar]
- [3].Miller CM, Boulter NR, Ikin RJ, Smith NC. The immunobiology of the innate response to Toxoplasma gondii, Int. J. Parasitol. 2009;39:23–39. doi: 10.1016/j.ijpara.2008.08.002. [DOI] [PubMed] [Google Scholar]
- [4].Yap GS, Sher A. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiology. 1999;201:240–247. doi: 10.1016/S0171-2985(99)80064-3. [DOI] [PubMed] [Google Scholar]
- [5].Khan IA, Ely KH, Kasper LH. A purified parasite antigen (p30) mediates CD8+ T cell immunity against fatal Toxoplasma gondii infection in mice, J. Immunol. 1991;147:3501–3506. [PubMed] [Google Scholar]
- [6].Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii, J. Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- [7].Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine, J. Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- [8].Khan IA, Ely KH, Kasper LH. Antigen-specific CD8+ T cell clone protects against acute Toxoplasma gondii infection in mice. J. Immunol. 1994;152:1856–1860. [PubMed] [Google Scholar]
- [9].Tait ED, Jordan KA, Dupont CD, Harris TH, Gregg B, Wilson EH, Pepper M, Dzierszinski F, Roos DS, Hunter CA. Virulence of Toxoplasma gondii is associated with distinct dendritic cell responses and reduced numbers of activated CD8+ T cells. J. Immunol. 2010;185:1502–1512. doi: 10.4049/jimmunol.0903450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hakim FT, Gazzinelli RT, Denkers E, Hieny S, Shearer GM, Sher A. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J. Immunol. 1991;147:2310–2316. [PubMed] [Google Scholar]
- [11].Kasper LH, Khan IA, Ely KH, Buelow R, Boothroyd JC. Antigen-specific (p30) mouse CD8+ T cells are cytotoxic against Toxoplasma gondii-infected peritoneal macrophages. J Immunol. 1992;148:1493–1498. [PubMed] [Google Scholar]
- [12].Denkers EY, Yap G, Scharton-Kersten T, Charest H, Butcher BA, Caspar P, Heiny S, Sher A. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J. Immunol. 1997;159:1903–1908. [PubMed] [Google Scholar]
- [13].Suzuki Y, Wang X, Jortner BS, Payne L, Ni Y, Michie SA, Xu B, Kudo T, Perkins S. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am. J. Pathol. 2010;176:1607–1613. doi: 10.2353/ajpath.2010.090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- [15].Guiton R, Zagani R, Dimier-Poisson I. Major role for CD8 T cells in the protection against Toxoplasma gondii following dendritic cell vaccination. Parasite Immunol. 2009;31:631–640. doi: 10.1111/j.1365-3024.2009.01146.x. [DOI] [PubMed] [Google Scholar]
- [16].Kasper LH, Matsuura T, Khan IA. IL-7 stimulates protective immunity in mice against the intracellular pathogen, Toxoplasma gondii. J. Immunol. 1995;155:4798–4804. [PubMed] [Google Scholar]
- [17].Khan IA, Kasper LH. IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondii infection in mice. J. Immunol. 1996;157:2103–2108. [PubMed] [Google Scholar]
- [18].Khan IA, Moretto M, Wei XQ, Williams M, Schwartzman JD, Liew FY. Treatment with soluble interleukin-15Ralpha exacerbates intracellular parasitic infection by blocking the development of memory CD8+ T cell response. J. Exp. Med. 2002;195:1463–1470. doi: 10.1084/jem.20011915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bhadra R, Guan H, Khan IA. Absence of both IL-7 and IL-15 severely impairs the development of CD8 T cell response against Toxoplasma gondii. PLoS One 5. 2011:e10842. doi: 10.1371/journal.pone.0010842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bhadra R, Gigley JP, Weiss LM, Khan IA. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc. Natl. Acad. Sci. U S A. 2011;108:9196–9201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bhadra R, Gigley JP, Khan IA. Cutting edge: CD40-CD40 ligand pathway plays a critical CD8-intrinsic and -extrinsic role during rescue of exhausted CD8 T cells. J. Immunol. 2011;187:4421–4425. doi: 10.4049/jimmunol.1102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bhadra R, Gigley JP, Khan IA. PD-1-Mediated Attrition of Polyfunctional Memory CD8+ T Cells in Chronic Toxoplasma Infection. J. Infect.Dis. 2012;206:125–134. doi: 10.1093/infdis/jis304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Burg JL, Grover CM, Pouletty P, Boothroyd JC. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J. Clin. Microbiol. 1989;27:1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J. Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Weng NP, Liu K, Catalfamo M, Li Y, Henkart PA. IL-15 is a growth factor and an activator of CD8 memory T cells. Ann. N. Y. Acad. Sci. 2002;975:46–56. doi: 10.1111/j.1749-6632.2002.tb05940.x. [DOI] [PubMed] [Google Scholar]
- [26].Ohteki T, Suzue K, Maki C, Ota T, Koyasu S. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat. Immunol. 2001;2:1138–1143. doi: 10.1038/ni729. [DOI] [PubMed] [Google Scholar]
- [27].Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Verbist KC, Cole CJ, Field MB, Klonowski KD. A role for IL-15 in the migration of effector CD8 T cells to the lung airways following influenza infection. J. Immunol. 2011;186:174–182. doi: 10.4049/jimmunol.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vezys V, Masopust D, Kemball CC, Barber DL, O’Mara LA, Larsen CP, Pearson TC, Ahmed R, Lukacher AE. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J. Exp. Med. 2006;203:2263–2269. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wherry EJ. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- [31].Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival Nat. Rev. Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- [32].Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- [33].Bhadra R, Gigley JP, Khan IA. The CD8 T-cell road to immunotherapy of toxoplasmosis. Immunotherapy. 2011;3:789–801. doi: 10.2217/imt.11.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Khan IA, Casciotti L. IL-15 prolongs the duration of CD8+ T cell-mediated immunity in mice infected with a vaccine strain of Toxoplasma gondii. J. Immunol. 1999;163:4503–4509. [PubMed] [Google Scholar]
- [35].Combe CL, Moretto MM, Schwartzman JD, Gigley JP, Bzik DJ, Khan IA. Lack of IL-15 results in the suboptimal priming of CD4+ T cell response against an intracellular parasite. Proc. Natl. Acad. Sci. U S A. 2006;103:6635–6640. doi: 10.1073/pnas.0506180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Heath SL, Sabbaj S, Bansal A, Kilby JM, Goepfert PA. CD8 T-cell proliferative capacity is compromised in primary HIV-1 infection. J. Acquir. Immune Defic. Syndr. 2011;56:213–221. doi: 10.1097/QAI.0b013e3181ff2aba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cox MA, Harrington LE, Zajac AJ. Cytokines and the inception of CD8 T cell responses. Trends Immunol. 2011;32:180–186. doi: 10.1016/j.it.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Carvalho LH, Sano G, Hafalla JC, Morrot A, Curotto de Lafaille MA, Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat .Med. 2002;8:166–170. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- [39].Morrot A, Hafalla JC, Cockburn IA, Carvalho LH, Zavala F. IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites J. Exp. Med. 2005;202:551–560. doi: 10.1084/jem.20042463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Moretto MM, Lawlor EM, Khan IA. Lack of interleukin-12 in p40-deficient mice leads to poor CD8+ T-cell immunity against Encephalitozoon cuniculi infection. Infect. Immun. 2010;78:2505–2511. doi: 10.1128/IAI.00753-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Henry CJ, Grayson JM, Brzoza-Lewis KL, Mitchell LM, Westcott MM, Cook AS, Hiltbold EM. The roles of IL-12 and IL-23 in CD8+ T cell-mediated immunity against Listeria monocytogenes: Insights from a DC vaccination model Cell. Immunol. 2010;264:23–31. doi: 10.1016/j.cellimm.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Novy P, Huang X, Leonard WJ, Yang Y. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J. Immunol. 2010;186:2729–2738. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Haque S, Khan I, Haque A, Kasper L. Impairment of the cellular immune response in acute murine toxoplasmosis: regulation of interleukin 2 production and macrophage-mediated inhibitory effects. Infect. Immun. 1994;62:2908–2916. doi: 10.1128/iai.62.7.2908-2916.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Candolfi E, Hunter CA, Remington JS. Mitogen-and antigen-specific proliferation of T cells in murine toxoplasmosis is inhibited by reactive nitrogen intermediates. Infect Immun. 1994;62:1995–2001. doi: 10.1128/iai.62.5.1995-2001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Khan IA, Matsuura T, Kasper LH. IL-10 mediates immunosuppression following primary infection with Toxoplasma gondii in mice, Parasite. Immunol. 1995;17:185–195. doi: 10.1111/j.1365-3024.1995.tb00888.x. [DOI] [PubMed] [Google Scholar]
- [46].Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12, J. Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- [47].Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2011;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]