Abstract
Protozoan parasites cause severe morbidity and mortality in humans worldwide, especially in developing countries where access to chemotherapeutic agents is limited. Although parasites initially evoke a robust immune response, subsequent immunity fails to clear infection, ultimately leading to the chronic stage. This enigmatic situation was initially addressed in chronic viral models, where T cells lose their function, a phenomenon referred to as ’exhaustion‘. However, recent studies demonstrate that this paradigm can be extended to protozoan diseases as well, albeit with notable differences. These studies have revealed that T cell responses generated against Toxoplasma gondii, Plasmodium sp. and Leishmania sp. can become dysfunctional. This Review discusses T cell exhaustion in parasitic infection, mechanisms of development, and a possible role in disease outcome.
Keywords: protozoan, exhaustion, parasite, Toxoplasma, Leishmania, Plasmodium, T cell
A brief overview of T cell exhaustion in infectious diseases
A hallmark of potent immunity against intracellular pathogens is the development of an optimal T cell response that exhibits low apoptosis, rapid proliferative potential and polyfunctionality [1]. The fact that the quantum of polyfunctional T cell rather than absolute CD8 T cell number is directly correlated with improved viral clearance in HIV+ non-progressors highlights the critical importance of this subset [2]. During acute infections, such T cells clear the pathogen, ultimately leading to the development of robust antigen-independent memory T cells characterized by the following cardinal features -the ability to mount rapid recall response and reactivate polyfunctional effector mechanisms upon antigen re-exposure [3]. In contrast to acute infections, during the chronic stage, antigen-specific T cells become functionally impaired and even get deleted [4]. Persistence of antigen-specific T cells exhibiting inferior effector functions, poor recall response and suboptimal antigen-independent homeostatic proliferation is referred to as exhaustion. In various chronic viral models of infection such as LCMV (Lymphocytic choriomeningitis virus), HIV (Human immunodeficiency virus), SIV (Simian immunodeficiency virus), HBV (Hepatitis B virus) and HCV (Hepatitis C virus), it has been demonstrated that CD8 T cells lose their polyfunctionality in a hierarchical manner (Figure 1). It begins with the inability to produce IL-2, exhibit cytotoxic activity, and proliferate, followed by impaired TNFα and IFNγ production. Concurrent with this loss of function, T cells exhibit increased apoptotic potential, leading to their deletion (Figure 1) [3]. Moreover, in the LCMV model, it has been reported that during exhaustion, epitopes presented at higher levels in vivo result in physical deletion, while those presented at lower levels induce functional exhaustion [5]. It will be interesting to study in parasitic models if epitope dependent hierarchal loss of T cell function follows a pattern observed during viral infections. However, these studies are impeded by the paucity of information regarding immunodominant and subdominant MHC (Major Histocompatibility Complex) restricted epitopes in parasitic models.
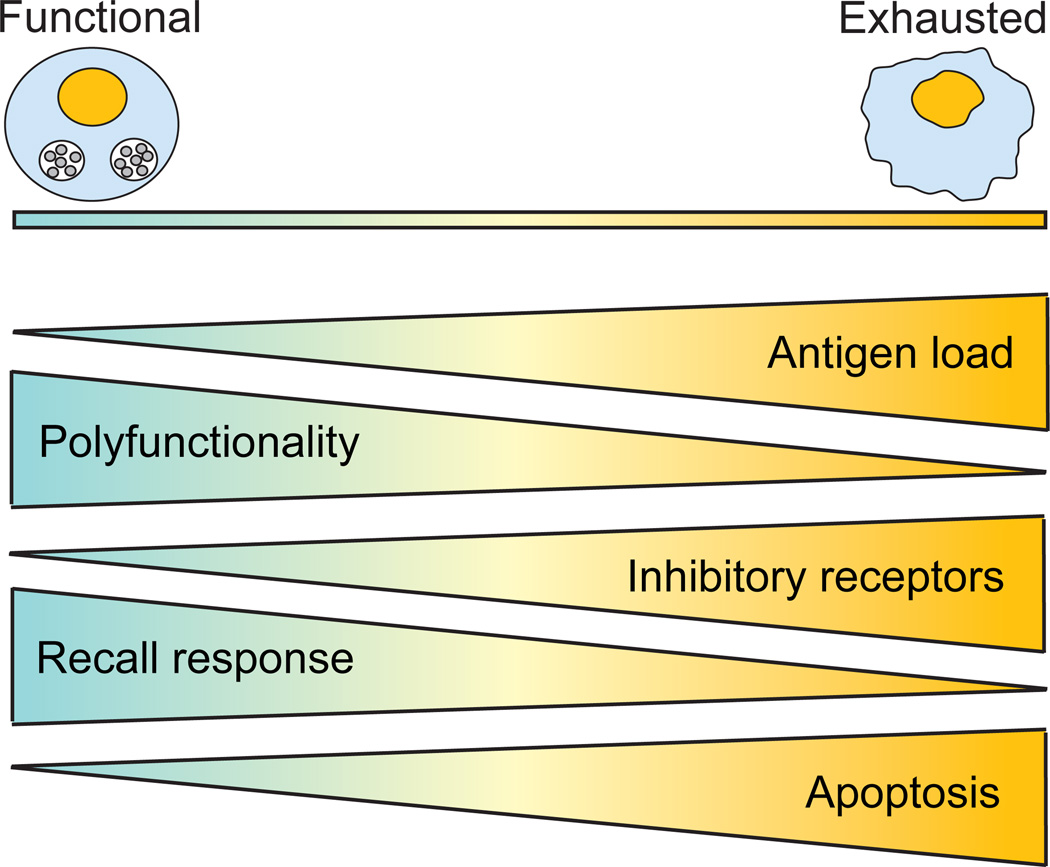
Figure 1. T cell exhaustion.
During acute infections, the host develops a successful T cell immune response against the pathogen, characterized by rapid proliferation and robust polyfunctionality (cytotoxicity and production of IFNγ, TNFα and IL-2). However, during chronic infection, T cells become progressively exhausted and gradually lose the ability to mount an effective recall response to the infection as well as their polyfunctionality ability. At first, reduced IL-2 production and proliferative response are detected. Then, as exhaustion progress, cells lose the ability to produce TNFα. Finally, cells exhibiting the most severe phenotype are unable to secrete IFNγ in response to the infection. At the same time, gradual upregulation of inhibitory receptors (PD-1, LAG3, CD160, CTLA4, 2B4) plays a central role in T cell exhaustion and ultimately, concomitant expression of multiple inhibitory receptors leads to severe T cell exhaustion. Concurrently, exhausted T cells exhibit increased apoptosis potential, leading to their complete deletion. T cell exhaustion is highly dependent on antigen load, and as antigen burden increases, T cells become more exhausted.
Multiple factors such as antigen load, duration of infection, CD4 help, regulatory T cells, and type of antigen presenting cell affect the intensity of CD8 T cell exhaustion (Figure 1) [6]. Recent studies have demonstrated that inhibitory receptors, especially the PD-1-PD-L1 pathway, play a central role in regulating T cell exhaustion [7]. Although T cells in acute infection models transiently express inhibitory receptors upon activation, exhausted CD4 and CD8 T cells exhibit sustained expression of these molecules. Blockade of these inhibitory receptor pathways (especially PD-1-PD-L1) reinvigorates exhausted CD8 T cells, leading to reduced pathogen burden [4]. Apart from inhibitory receptors, cytokines such as IL-10 or TGFβ [8, 9] also play a role in exacerbation of CD8 exhaustion in viral models. Akin to CD8 T cells, during chronic infection CD4 T cells can also become dysfunctional [3], although information in this area is limited.
Most of the information presented above was derived from chronic viral models, and it was only recently that the paradigm of CD8 exhaustion has begun to be explored in non-viral models. Recent studies in Toxoplasma, Plasmodium sp., and Leishmania sp. models strongly suggest that T cell exhaustion is occurring in parasitic diseases (Figure 2). Understanding the mechanistic and molecular basis of T cell exhaustion during parasite infection is an important future goal. Considering the human and economic toll associated with the three protozoan infections discussed here, a better perception of T cell exhaustion in these diseases is imperative for the development of effective immunotherapeutic approaches. This article reviews the recently emerging field of T cell exhaustion in chronic parasite infections and discusses the mechanisms involved in the process.
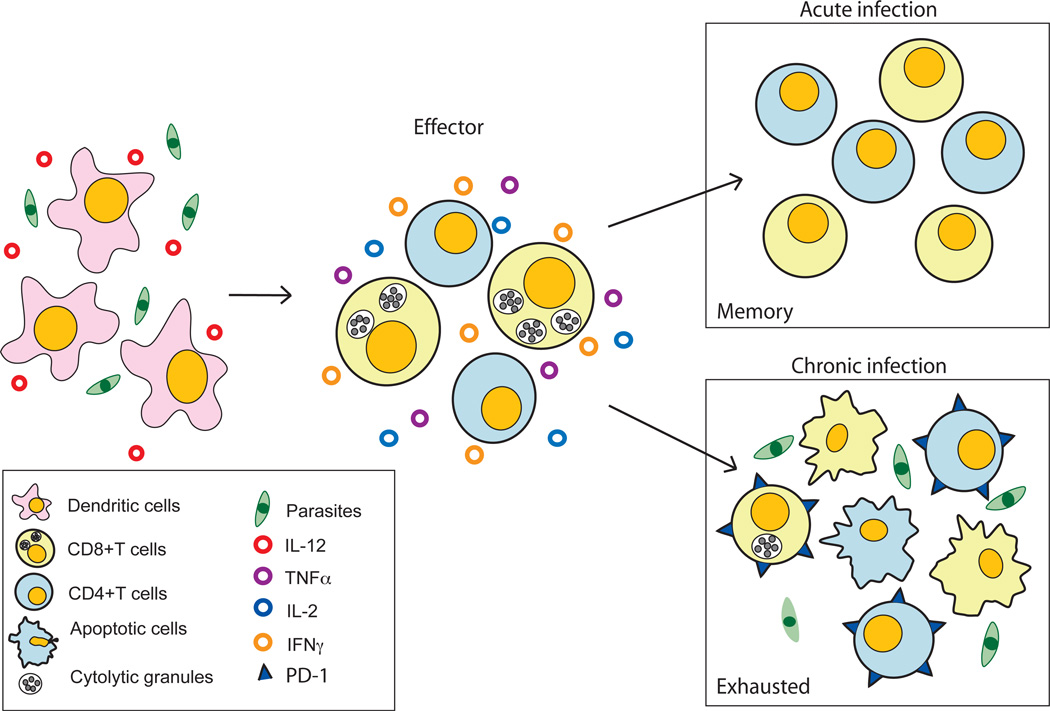
Figure 2. Immune response to protozoan parasites and development of T cell exhaustion.
Protozoan parasites evoke a strong immune response which begins with their encounter with a potent antigen presenting population such as dendritic cells (DC). This leads to DC activation which is manifested by strong IL-12 production and expression of multiple co-stimulatory molecules (CD80/86, CD40/40L, etc.) on the surface. Subsequently, the antigen is processed and the resulting peptides are presented to T cells in the context of MHC molecules, leading to their activation, clonal expansion and differentiation into an effector population. Due to their ability to exhibit polyfunctional ability (cytotoxic activity and production of inflammatory cytokines such as IL-2, TNFα and IFNγ) the effector T cells (both CD4 and CD8 T cell subset) are able to resolve the acute infection, and a memory response against the pathogen is developed, which is highly efficient in controlling re-infection with the same pathogen. However, in the case of parasites which lead to chronic infection, the presence of high antigenic load causes T cells to express inhibitory molecules such as PD-1 in a graded manner, resulting in loss of their polyfunctional ability with a concomitant increase in apoptotic potential (bottom panel). The dysfunctional or exhausted T cells are unable to clear the parasites and in severe cases undergo deletion.
Plasmodium sp. and T cell exhaustion
Plasmodium sp. are the causative agent of malaria, infecting over 500 million people worldwide with ~ 2 million deaths per year associated with the disease [10]. While overall protection against Plasmodium infection during liver stages is mediated by IFNγ secreting CD4 and CD8 T cells (Figure 3) [11], antibody producing B cells play an important role during blood stages of infection [12, 13]. Despite early robust responses, long-term immunity against this stage of infection remains somewhat elusive [14], and it has also been suggested that the parasite may develop a unique survival strategy by hiding in plasmacytoid dendritic cells thus preventing exposure to T cells [15]. Moreover, recent studies with human malaria have reported significant expansion of regulatory T cell levels and shift in DC population which most likely is linked to higher parasite burden in the infected individuals [16].
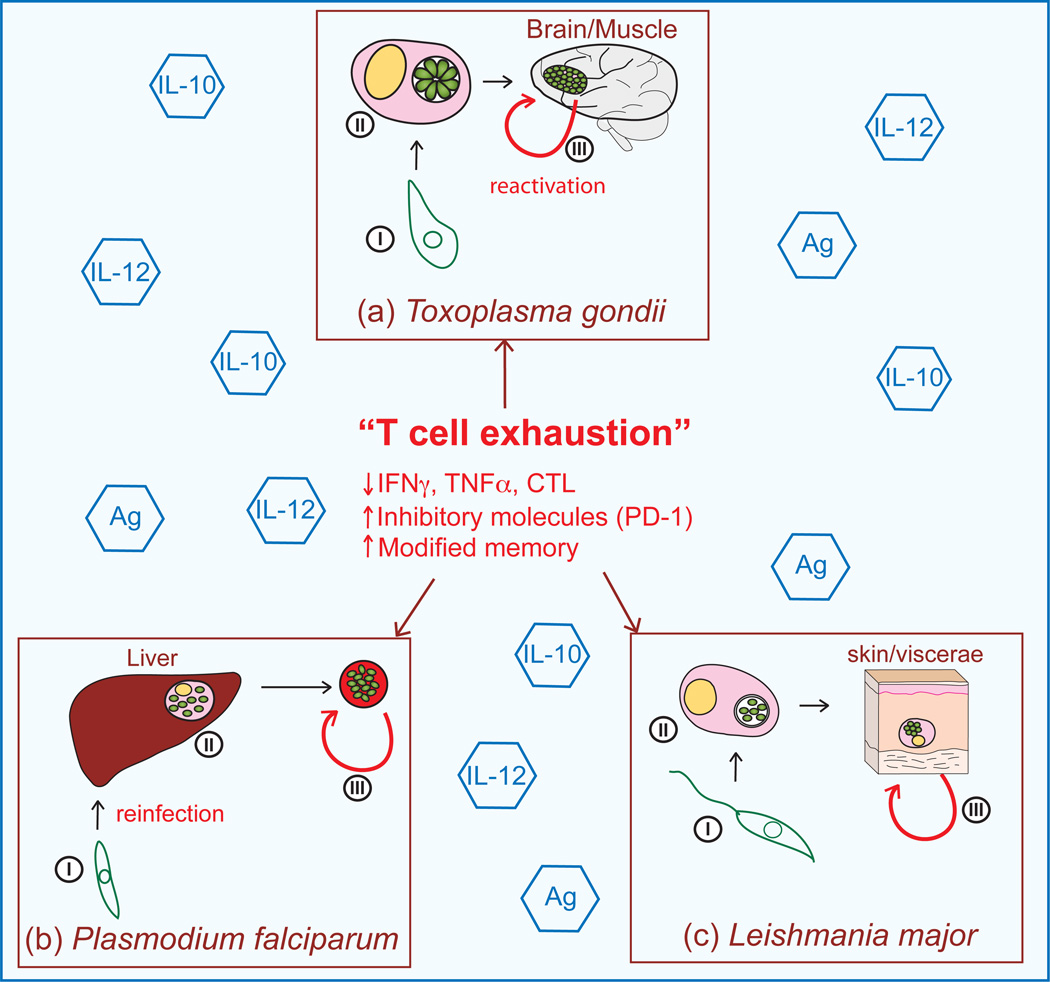
Figure 3. Parasite life cycle and consequences of T cell exhaustion on disease.
Infection with protozoan parasites Toxoplasma gondii, Plasmodium sp. and Leishmania sp. results in an intricate host-pathogen interaction and the development of persistent disease as a consequence of T cell exhaustion (a) (i) T. gondii infection occurs after ingestion of the sporozoite in contaminated ground water, vegetable matter or cat litter or via ingestion of bradyzoite cysts in undercooked meat [21]. (ii) After acute infection the parasite establishes a chronic infection by becoming encysted in immune privileged sites such as the brain, central nervous system (CNS), testes, and deep muscle tissue. (iii) Reactivation of CNS infections leads to the development of toxoplasmic encephalitis and death of the host. (b) (i) Infection with Plasmodium sp. is initiated when an anopheles mosquito harboring sporozoites takes a blood meal from an individual [13, 51]. (ii) Depending on the species of Plasmodium, within 24 hours of infection, the sporozoites either migrate to the liver and invade hepatocytes, remain in the skin, or enter the lymphatics draining to local lymph nodes where they either establish infection or are degraded [52]. Once inside the host cells, the sporozoites undergo asexual amplification as schizonts containing merozoites, which are released into the blood circulation where they invade the erythrocytes [13]. (iii) During the blood stage of the infection the parasite can further propagate the infection as merozoites, become chronic and spontaneously reactivate, or differentiate into gametes, completing the life cycle for further transmission to another host. The repeat of the life cycle via re-infection in endemic areas occurs continually at high levels. (c) (i) Leishmania sp. transmission occurs via sand fly bite which regurgitates the motile flagellated promastigote into the bloodstream of the host. The non-dividing promastigote attaches to professional phagocytes or macrophages where it is then internalized. (ii) Once intracellular, the promastigote transforms into the amastigote and replicates at a high rate, eventually rupturing the parasitized host cell, releasing amastigotes that can re-infect surrounding cells and disseminate throughout the host [27, 53]. (iii) Reactivation of the parasite leads to varied clinical manifestations including visceral (Kala-azar), local and diffuse cutaneous, dermal and mucocutaneous leishmaniasis. Steps leading to T cell exhaustion, including high levels of antigen (Ag), inflammation (IL-12), and immunoregulatory cytokines (IL-10) probably contribute to the sequelae associated with these infections.
In addition to the aforementioned factors, recent studies have attributed PD-1 mediated T cell exhaustion to be a major contributory factor in the development of subdued immune response against the parasite [17]. Although elevated PD-1 expression on T cells during blood-stage infection was previously reported [18], it was the recent study from Butler et al. that definitively established that CD4 T cells underwent exhaustion [17]. The first suggestion of this happening came from studying CD4 T cells in children from Mali, an endemic area. Increased frequencies of PD-1 expressing CD4 T cells were detected in their blood, suggesting exhaustion of these cells. To fully determine the significance of the observation made in human population, they continued their studies with a murine model of infection. In agreement with the hypothesis introduced from human studies, CD4 T cell dysfunction was observed and was attributed to high expression of PD-1 and LAG-3 [17]. Treatment of infected animals with a combination of neutralizing antibodies against these inhibitory molecules restored the protective immunity and also led to enhanced control of parasitic infection in outbred mice, suggesting that T cell exhaustion is independent of MHC alleles and is not restricted to any particular strain of mice. Furthermore, treatment of infected mice with the antimalarial drug, chloroquine on day 8 and 9 post infection reduced dysfunction of both CD4 and CD8 T cell subsets and reversed the exhausted phenotype in 40 to 70% of these cells [17].
Control of Plasmodium infection is also dependent on the ability of CD4 T cells to help antibody producing B cells. Critical for this process are CD4 T follicular helper cells (Tfh), which are required for germinal center formation and once these are formed, contribute to B cell differentiation into plasma and memory cells [19]. Again in the aforementioned study, PD-1 and LAG-3 blockade drove increased Tfh responses and greater antibody protection, which controlled the blood stage of the parasite [17]. Whether or not Tfh were becoming exhausted during this infection is still enigmatic and will be interesting to pursue. While PD-1 and LAG-3 blockade mediated protection was attributed to amplified antibody production [17], another study suggested that cytokine production by the effector memory CD4 population was a major factor responsible for enhanced parasite control [20]. Nevertheless, T cell exhaustion is a major event during Plasmodium infection, which in order to ensure robust protection in the host, needs to be further evaluated. Overall, malarial infection can be categorized amongst the chronic infections in which T cell exhaustion hampers parasite clearance and for successful therapeutic intervention, it may be prudent to include antibodies for multiple co-inhibitory molecules along with vaccine/drug regimen.
Toxoplasma and T cell exhaustion
In the post HAART (highly active anti-retroviral therapy era), toxoplasmic encephalitis from the reactivation of chronic Toxoplasma infection still remains a lethal risk for Toxoplasma seropositive HIV patients in developing countries [21, 22]. Effective CD8 and CD4 T cell response are critical for control of toxoplasmosis (Figure 3). In susceptible mice strains, enigmatically, this does not ensure their long-term survival. Recent studies have demonstrated that during chronic toxoplasmosis, CD8 T cells exhibit progressive functional exhaustion, poor recall response, and elevated apoptosis [23]. This dysfunction leads to parasite stage-conversion involving differentiation of chronic stage-associated bradyzoite to acute stage-associated tachyzoite, which eventually leads to the death of the infected animals. Concomitant with this loss of polyfunctional T cell response, CD8 T cells exhibit high PD-1 expression. Analysis of PD-1 expressing subsets revealed that this inhibitory receptor was preferentially expressed on polyfunctional memory CD8 T cells resulting in apoptosis of this important subset [24]. This is in contrast to HIV infection where PD-1 expression is not restricted to the memory subset [25]. Treatment of chronically infected mice with blocking αPD-L1 antibody reduced CD8 apoptosis and rescued CD8 proliferation and polyfunctionality, albeit partially. Significantly, blockade of the PD-1-PDL-1 interaction not only reduced parasite stage conversion and parasitemia but prevented host mortality.
Although the numerous studies in chronic viral models have reported CD8 rescue via αPD-L1 therapy, the mechanistic basis of αPD-L1 rescue has remained under-explored in both viral and parasitic models. A subsequent study revealed that mere blockade of inhibitory receptor pathway in absence of positive co-stimulatory signals, namely CD40-CD40L, is insufficient for T cell rescue [26]. Significantly, both T cell intrinsic and extrinsic CD40 signaling play a critical role not only in rescuing CD8 T cells but also augmenting Tfh response during αPD-L1 therapy. Whether a combinatorial treatment, with agonistic CD40 and αPD-L1, would be more potent at rescuing exhausted T cells than αPD-L1 alone is an interesting question.
Overall, there is strong evidence that CD8 T cell exhaustion plays an important role in the reactivation of chronic toxoplasmosis. Although αPD-L1 treatment reinvigorated CD8 T cell response, the PD-1hi subset remained refractory to this ’rescue effect‘. Whether poor response of PD-1hi cells is due to co-expression of multiple inhibitory receptors is an area of active investigation in our laboratory. Another important question that needs to be addressed is whether this phenomenon occurs in resistant mice strains such as BalB/c or if improved, CD4 help and rapid antigen control due to haplotype dependent and independent mechanisms prevent such T cell dysfunction from developing in these animals. Considering the wide sero-prevalence of Toxoplasma in the HIV+ population and the prospect of synergistic or additive T cell exhaustion, addressing these questions is vital for development of robust immunotherapeutic agents against this pathogen.
Leishmania and T cell exhaustion
Leishmania sp. causes another significant parasitic disease of humans, affecting hundreds of millions of people worldwide with 50 000 to 70 000 deaths per year [27]. Despite being a silent invader of the host and exquisitely capable of immune evasion, MyD88 dependent triggering of IL-12 production stimulates a CD4 and CD8 T cell response (Figure 3). IFNγ produced by these cells activates macrophages and dendritic cells (DC) to kill the parasite [28–32]. Both CD4 and CD8 T cells are required for this protection; however, as with other parasite infections discussed in this review, their ability to respond to infection is delayed, causing severe disease in the host. CD8 T cell numbers are elevated in the blood and lesions of chronically infected humans. Nevertheless, individuals who exhibit the clinically severe disease (diffuse cutaneous leishmaniasis or kala-azar) have reduced CD8 T cell numbers with a hampered ability to proliferate and produce cytokines (IL-2 and IFNγ), and in the murine model of infection blockade of PD-1-PD-L1 pathway increased the survival of exhausted CD8 T cells [33, 34]. This situation is similar to the hierarchical loss of immune function defined in the T cell exhaustion model [3, 33–35]. To better understand the transient nature of effector immunity, a recent study investigated the fate of antigen-specific CD8 T cells over the course of infection in mice [35]. Using the transgenic Leishmania donovani parasites which express a non-endogenous model epitope OVA, it was noted that after an initial wave of OVA-specific donor CD8 T cell expansion and contraction, these cells adopt a phenotype similar to central memory cells. However, these antigen-specific memory CD8 T cells reactivate and soon thereafter exhibit increased PD-1 expression. This elevated PD-1 expression is concomitant with loss of their effector function (IFNγ, TNFα and IL-2) and subsequent decline in numbers, suggestive of T cell exhaustion [35]. Interestingly, the exhaustion phenotype of these OTI cells is organ specific and only occurs in the spleen where chronic Leishmania infection persists. A similar functional decrease in CD8 T cells was found to occur in humans infected with Leishmania mexicana who had developed diffuse cutaneous leishmaniasis [34]. Taken together, the data from these studies suggest that T cell exhaustion may occur during Leishmania sp. infections, and consistent with other chronic parasite infections, is responsible for exacerbation of the infection in the host. Further investigation of the mechanism behind the decrease of CD8 T cell function in response to this infection revealed that PD-L1 was expressed at a higher level on splenic DCs when the exhaustion phenotype was observed [35]. Importantly, blockade of PD-1/PD-L1 interaction in vivo in L. donovani OVA infected mice or ex vivo treatment of CD8 T cells from diffused cutaneous leishmaniasis patients with TLR2 agonists partially restored the functions of these cells [34]. This strongly suggests that the exhausted CD8 T cell restoration could be a viable therapy to combat chronic leishmaniasis. Nevertheless, CD8 T cell exhaustion in an organ-specific manner is an intriguing phenomenon, which although not reported to date may not be exclusive to Leishmania sp. Obviously further information is needed to understand underlying causes for the tissue based selective T cell exhaustion before any therapeutic measures are considered.
Concluding remarks and future perspectives
The mechanistic basis for the development of T cell exhaustion in chronic viral models has begun to take shape over the last decade. However, these paradigms are now being explored in chronic protozoan infection models. One of the most important factors influencing the intensity of exhaustion is antigen load and duration of exposure (Figures 1 and 3). As such, it is hardly surprising that CD8 exhaustion occurs in, viral models which are characterized by persistent high viremia [3]. By contrast, during parasitic infections after high-level parasitemia during the acute phase, there is a significant reduction in pathogen load before the onset of exhaustion. Interestingly, in viral models such as murine cytomegalovirus which is characterized by low-level persistent viremia, CD8 T cells do not undergo exhaustion [36]. Similarly in Chagas disease caused by Trypanosoma cruzi, CD8 T cells do not exhibit PD-1 up-regulation or functional exhaustion [37]. This suggests that high persistent antigen level is not the sole determinant of CD8 T cell exhaustion in parasitic models. The observation that early drug treatment rather than later during T. gondii infection ameliorates T cell exhaustion highlights that a transient antigen spike during acute stage infection may be sufficient to program CD8 exhaustion. This difference alone emphasizes that although similarities exist between viral and parasitic models of T cell exhaustion, there are significant differences as well. Considering that antigen burden, duration and time of antigen exposure as well as antigen affinity can potentially play a role in CD8 exhaustion, it will be important to address these questions in parasitic models. Development of transgenic parasites that express altered peptide ligand (with different affinity for MHC) or permit inducible expression of a model epitope under the control of a weak or strong promoter, will be critical in unraveling the role of antigen in mediating both CD8 and CD4 T cell exhaustion
Apart from antigen burden, immunosuppressive cytokines such as IL-10 and TGFβ play an important role in modulating T cell exhaustion in viral models [9, 38]. T. gondii infection has been shown to induce high levels of IL-10. While IL-10-IL10R blockade has been shown to ameliorate T cell exhaustion in chronic viral models [3], a similar strategy of αIL-10R treatment in chronically T. gondii infected mice resulted in rapid mortality, presumably due to immunopathology (I.A. Khan et al., unpublished). Surprisingly, conventional CD4 T cells but not Tregs are the major producers of this cytokine during Toxoplasma infection [21, 22]. Interestingly, BLIMP1, a transcription factor involved in mediating CD8 exhaustion in chronic viral models, plays a critical role in regulating IL-10 production by CD4 T cells [39]. Currently, our group is addressing whether reduced IL-10 production by CD4 T cells during chronic toxoplasmosis rescues CD8 exhaustion using conditional BLIMP1 heterozygotes mice. Similarly, elevated IL-10 levels have a direct correlation with increased pathogen burdens in patients infected with Plasmodium [40]. Additionally, patients with Kala-azar or disseminated visceral leishmaniasis exhibit elevated IL-10 [33, 41–43]. Interestingly blockade of the IL-10-IL-10R pathway promotes parasite clearance and near complete resolution of disease in experimental models of visceral leishmaniasis [43, 44]. Whether this is a consequence of CD8 T cell rescue needs to be further defined and carefully dissected. In summary, the mechanistic basis of T cell exhaustion is poorly understood in parasitic models and future studies in this regard would be imperative for the development of superior immunotherapeutic interventions. Currently, the molecular basis of CD8 exhaustion is poorly understood in parasitic models. While transcription factors such as Blimp1, T-bet and BATF among others, have been shown to play a critical role in viral models of CD8 exhaustion, the mechanistic underpinnings of CD4 exhaustion remain severely underexplored in both viral and parasitic models. It will be critical to use high throughput approaches such as microarrays as well as whole genome DNA methylation arrays, to unravel potential targets that may be involved in ameliorating or exacerbating CD4 and CD8 T cell exhaustion in parasitic models.
The parasites covered in this review have developed an intricate interaction with the host which promotes their ability to be transmitted and consequently survive. Like chronic viral infections, these parasites can persist for the life of the host and have developed mechanisms to evade attack by the immune system. After control of the acute stage of infection, one of these evasion mechanisms could be the development of T cell exhaustion which allows the pathogens to persist either in a chronic stage within the host or via productive continual re-infection to promote transmission. The causes behind the development of T cell exhaustion are still unclear in parasitic disease and many questions remain (Table 1). Whether high-level antigen or regulatory cytokine production such as IL-10 produced as a consequence of high inflammation play a role, is not well understood and will be important to elucidate. Does re-infection in T. gondii, Leishmania sp. and Plasmodium sp. and/or continual exposure to antigen act to provide high enough persistent TCR signaling to promote dysfunction? Finally, which molecular mechanisms are involved, including transcription factors (Box 1), to promote immune exhaustion with these pathogens? CD8 T cells expressing intermediate levels of PD-1 are most responsive to αPD-L1 therapy while cells with high level expression seem to be unrecoverable (Figure 4). As such, a multipronged approach may be very useful and more efficient for rescuing cellular function (Figure 4). An important issue which needs to be considered is the role of CD4 T cells in the regulation of CD8 T cell exhaustion. Importance of this T cell subset in the maintenance of robust CD8 immunity has been reported both for Toxoplasma and Plasmodium infection [21, 45]. In this regard, a recent study has shown that αPD-L1 blockade augments IL-21 production by CD4 T cells [26]. Incidentally, IL-21 has been shown to ameliorate CD8 exhaustion in chronic viral models [46]. The significance of IL-21 and the role of Tfh, a CD4 subset known to produce copious IL-21, needs to be investigated in the protozoan models [19].
Table 1.
Parasite infections and T cell exhaustion characteristics
| Pathogen | CD4 Exhaustion |
CD8 Exhaustion |
Inhibitory Receptors a | Treatment b | References |
|---|---|---|---|---|---|
| LCMV clone 13 | + | + | PD-1, LAG-3, CD160, 2B4, Tim3 | i. αPD-L1 ii. αPD-L1 + αLAG-3 iii. αPD-L1 + Tim3-Ig |
[4, 54] |
| Leishmania sp. | ? | + | PD-1 | i. αPD-L1 | [35] |
| Plasmodium sp. | + | + | PD-1, LAG-3, CD160 (?)c, 2B4 (?)c | i. αPD-L1 + αLAG-3 | [17] |
| T. gondii | ? | + | PD-1 | i. αPD-L1 | [23, 24, 26] |
Inhibitory receptor profile of T cells in various models of exhaustion is shown.
Strategies to rescue T cell exhaustion by blocking inhibitory receptor interaction with it ligand via use of blocking antibodies (αPD-L1, αLAG3) or fusion proteins (Tim3-Ig).
These two inhibitory receptors are upregulated on T cells during exhaustion in malaria. However their role in mediating exhaustion in Plasmodium sp. model is unknown.
Box 1. Molecular mechanisms of T cell exhaustion.
The critical role of transcription factors in modulating differentiation, survival and function of T cells is well established. However, how these molecules induce or prevent CD8 T cell exhaustion has only recently been explored. Distinct from its role in acute infection, during chronic infection, T-bet, a T-box transcription factor, is downregulated in exhausted CD8 T cells leading to high expression of PD-1 and other inhibitory receptors [48]. While conditional ablation of T-bet exacerbated CD8 exhaustion, overexpression of this molecule partially ameliorated the exhaustion phenotype. In contrast to T-bet, BLIMP-1, a zinc finger containing transcriptional repressor, is expressed at high levels in exhausted CD8 T cells resulting in poor memory CD8 differentiation and elevated PD-1 expression during chronic viral infection [7]. Surprisingly, while conditional deletion of BLIMP-1 ameliorated CD8 exhaustion, heterozygous conditional knockout mice were superior to wild type or even homozygous conditional knockout mice at controlling the pathogen. This potentially suggests that BLIMP-1 acts as a rheostat whereby at moderate levels it regulates CD8 T cell effector function whereas at high levels it causes CD8 exhaustion. Similar to BLIMP-1, another transcription factor, BATF, a member of the AP-1 family, has been shown to downregulate HIV+ CD8 cytokine response and proliferation in response to PD-1 engagement [49]. Significantly, knocking down BATF in T cells from patients with chronic viremia rescued their functionality. In another study, impaired NFAT translocation was shown to downregulate cytokine response but not degranulation during chronic LCMV and HIV infection [50]. Overall, the present knowledge regarding transcriptional control of T cell exhaustion is based almost entirely on viral models. While αPD-L1 therapy during chronic toxoplasmosis has been shown to upregulate T-bet and Eomes, the significance of these molecules during T cell exhaustion or rescue needs to be investigated more thoroughly in chronic protozoan models.
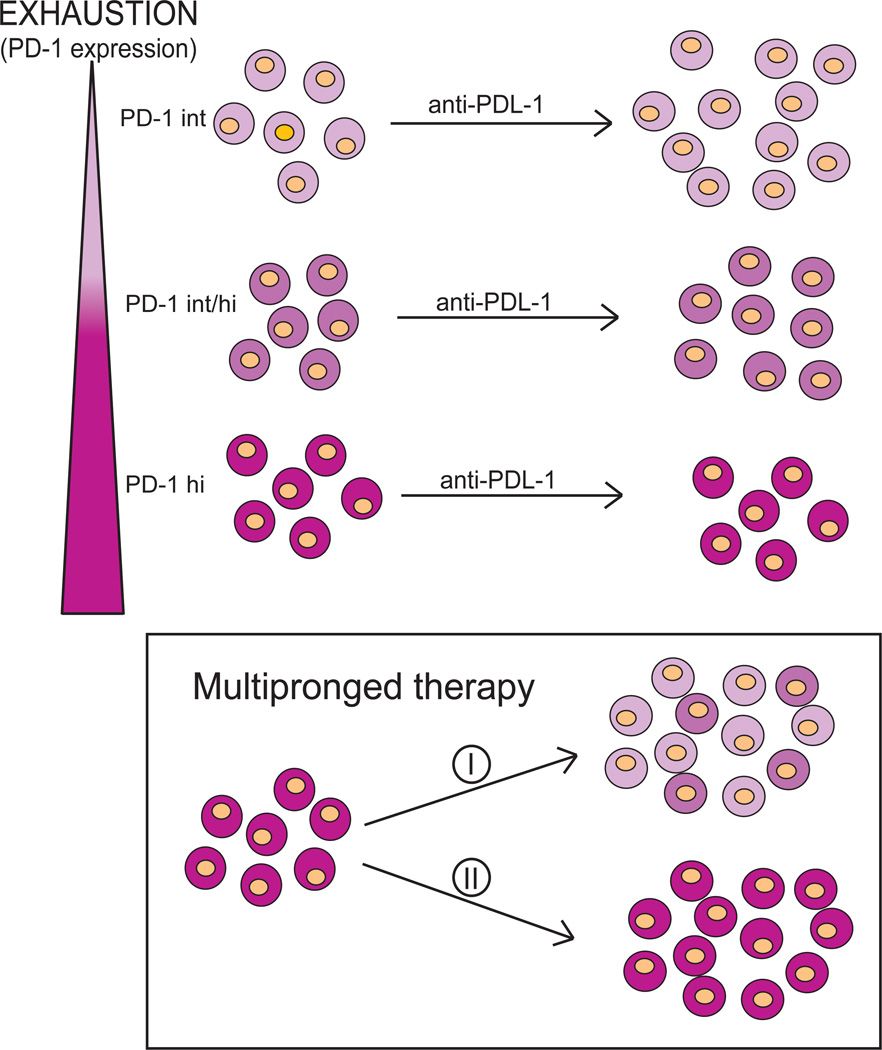
Figure 4. Therapeutic potential of the blockade of inhibitory receptor-ligand interaction on exhausted T cells.
T cells that express intermediate levels of PD-1 (light pink) are highly responsive to αPD-L1 administration and can be rescued. With the progressive increase of PD-1 expression (light pink-dark pink), antibody treatment becomes less efficacious. Ultimately, with high expression of the PD-1, the therapy becomes ineffective (dark pink). For rescue of the PD-1hi population, a multi-pronged approach such as treatment with combinations of antibodies to more than one receptor may be needed. Also, to restore T cell functionality, use of CD40 agonist or IL-21 treatment along with blocking antibodies may be needed. This could have two possible outcomes: (i) the treatment regimen may be successful, and reversal of exhaustion in PD-1hi expressing cells could be achieved due to conversion of PD-1hi cells to PDint and PD-1int/hi cell populations, which respond better to treatment. (ii) Alternatively, CD8 T cells may still retain their high PD-1 expression but regain effector functions only for the duration of treatment.
Importance of T cell exhaustion by parasitic pathogens gains further importance in a co-infection scenario with HIV or other chronic viral pathogen. In a recent study in Kenya, it was estimated that interaction between HIV and Plasmodium in a dual infected population may result in substantially enhanced HIV and malarial infection [47]. Similarly, a recent WHO (World Health Organization) report found that concomitant HIV infection increases the risk of developing visceral leishmaniasis by 100 to 2320 times (http://www.who.int/leishmaniasis/burden/hiv_coinfection/burden_hiv_coinfection/en/index.html). As mentioned earlier, before the advent of HAART therapy, T. gondii was a major opportunistic infection in the HIV infected population [21]. Considering that chronic parasitic/viral co-infection can have a potentially additive/synergistic effect on T cell dysfunction and rapid host mortality, the state of T cell exhaustion in the dually infected population, especially in the context of HIV infection, needs to be extensively studied. Overall, understanding the mechanism of T cell exhaustion is critical for strategizing the design of effective immuno-therapeutic treatment specific to each disease without the risk of immuno-pathology or autoimmunity.
Acknowledgments
J.P.G., R.B. M.M. and I.A.K. contributed equally to the formulation, outline, writing and discussion of this manuscript.
Glossary
- Acute infection
An acute infection is caused by an extensive expansion of pathogens soon after primary infection. Acute infection is usually symptomatic and is often controlled by the development of an efficient immune response specific for the pathogen.
- Apoptosis
Apoptosis is the process of programmed cell death used to control proliferative expansion of lymphoid T cells in response to an infection. However in certain infections, apoptosis can have pathological consequences. The process is triggered by a diverse range of cell signals leading to organized degradation of cellular organelles. Afterward, phagocytic cells remove the resulting cell fragments.
- Basic leucine zipper transcription factor (BATF)
BATF is a transcription factor that directly regulates differentiation and function of IL-17 and IL-21 producing T cells (T helper 17 and T follicular helper cells) as well as antibody class switch in B cells.
- B lymphocyte-induced maturation protein 1 (BLIMP-1)
BLIMP-1 is a transcription factor responsible for plasma B cell differentiation, but it is also involved in maintenance of T cell homeostasis. BLIMP-1 is required for the differentiation of CD8 cytotoxic effector T cells but suppresses primary memory CD8 T cell development. BLIMP-1 acts as a transcriptional repressor of T cell proliferation.
- Bradyzoite
Bradyzoite is a sessile, long-lived, slow growing form of Toxoplasma gondii found during the chronic phase of toxoplasmosis. Intracellular bradyzoites form cysts in tissues such as muscles and brain where they can evade the immune response.
- CD40
CD40, a member of the tumor necrosis factor superfamily, is a costimulatory molecule required for activation of antigen presenting and T cells. With its ligand CD40L, CD40 is a critical contributor to the inflammatory process.
- CD160
CD160 is a membrane protein found on normal natural killer and T cells. CD160 improve proliferation and cytotoxic activity in CD8 T cells. It has recently been recognized as an inhibitory receptor.
- CD244 (2B4)
2B4, a transmembrane protein belonging to the Ig superfamily is a costimulatory receptor expressed by memory CD8 T cells and natural killer cells. 2B4 can elicit both activating and inhibitory signals.
- Central memory T cells
Central memory cells exhibit elevated expression of lymphoid homing molecules, high proliferative potential and produce copious levels of IL-2 in response to an antigenic stimulus. Central memory T cells home preferentially in lymphoid tissue.
- Chronic infection
A chronic infection is usually caused when the host is unable to fully clear the pathogen. This may be a consequence of host immune dysfunction or immune evasion by the pathogen or even both.
- Cytolytic granules
The cytolytic granules present in cytotoxic T cells and natural killer cells contain an array of proteins with varied functions from forming pores in the cell membrane and entering the cytoplasm of a target cell to initiating a cascade of events that ultimately leads to the destruction of the target cell.
- Cytotoxic T-lymphocyte Antigen 4 (CTL4)
CTLA4, a co-inhibitory molecule expressed by activated T cells and regulatory T cells, is essential to immune homeostasis maintenance but can also down-regulate T cell response and proliferation.
- Degranulation
Degranulation refers to the release of the content of the cytolytic/cytotoxic granules in response to an antigenic stimulus.
- Deletion
Deletion refers to the removal of T cells usually by apoptosis.
- Effector memory T cells
effector memory cells are characterized by high cytotoxicity, lack of lymphoid homing receptor. These cells reside preferentially in non-lymphoid tissue.
- Eomesodermin (Eomes)
Eomes, also known as Tbr2, is a T-Box transcription factor related to T-bet. Eomes, like T-bet, is involved in the regulation of the CD8 T cell response. Eomes expressing cells preferentially develop in long-lived memory cells while T-bet expressing cells become preferentially terminal effector cells.
- Exhaustion
is a state of T cell dysfunction characterized by attrition of effector functions, prolonged expression of inhibitory receptors and inability to control infections or tumors.
- Germinal center
Germinal center is an activated center of a lymphoid follicle in a secondary lymphoid tissue where mature B cells undergo rapid proliferation, differentiation and antibody class switch in response to antigen stimulation.
- Highly active antiretroviral therapy (HAART)
HAART is a combination of protease inhibitors and nucleoside reverse transcriptase inhibitor used for treating AIDS and HIV.
- HIV+ non progressor
HIV positive individuals who do not take HIV treatment, do not present any symptoms and have a CD4 cell count above 500 cells/mm3 for at least 8 years after initial HIV infection.
- Lymphocyte-activation gene 3 (LAG3)
LAG3, a member of the Ig superfamily, regulates expansion of activated primary T cells and development of the memory T cell pool. LAG3 is also an inhibitory molecule.
- Memory T cells
Memory T cells are long-lived, capable of antigen independent maintenance and can mediate a robust recall response upon antigen re-encounter.
- Myeloid differentiation primary response gene (88) (MyD88)
MYD88 is an adapter protein responsible for activating the early innate immune response against an antigenic stimulus via the Toll-like receptor and IL-1 signaling pathway.
- Nuclear factor of activated T cells (NFAT)
NFAT is a family of transcription activators that play a significant role in T cell activation and differentiation. NFAT was first identified as an inducible DNA-binding protein able to bind the IL-2 promoter. NFAT is also involved in other immune cell functions (dendritic cells, B cells).
- Polyfunctionality
Polyfunctionality is defined by the ability of a single cell to display multiple functions like cytotoxicity and secretion of multiple cytokines in response to an antigenic stimulus.
- Programmed Death 1 (PD-1)
PD-1, a surface membrane protein that belongs to the Ig superfamily, is expressed by a wide variety of cells, including T cells, B cells and natural killer cells. PD-1 interaction with its ligand PD-L1 negatively regulates the immune response. In the context of exhaustion, progressive expression of PD-1 correlates with exacerbation of CD8 dysfunction (PD-1 intermediate < PD-1 intermediate/high < PD-1 high).
- Programmed death ligand 1 (PD-L1)
PD-L1, one of the ligands for PD-1 receptor, is constitutively expressed on antigen presenting cells, T cells and a wide range of non-hematopoietic cell types. PD-L1 expression is upregulated after activation of antigen presenting and T cells. The PD-1/PD-L1 pathway controls the balance between stimulatory and inhibitory signals needed for optimal immunity.
- Recall response
A recall response occurs after a second encounter with a pathogen or antigen. The ensuing immune response is usually faster and stronger than a primary response and involves memory cells.
- Tachyzoite
Tachyzoite is the fast replicative form of Toxoplasma gondii responsible for acute toxoplasmosis.
- T box expressed in T cells (T-bet)
T-bet, a lineage specific transcription factor expressed by T helper 1 cells, belongs to the T box family. Also known as Tbx21, T-bet with its sister protein Eomes, is involved in important aspects of effector and memory CD8 T cell differentiation. T-bet plays a critical role in T helper 1 response development.
- T-cell immunoglobulin and mucin domain 3 (TIM3)
TIM3, expressed by several lymphocytes subsets including IFNg secreting T helper 1 cells, negatively regulates Th1 response.
- T follicular helper cells (Tfh)
Tfh are a newly described CD4 T cell type with a distinct differentiation lineage. Tfh are distinguished by their expression of CXCR5 (a chemokine receptor), IL-21 and ICOS. Tfh are critical for B cell help through IL-21 expression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicting interests.
References
- 1.Lukens JR, et al. Blockade of PD-1/B7-H1 interaction restores effector CD8+ T cell responses in a hepatitis C virus core murine model. J Immunol. 2008;180:4875–4884. doi: 10.4049/jimmunol.180.7.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betts MR, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wherry EJ, et al. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angelosanto JM, Wherry EJ. Transcription factor regulation of CD8+ T-cell memory and exhaustion. Immunol Rev. 2010;236:167–175. doi: 10.1111/j.1600-065X.2010.00927.x. [DOI] [PubMed] [Google Scholar]
- 7.Shin H, et al. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ejrnaes M, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tinoco R, et al. Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity. 2009;31:145–157. doi: 10.1016/j.immuni.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerra CA, et al. Mapping the global extent of malaria in 2005. Trends Parasitol. 2006;22:353–358. doi: 10.1016/j.pt.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues MM, et al. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int Immunol. 1991;3:579–585. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- 12.Cockburn IA, et al. Memory CD8+ T cell responses expand when antigen presentation overcomes T cell self-regulation. J Immunol. 2008;180:64–71. doi: 10.4049/jimmunol.180.1.64. [DOI] [PubMed] [Google Scholar]
- 13.Douradinha B, Doolan DL. Harnessing immune responses against Plasmodium for rational vaccine design. Trends Parasitol. 2011;27:274–283. doi: 10.1016/j.pt.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Borrmann S, Matuschewski K. Protective immunity against malaria by 'natural immunization': a question of dose, parasite diversity, or both? Curr Opin Immunol. 2011;23:500–508. doi: 10.1016/j.coi.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Wykes MN. Are plasmacytoid dendritic cells the misguided sentinels of malarial immunity? Trends Parasitol. 2012;28:182–186. doi: 10.1016/j.pt.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Goncalves RM, et al. CD4+ CD25+ Foxp3+ regulatory T cells, dendritic cells, and circulating cytokines in uncomplicated malaria: do different parasite species elicit similar host responses? Infect Immun. 2010;78:4763–4772. doi: 10.1128/IAI.00578-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butler NS, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2011;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandele A, et al. Phenotypic and functional profiling of malaria-induced CD8 and CD4 T cells during blood-stage infection with Plasmodium yoelii. Immunology. 2010;132:273–286. doi: 10.1111/j.1365-2567.2010.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 20.Stephens R, Langhorne J. Effector memory Th1 CD4 T cells are maintained in a mouse model of chronic malaria. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001208. e1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhadra R, et al. The CD8 T-cell road to immunotherapy of toxoplasmosis. Immunotherapy. 2011;3:789–801. doi: 10.2217/imt.11.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gigley JP, et al. CD8 T Cells and Toxoplasma gondii: A New Paradigm. J Parasitol Res. 2011;2011 doi: 10.1155/2011/243796. 243796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhadra R, et al. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc Natl Acad Sci U S A. 2011;108:9196–9201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhadra R, et al. PD-1 mediated attrition of polyfunctional memory CD8+ T cells in chronic Toxoplasma infection. J Infect Dis. 2012 doi: 10.1093/infdis/jis304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrovas C, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhadra R, et al. Cutting edge: CD40-CD40 ligand pathway plays a critical CD8-intrinsic and -extrinsic role during rescue of exhausted CD8 T cells. J Immunol. 2011;187:4421–4425. doi: 10.4049/jimmunol.1102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 28.Stern JJ, et al. Role of L3T4+ and LyT-2+ cells in experimental visceral leishmaniasis. J Immunol. 1988;140:3971–3977. [PubMed] [Google Scholar]
- 29.Muller I, et al. Gamma interferon response in secondary Leishmania major infection: role of CD8+ T cells. Infect Immun. 1993;61:3730–3738. doi: 10.1128/iai.61.9.3730-3738.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belkaid Y, et al. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J Immunol. 2002;168:3992–4000. doi: 10.4049/jimmunol.168.8.3992. [DOI] [PubMed] [Google Scholar]
- 31.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 32.de Veer MJ, et al. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur J Immunol. 2003;33:2822–2831. doi: 10.1002/eji.200324128. [DOI] [PubMed] [Google Scholar]
- 33.Karp CL, et al. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993;91:1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez-Ruiz J, et al. CD8 cells of patients with diffuse cutaneous leishmaniasis display functional exhaustion: the latter is reversed, in vitro, by TLR2 agonists. PLoS Negl Trop Dis. 2010;4:e871. doi: 10.1371/journal.pntd.0000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joshi T, et al. B7-H1 blockade increases survival of dysfunctional CD8(+) T cells and confers protection against Leishmania donovani infections. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000431. e1000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder CM, et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bustamante JM, et al. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med. 2008;14:542–550. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brooks DG, et al. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A. 2008;105:20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cretney E, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 40.Bueno LL, et al. Plasmodium vivax: induction of CD4+CD25+FoxP3+ regulatory T cells during infection are directly associated with level of circulating parasites. PLoS One. 5:e9623. doi: 10.1371/journal.pone.0009623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghalib HW, et al. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J Clin Invest. 1993;92:324–329. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy ML, et al. IL-10 mediates susceptibility to Leishmania donovani infection. Eur J Immunol. 2001;31:2848–2856. doi: 10.1002/1521-4141(2001010)31:10<2848::aid-immu2848>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 43.Salhi A, et al. Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis. J Immunol. 2008;180:6139–6148. doi: 10.4049/jimmunol.180.9.6139. [DOI] [PubMed] [Google Scholar]
- 44.Murray HW, et al. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect Immun. 2002;70:6284–6293. doi: 10.1128/IAI.70.11.6284-6293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carvalho LH, et al. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med. 2002;8:166–170. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 46.Elsaesser H, et al. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abu-Raddad LJ, et al. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314:1603–1606. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- 48.Kao C, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quigley M, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med. 2010;16:1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agnellini P, et al. Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. Proc Natl Acad Sci U S A. 2007;104:4565–4570. doi: 10.1073/pnas.0610335104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doolan DL, et al. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chakravarty S, et al. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med. 2007;13:1035–1041. doi: 10.1038/nm1628. [DOI] [PubMed] [Google Scholar]
- 53.Beattie L, Kaye PM. Leishmania-host interactions: what has imaging taught us? Cell Microbiol. 2011;13:1659–1667. doi: 10.1111/j.1462-5822.2011.01658.x. [DOI] [PubMed] [Google Scholar]
- 54.Jin HT, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]