Abstract
Lakes are a central component of the carbon cycle, both mineralizing terrestrially derived organic matter and storing substantial amounts of organic carbon (OC) in their sediments. However, the rates and controls on OC burial by lakes remain uncertain, as do the possible effects of future global change processes. To address these issues, we derived OC burial rates in 210Pb-dated sediment cores from 116 small Minnesota lakes that cover major climate and land-use gradients. Rates for individual lakes presently range from 7 to 127 g C m–2 yr–1 and have increased by up to a factor of 8 since Euro-American settlement (mean increase: 2.8×). Mean pre-disturbance OC burial rates were similar (14–22 g C m–2 yr–1) across all land-cover categories (prairie, mixed deciduous and boreal forest), indicating minimal effect of the regional temperature gradient (approx. 4°C) on background carbon burial. The relationship between modern OC burial rates and temperature was also not significant after removal of the effect of total phosphorus. Contemporary burial rates were strongly correlated with lake-water nutrients and the extent of agricultural land cover in the catchment. Increased OC burial, documented even in relatively undisturbed boreal lake ecosystems, indicates a possible role for atmospheric nitrogen deposition. Our results suggest that globally, future land-cover change, intensification of agriculture and associated nutrient loading together with atmospheric N-deposition will enhance OC sequestration by lakes.
Keywords: eutrophication, nitrogen, phosphorus, land-cover, deforestation, disturbance
1. Introduction
Consideration of lakes as an important component of the terrestrial carbon (C) cycle has focused primarily on their processing of externally derived dissolved organic carbon (DOC) [1–3]. At high DOC loading, lakes are often heterotrophic (net ecosystem respiration is greater than primary productivity), which results in a net efflux of CO2 to the atmosphere [4]. However, lakes can also bury considerable amounts of organic carbon (OC) in sediments that have accumulated since the formation of the lake [5]. Burial of OC in lakes reflects the difference between inputs of aquatic primary production and terrestrially fixed organic matter (as catchment inputs of DOC and particulate organic carbon; POC) and losses via respiration (within the water column and sediments) and outflow [2].
Despite the recognition that OC burial in lakes is an important component of the terrestrial C cycle, there has been little consideration of how rates may have changed over time, particularly in response to anthropogenic changes in land cover that influence soil (and particulate carbon) erosion and nutrient export that results in lake eutrophication. The ‘natural’ OC burial rate by lakes is assumed to reflect major gradients in climate, terrestrial vegetation and geology [6], although most OC burial estimates are largely derived from Northern temperate and boreal lakes [5,7]. Land-cover and land-use change strongly influence both DOC export from terrestrial ecosystems as well as the primary productivity of lakes [8]. Although OC burial rates are relatively constant in the absence of significant land-cover change [5,9], the effect of changing land cover on burial/storage in lakes has only been examined at a limited number of sites [10].
The state of Minnesota is an excellent natural laboratory to examine the role of land-cover type, and its conversion for human use and interaction with climate on lake OC burial rates. Minnesota possesses many lakes (approx. 104 with an area >1 ha), which are distributed across three major biomes [11], from boreal forest in the northeast, through mixed deciduous forest to prairie in the southwest (figure 1). This vegetation/land-cover gradient reflects two pronounced climate gradients: a north–south 4°C increase in mean annual air temperature (MAT) and an east–west decrease in effective moisture (precipitation minus evaporation). The temperature range itself is comparable to future warming predicted for this region by climate models. Minnesota has also undergone a range of anthropogenic land-cover changes over the last approximately 150 years: logging and forest regeneration, land clearance for agriculture and its intensification and urbanization. Here, we present OC burial rates for 116 lakes from four ecoregions in Minnesota, determined from 210Pb-dated sediment cores, corrected for sediment focusing [12] and covering the last approximately 150 years. Using both contemporary and pre-disturbance C burial rates from sites across the latitudinal (i.e. 4°C temperature) gradient within Minnesota, we test the hypothesis that climate controls on C burial are not significant compared to land-use (and nutrient) effects.
Figure 1.
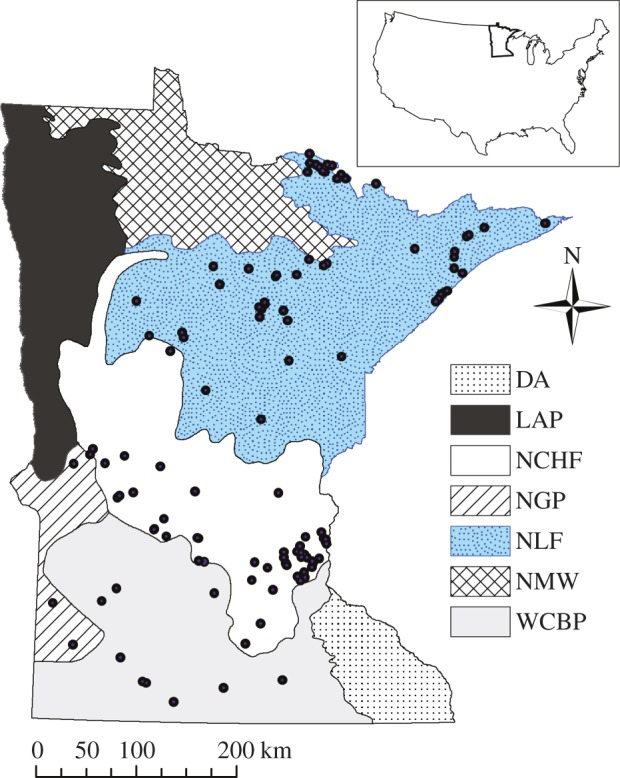
Location of the study lakes in Minnesota and their associated ecoregion. WCBP and NGP were formerly tallgrass prairie and have fertile soils; today they are primarily cultivated (approx. 80% of land use) but contain some pasture and open land. NCHF is a transition zone consisting of mixed woodlands (eastern broadleaf trees) and savannah vegetation. The NLF is extensively forested and largely removed from direct human influences, and has less fertile soils. NLF, northern lakes and forests; NCHF, north central hardwood forests, NCHF; WCBP, western corn belt plains; NGP, northern glaciated plains; LAP, lake Agassiz plain; DA, driftless area and NMW, northern Minnesota wetlands [11]. (Online version in colour.)
2. Material and methods
The sediment cores reported here were originally sampled for a range of palaeolimnological studies. Briefly, sediment cores were extruded at 0.5–4 cm intervals, and dry density and organic content determined using standard loss-on-ignition (at 550°C) methods. All cores were dated by 210Pb and chronologies and dry mass accumulation rates (DMARs) derived using the c.r.s. (constant rate of supply) model. As OC was not determined directly, organic content was converted to C using a standard correction factor (0.469) [13]. The fractional C content was used to convert the DMAR to OC accumulation rates (OCAR) and expressed as g C m–2 yr–1. In this study, all OCARs were corrected for sediment focusing (the preferential deposition of fine-grained sediments in deeper regions of the lake basin) using the ratio of core-specific 210Pb flux to atmospheric 210Pb flux as measured for Minnesota [14]. This correction is applied to the whole core and therefore assumes that focusing is constant over time, which may not be a valid assumption in some cases. However, the benefits of using focusing-corrected C burial estimates considerably outweigh possible problems associated with the approach [15].
OCARs were calculated for four periods: approximately 2000, 1950, 1900 and 1850. The ca 2000 values represent the average rate of OC accumulation in lake sediments dated between 1990 (±5 years) and the present. The ca 1950, 1900 and 1850 values are average accumulation rates for the periods 1940–1960, 1890–1910 and 1800–1860, respectively. For each lake, period means of OCAR were calculated as the product of the mean OC content and mean DMAR for the given date range, assigning equal weight to each sediment interval within the range. Ecoregion OCARs were then constructed for each period by averaging the period means of OCAR for all lakes within the region. Ecoregion designations were determined from USEPA Level III ecoregion boundaries, revised in 2007 and based on those of Omernik [11].
Limnological data, obtained for most of the 116 study lakes, derive from databases maintained by the Minnesota Department of Natural Resources and the Minnesota Pollution Control Agency (MPCA), as well as from the STORET data repository developed by USEPA. Concentration of total phosphorus, chlorophyll a, and total nitrogen are based on summer water samples collected by MPCA and others between 1990 and 2010. The relationship between contemporary OCAR and land-use variables was determined for a 55-lake subset, where detailed land-cover data were available [12].
For lake–climate interactions, although lake-water temperatures are not available for Minnesotan lakes except in recent decades, it is reasonable to assume that past lake temperatures reflect the modern climate gradient across the state, and so we used latitude as an analogue for mean temperature. In the present study, lake locations span 5° of latitude, across which MAT ranges 4°C (Minnesota State Climatology Office, http://climate.umn.edu/doc/historical/temp_norm_adj.htm). Latitude and MAT are essentially collinear (r = –0.97, p < 0.0001). Annual irradiance can vary by as much as 10% across the state (based on 1961–1990 data from the National Solar Radiation Database, http://rredc.nrel.gov/solar/old_data/nsrdb/) and median ice-out dates differ by nearly 1 month (Minnesota Department of Natural Resources, http://www.dnr.state.mn.us/ice_out/index.html?year=median).
Statistical analyses were undertaken using JMP v. 10 (SAS Institute, Cary, NC) and variables were log-transformed where appropriate.
3. Results
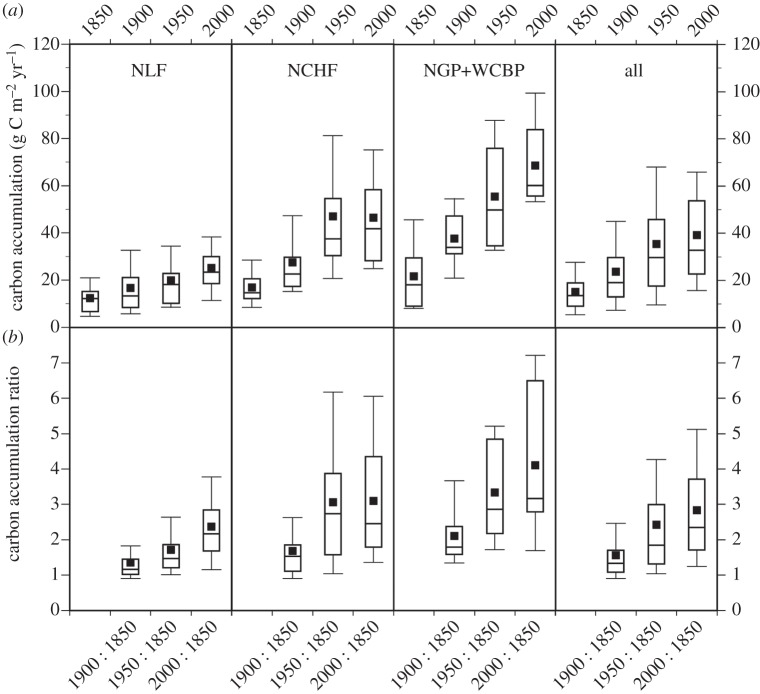
Carbon burial rates for individual lakes (all time periods) range from 3 to 184 g C m–2 yr–1 (figure 2). Mean pre-disturbance rates (i.e. prior to Euro-American settlement) were relatively similar for all regions (14–22 g C m–2 yr–1) and are comparable to the average ‘global’ OCAR burial rates reported by Tranvik et al. [2] (5–14 g C m–2 yr–1). Mean contemporary rates for the different ecoregions range from 25 g C m–2 yr–1 in the northern lakes and forest (NLF) ecoregion to 70 g C m–2 yr–1 in the agriculturally impacted western corn belt plains (WCBPs) and northern glaciated plains (NGPs) ecoregions in the southwest (figure 1). Around 89% of agriculturally impacted lakes have OCAR more than 50 g C m–2 yr–1, while the mean contemporary burial rate for all lakes is 39 g C m–2 yr–1. Burial rates have increased across all ecoregions over the last 150 years; maximum C accumulation ratios (relative to 1850) (see Material and methods) occur in the most recent period (ca 2000) except in the north central hardwood forest (NCHF) ecoregion, where they have been relatively constant (approximately 50 g C m–2 yr–1) since ca 1950 (figure 2). Both the lowest OCAR and the smallest increase are recorded in the NLF lakes.
Figure 2.
(a) Organic carbon accumulation rates (g C m–2 yr–1) by ecoregion and time period. (b) Carbon accumulation ratios (selected time period relative to ca 1850) by ecoregion (see Material and methods). Boxes represent the inter-quartile range, midlines indicate median values, solid squares are the means, and whiskers denote 90% CIs.
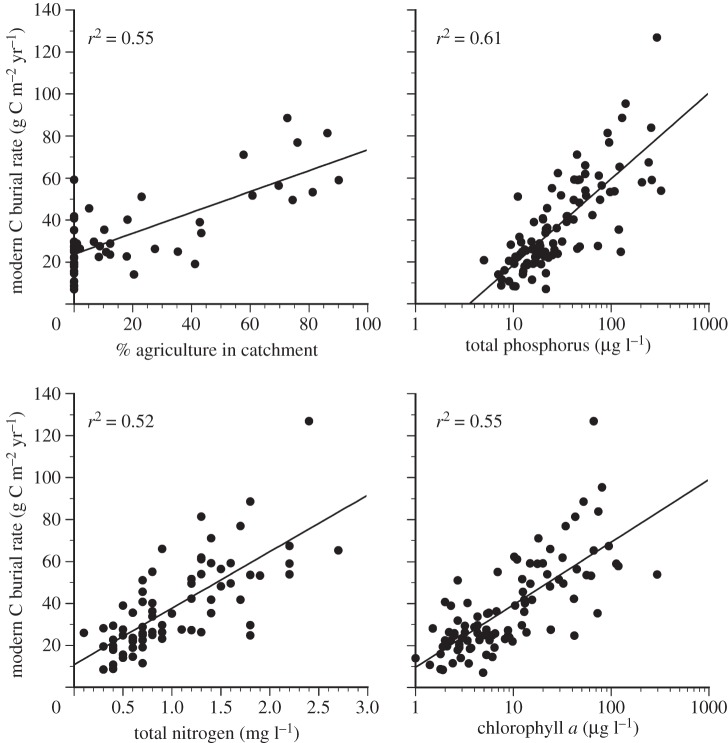
Contemporary OCAR in Minnesota lakes is significantly correlated with two key biogeochemical indicators of altered nutrient cycling and limnological productivity: epilimnetic logTP (r2 = 0.61) and logChl. a (r2 = 0.55) (figure 3). A significant relationship was also found between OCAR and total nitrogen (r2 = 0.53), and with the extent of agricultural land cover in the catchment (r2 = 0.55) (figure 3).
Figure 3.
Relationships between contemporary C accumulation rates (g C m–2 yr–1) and selected limnological variables (total phosphorus, total nitrogen and chlorophyll a; n = 97, 74 (after three extreme outliers being removed) and 95, respectively; see Material and methods for data sources) and percentage agricultural land-use in catchment (n = 52; see [12]).
Lake morphometry was shown to be an important control on C accumulation in boreal lakes in Quebec [16]. In the present study, however, lake area, catchment area and CA : LA had no significant relationship to C burial rates, not even in the pre-settlement period when the effects of land-use change were not present. Stepwise multiple regression analysis on a 52-lake subset (i.e. for those lakes with data for all key variables) shows that after accounting for TP, latitude and/or the extent of agriculture land (%Ag), there was not a statistically significant relationship with any morphometric variable.
Contemporary OCAR is correlated with latitude (r2 = 0.40, p < 0.0001), as are ‘natural’, pre-Euro-American rates (r2 = 0.14; p < 0.0001; figure 4), suggesting a possible relationship between carbon burial and regional climate. However, the role of climate is confounded by the fact that in-lake phosphorus availability is also correlated with latitude, reflecting intensification of agriculture southwards within the state. In a stepwise multiple regression of C burial rates for the modern period on logTP and latitude, the latter explains insignificant variance (p = 0.055) after accounting for logTP. An interactive term (logTP × latitude) was also insignificant (p = 0.7).
Figure 4.
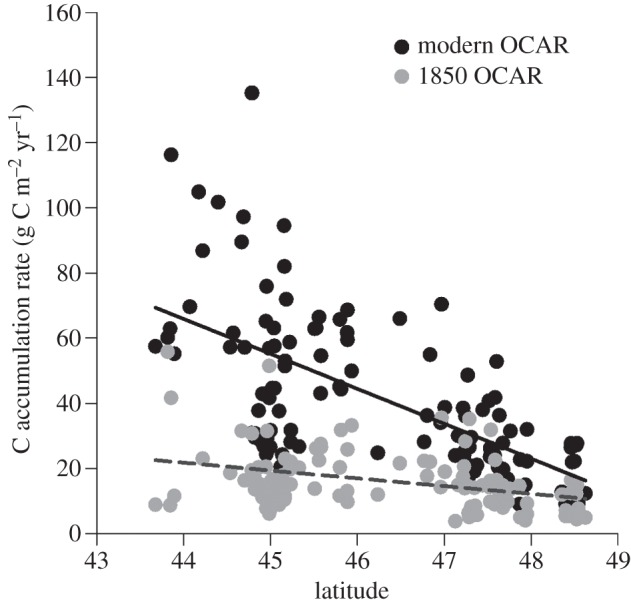
Contemporary and pre-disturbance (i.e. pre-Euro-American settlement, pre-1850) C accumulation rates (g C m–2 yr–1) plotted against latitude (used here as a surrogate for climate). The 1850-C AR : latitude relationship (grey; r2 = 0.14; p < 0.0001) is significant and suggests a possible weak climate control on C burial rates or the effect of more nutrient rich prairie soils on background lake productivity. Removal of the nutrient effect (TP; see figure 3) for the contemporary C AR : latitude relationship (black; r2 = 0.40, p < 0.0001) results in a non-significant effect of latitude (i.e. temperature).
4. Discussion
It is important to recognize that recent rates of sediment carbon accumulation can be inflated relative to long-term burial rates owing to incomplete remineralization of recently deposited organic matter. Although this effect will probably vary among lakes, repeat coring of laminated sediments from a Swedish boreal lake indicates that most of the remineralization occurs within the first 5 years of deposition [17]. If that period of the record is removed from the core data in this study, the effect is to reduce contemporary rates of C burial by less than 10%. Furthermore, many of the records from this study exhibit constant or decreasing C concentrations near the sediment–water interface, which suggests that organic matter diagenesis has not contributed appreciably to the shape of these profiles. Moreover, the timing of OC burial changes does not match what would be expected if the profiles were mainly driven by mineralization: most of the change occurs between pre-1850 and 1950 (figure 2). The point is not to discount the importance of carbon remineralization in affecting C burial rates, only that the contemporary C burial rates and their large historical increases documented in our cores are probably not an artefact of incomplete diagenesis.
Increased carbon burial by lakes following clearance for agriculture probably reflects both lateral transfer and burial of soil C [18,19] as well as increased lake productivity driven by fertilizer application and runoff [8]. Terrestrial inputs of OC may explain why OCAR is not directly proportional to chlorophyll a (figure 3), but rather increases as a power function with a Y-intercept of approximately 14.5 g C m–2 yr–1. Thus there is an unknown, but significant portion of present-day OC coming from the terrestrial environment. In some lakes in Minnesota, the historical input of terrestrial C may also have been substantial.
Disruption of the biogeochemical cycles of phosphorus and nitrogen has had considerable impacts on lake productivity globally [20]. The OCAR observed in lakes in the agriculturally dominated landscape of southern and western Minnesota are mainly explicable in terms of agriculturally derived nutrients (figure 3), but lateral transfer of soil carbon will also have contributed. The relatively constant mean OCAR rate in the NCHF ecoregion after 1950 probably reflects the expansion of the Minneapolis–St Paul metropolitan area, which has seen agricultural land taken out of production, sold and subdivided for housing development, with a corresponding improvement in water quality, lower primary productivity and hence constant or declining C burial in some lakes after 1950 (figure 2).
While agriculture is clearly an important control of OCAR in many lakes in Minnesota (figure 3), the maximum OC burial rates during the most recent period in the NLF lakes are more difficult to explain. The main period of catchment disturbance in northeastern Minnesota (NFL ecoregion) was associated with selective logging in the late nineteenth, early twentieth centuries [21]. And despite more than 80–100 years of forest re-growth (i.e. increasing catchment stability), average OCAR in NLF lakes has continued to increase (ca 2000 mean ≈ 25 g C m–2 yr–1) and has not returned to pre-disturbance levels (ca 1850 ≈ 12 g C m–2 yr–1). Catchment carbon and nutrient dynamics change as forests recover from disturbance [22], influencing element export [23]. That the OCAR in the NLF has not declined may indicate that catchment stabilization is not yet complete and/or there is continued nutrient export that would support in-lake production. Alternatively, the C burial in these lakes may be dominated by changing inputs of terrestrial C, possibly from flocculation of exported DOC as much as POC inputs [24]. There are now documented regional increases in DOC to streams and lakes [25], a change hypothesized to be caused by reduced SO4 deposition [26] and/or climate warming [27]. That said, DOC's influence on OCAR in this study is only marginally significant (r2 = 0.08; p = 0.04; data not shown). Finally, Fe has been highlighted as having an important role in sequestering terrestrial C in some systems [28], but its role in Minnesotan lakes is unknown.
An alternative explanation for increasing C burial in Minnesota's boreal lakes is that atmospheric inputs of reactive nitrogen are increasing lake productivity [29,30]. Disruption of the global N cycle is now comparable to alteration of the carbon cycle [31], and there is increasing evidence for direct effects of atmospheric N deposition on lakes [32]. A positive effect on algal productivity due to increased atmospheric nitrogen inputs for the western Great Lakes region was postulated by Axler et al. [33]. Using a bioassay approach, they showed that N enrichment resulted in a greater increase in biomass than P, although N + P generally yielded the greatest effect, and concluded that atmospheric inputs could sustain algal growth and may lead to lake eutrophication. There is now growing evidence that medium-range transport and deposition of NOx derived from the intensive agricultural and industrial/urban areas is affecting ‘pristine’ limnic ecosystems by increasing lake productivity (and hence OC burial) [30]. The observation that the largest increase in OCAR in NFL lakes occurs during the latter half of the twentieth century is consistent with an N-deposition driver [31]. Kortelainen et al. [34] have highlighted the key role that N plays in the long-term C balance of N-limited boreal ecosystems.
The NLF ecoregion contains nearly 50% of the total lake area in Minnesota and accounts for 35% of the total state-wide lake-C burial. Although the NLF lakes are located at the southern edge of the boreal biome, the global dominance of boreal lakes (total area = 142 Mha), combined with projected changes in the distribution of boreal forest suggests that a critical assessment of C burial by boreal lakes is needed [35]. Results from this study suggest that OC burial rates may be increasing throughout the boreal zone in response to global environmental change drivers (N deposition, increased DOC export), while at the same time the actual present-day C burial by lakes may be substantially underestimated. The Holocene OC burial rate for small boreal lakes (less than 1 km2) in Finland was estimated at less than 5 g C m–2 yr–1 by Kortelainen et al. [5], 4–5 times lower than the recent burial rate for NLF lakes in Minnesota. Furthermore, the mean pre-disturbance C burial rate in the NLF, approximately 12 g C m–2 yr–1, is close to the maximum global range quoted by Tranvik et al. [2]. While the differences in these rates may reflect contrasting methodologies and time scales (210Pb with focusing correction versus 14C-dated Holocene averages), there is some suggestion that C burial rates in boreal lakes have increased in northern Minnesota and elsewhere. Extrapolating C retention budgets to the global boreal lake area, Molot & Dillon [36] estimated the average contemporary annual C accumulation rate to be 19–24 g C m–2 yr–1. OCAR (focusing-corrected) for boreal lakes in northern Wisconsin (i.e. essentially the same ecoregion as Minneosta's NLF) was estimated to be 20 (range 9–31) g C m–2 yr–1 [37], very similar to the post-1950 rates reported in this study (figure 2). Using an areally corrected, 210Pb-dating method (an approach similar to that used in this study), Molot & Dillon [36] determined a rate of 8.3 ± 3.4 g C m–2 yr–1; taken together these results suggest that approximately 5 g C m–2 yr–1 is an underestimate of contemporary C burial in boreal lakes.
Lake productivity is widely assumed to be influenced by temperature [38], although post-depositional mineralization rates are also temperature-dependent [39]. Latitude, our analogue for temperature, can therefore potentially shape OCAR patterns in lakes. Pre-disturbance OCARs in Minnesota lakes show a weak relationship to latitude (figure 4), suggesting that the 4°C north-south temperature gradient is a statistically significant but minor control on OCAR. However, because latitude also exerts strong control over other climate variables that influence productivity (i.e. incident irradiance and ice cover [38]), the correlation between OCAR and latitude may not be exclusively driven by temperature. Furthermore, because there is also latitudinal variation in geology and natural landscape fertility [40], and therefore nutrient supply, it is unlikely that climatic variables account for all of the variance explained. Although contemporary OCARs are more strongly correlated with latitude (figure 4), the removal of the effect of nutrient input (TP) by linear regression indicates that there is no significant effect of latitude on OCAR independent of nutrient enrichment. While there may be productivity increases associated with altered lake heat budgets [41], the evidence suggest that these warming effects are minor compared with the impacts of land-cover change and increased nutrient loadings.
Lakes are an important part of the annual terrestrial C cycle in lake-rich landscapes [2], and compared with terrestrial soils, which respire most OC inputs, lake sediments are a long-term sink [5,9]. Although lakes are not normally included in regional terrestrial C budgets [37], they can have a large effect on net ecosystem storage if the rates of either aquatic production or respiration (loss rates) are substantially altered [39]. Evidence from this study indicates that there has been an increase in C sequestration by lakes at the regional scale. This increase has been driven largely by land-cover change (logging, conversion of forest and prairie to intensive agriculture), which has increased nutrient availability, and hence aquatic productivity and C burial in lakes (figure 3). In addition to increasing storage of autochthonous carbon, catchment disturbance and land-cover change have also probably increased the storage of soil-derived C.
While rates of land-cover conversion have slowed in temperate regions, intensification of agriculture in less-developed regions will continue in order to meet future food demands. Similarly, warming trends may facilitate agricultural expansion within the boreal zone, with associated impacts on nutrient and C dynamics. Moreover, the P surplus documented in agricultural soils worldwide [42] has considerable long-term implications for carbon burial in aquatic ecosystems. Also, while P induced eutrophication is a major problem for lakes in more developed regions [8], the effect of atmospherically transported reactive N on remote lakes is unlikely to diminish in the immediate future [30,32]. Overall, it is probable that nutrient transfer from land to surface waters will maintain elevated levels of lake productivity and C burial in the short term [8]. Predictions of global land-use change, disruption of nutrient cycles and climate warming scenarios suggest that the elevated C burial rates reported in this study for a range of vegetation types, disturbance histories and climate gradients are relevant to lakes elsewhere.
References
- 1.Sobek S, Algesten G, Bergstrom AK, Jansson M, Tranvik LJ. 2003. The catchment and climate regulation of pCO2 in boreal lakes. Global Change Biol. 9, 630–641 (doi:10.1046/j.1365-2486.2003.00619.x) [Google Scholar]
- 2.Tranvik LJ, et al. 2009. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 54, 2298–2314 (doi:10.4319/lo.2009.54.6_part_2.2298) [Google Scholar]
- 3.Battin TJ, Luyssaert S, Kaplan LA, Aufdenkampe AK, Richter A, Tranvik LJ. 2009. The boundless carbon cycle. Nat. Geosci. 2, 598–600 (doi:10.1038/Ngeo618) [Google Scholar]
- 4.Cole JJ, et al. 2007. Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems 10, 171–184 (doi:10.1007/s10021-006-9013-8) [Google Scholar]
- 5.Kortelainen P, Pajunen H, Rantakari M, Saarnisto M. 2004. A large carbon pool and small sink in boreal Holocene lake sediments. Glob. Change Biol. 10, 1648–1653 (doi:10.1111/j.1365-2486.2004.00848.x) [Google Scholar]
- 6.Stallard RF. 1998. Terrestrial sedimentation and the carbon cycle: coupling weathering and erosion to carbon burial. Glob. Biogeochem. Cycles 12, 231–257 (doi:10.1029/98gb00741) [Google Scholar]
- 7.Dean WE, Gorham E. 1998. Magnitude and significance of carbon burial in lakes, reservoirs, and peatlands. Geology 26, 535–538 (doi:10.1130/0091-7613(1998)026<0535:MASOCB>2.3.CO;2) [Google Scholar]
- 8.Smith VH, Tilman GD, Nekola JC. 1999. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 100, 179–196 (doi:10.1016/S0269-7491(99)00091-3) [DOI] [PubMed] [Google Scholar]
- 9.Anderson NJ, D'Andrea W, Fritz SC. 2009. Holocene carbon burial by lakes in SW Greenland. Glob. Change Biol. 15, 2590–2598 (doi:10.1111/j.1365-2486.2009.01942.x) [Google Scholar]
- 10.Heathcote AJ, Downing JA. 2012. Impacts of eutrophication on carbon burial in freshwater lakes in an intensively agricultural landscape. Ecosystems 15, 60–70 (doi:10.1007/s10021-011-9488-9) [Google Scholar]
- 11.Omernik JM. 1987. Ecoregions of the Conterminous United States. Ann. Assoc. Am. Geographers 77, 118–125 (doi:10.1111/j.1467-8306.1987.tb00149.x) [Google Scholar]
- 12.Engstrom DR, Balogh SJ, Swain EB. 2007. History of mercury inputs to Minnesota lakes: influences of watershed disturbance and localized atmospheric deposition. Limnol. Oceanogr. 52, 2467–2483 (doi:10.4319/lo.2007.52.6.2467) [Google Scholar]
- 13.Dean WE. 1974. Determination of carbonate and organic-matter in calcareous sediments and sedimentary-rocks by loss on ignition: comparison with other methods. J. Sediment. Petrol. 44, 242–248 (doi:10.1306/74D729D2-2B21-11D7-8648000102C1865D) [Google Scholar]
- 14.Lamborg CH, Engstrom DR, Fitzgerald WF, Balcom PH. 2013. Apportioning global and non-global components of mercury deposition through 210Pb indexing. Sci. Total Environ. 448, 132–140 (doi:10.1016/j.scitotenv.2012.10.065) [DOI] [PubMed] [Google Scholar]
- 15.Engstrom DR, Rose NL. 2013. A whole-basin, mass-balance approach to paleolimnology. J. Paleolimnol. 49, 333–347 (doi:10.1007/s10933-012-9675-5) [Google Scholar]
- 16.Ferland M-E, del Giorgio PA, Teodoru CR, Prairie YT. 2012. Long-term C accumulation and total C stocks in boreal lakes in northern Quebec. Glob. Biogeochem. Cycles 26, 1–10 (doi:10.1029/2011gb004241) [Google Scholar]
- 17.Galman V, Rydberg J, de Luna SS, Bindler R, Renberg I. 2008. Carbon and nitrogen loss rates during aging of lake sediment: changes over 27 years studied in varved lake sediment. Limnol. Oceanogr. 53, 1076–1082 (doi:10.4319/lo.2008.53.3.1076) [Google Scholar]
- 18.Quinton JN, Govers G, Van Oost K, Bardgett RD. 2010. The impact of agricultural soil erosion on biogeochemical cycling. Nat. Geosci. 3, 311–314 (doi:10.1038/ngeo838) [Google Scholar]
- 19.Van Oost K, et al. 2007. The impact of agricultural soil erosion on the global carbon cycle. Science 318, 626–629 (doi:10.1126/science.1145724) [DOI] [PubMed] [Google Scholar]
- 20.Schindler DW. 1977. Evolution of phosphorus limitation in lakes. Science 195, 260–262 (doi:10.1126/science.195.4275.260) [DOI] [PubMed] [Google Scholar]
- 21.Friedman SK, Reich PB. 2005. Regional legacies of logging: departure from presettlement forest conditions in northern Minnesota. Ecol. Appl. 15, 726–744 (doi:10.1890/04-0748) [Google Scholar]
- 22.Houghton RA. 2005. Aboveground forest biomass and the global carbon balance. Glob. Change Biol. 11, 945–958 (doi:10.1111/j.1365-2486.2005.00955.x) [Google Scholar]
- 23.Bormann FH, Likens GE, Siccama TG, Pierce RS, Eaton JS. 1974. Export of nutrients and recovery of stable conditions following deforestation at Hubbard Brook. Ecol. Monogr. 44, 255–277 (doi:10.2307/2937031) [Google Scholar]
- 24.von Wachenfeldt E, Tranvik LJ. 2008. Sedimentation in boreal lakes: the role of flocculation of allochthonous dissolved organic matter in the water column. Ecosystems 11, 803–814 (doi:10.1007/s10021-008-9162-z) [Google Scholar]
- 25.Clark JM, Bottrell SH, Evans CD, Monteith DT, Bartlett R, Rose R, Newton RJ, Chapman PJ. 2010. The importance of the relationship between scale and process in understanding long-term DOC dynamics. Sci. Total Environ. 408, 2768–2775 (doi:10.1016/j.scitotenv.2010.02.046) [DOI] [PubMed] [Google Scholar]
- 26.Monteith DT, et al. 2007. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 450, 537–540 (doi:10.1038/nature06316) [DOI] [PubMed] [Google Scholar]
- 27.Weyhenmeyer GA, Karlsson J. 2009. Nonlinear response of dissolved organic carbon concentrations in boreal lakes to increasing temperatures. Limnol. Oceanogr. 54, 2513–2519 (doi:10.4319/lo.2009.54.6_part_2.2513) [Google Scholar]
- 28.Einola E, Rantakari M, Kankaala P, Kortelainen P, Ojala A, Pajunen H, Makela S, Arvola L. 2011. Carbon pools and fluxes in a chain of five boreal lakes: a dry and wet year comparison. J. Geophys. Res. Biogeosci. 116, 1–13 (doi:10.1029/2010jg001636) [Google Scholar]
- 29.Bergstrom AK, Blomqvist P, Jansson M. 2005. Effects of atmospheric nitrogen deposition on nutrient limitation and phytoplankton biomass in unproductive Swedish lakes. Limnol. Oceanogr. 50, 987–994 (doi:10.4319/lo.2005.50.3.0987) [Google Scholar]
- 30.Elser JJ, Andersen T, Baron JS, Bergstrom AK, Jansson M, Kyle M, Nydick KR, Steger L, Hessen DO. 2009. Shifts in lake N : P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326, 835–837 (doi:10.1126/science.1176199) [DOI] [PubMed] [Google Scholar]
- 31.Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA. 2008. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320, 889–892 (doi:10.1126/science.1136674) [DOI] [PubMed] [Google Scholar]
- 32.Bergstrom AK, Jansson M. 2006. Atmospheric nitrogen deposition has caused nitrogen enrichment and eutrophication of lakes in the northern hemisphere. Glob. Change Biol. 12, 635–643 (doi:10.1111/j.1365-2486.2006.01129.x) [Google Scholar]
- 33.Axler RP, Rose C, Tikkanen CA. 1994. Phytoplankton nutrient deficiency as related to atmospheric nitrogen deposition in northern Minnesota acid-sensitive lakes. Can. J. Fish. Aquat. Sci. 51, 1281–1296 (doi:10.1139/F94-128) [Google Scholar]
- 34.Kortelainen P, et al. 2013. Carbon evasion/accumulation ratio in boreal lakes is linked to nitrogen. Glob. Biogeochem. Cycles 27, 1–12 (doi:10.1002/gbc.20036) [Google Scholar]
- 35.Benoy G, Cash K, McCauley E, Wrona F. 2007. Carbon dynamics in lakes of the boreal forest under a changing climate. Environ. Rev. 15, 175–189 (doi:10.1139/A07-006) [Google Scholar]
- 36.Molot LA, Dillon PJ. 1996. Storage of terrestrial carbon in boreal lake sediments and evasion to the atmosphere. Glob. Biogeochem. Cycles 10, 483–492 (doi:10.1029/96gb01666) [Google Scholar]
- 37.Buffam I, Turner MG, Desai AR, Hanson PC, Rusak JA, Lottig NR, Stanley EH, Carpenter SR. 2011. Integrating aquatic and terrestrial components to construct a complete carbon budget for a north temperate lake district. Glob. Change Biol. 17, 1193–1211 (doi:10.1111/j.1365-2486.2010.02313.x) [Google Scholar]
- 38.Lewis WM. 2011. Global primary production of lakes: 19th Baldi Memorial Lecture. Inland Waters 1, 1–28 (doi:10.5268/LW-1.1.384) [Google Scholar]
- 39.Gudasz C, Bastviken D, Steger K, Premke K, Sobek S, Tranvik LJ. 2010. Temperature-controlled organic carbon mineralization in lake sediments. Nature 466, 478–481 (doi:10.1038/nature09186) [DOI] [PubMed] [Google Scholar]
- 40.Gorham E, Dean WE, Sanger JE. 1983. The chemical-composition of lakes in the north-central United States. Limnol. Oceanogr. 28, 287–301 (doi:10.4319/lo.1983.28.2.0287) [Google Scholar]
- 41.Magnuson JJ, et al. 1997. Potential effects of climate changes on aquatic systems: Laurentian Great Lakes and Precambrian Shield Region. Hydrol. Process. 11, 825–871 (doi:10.1002/(SICI)1099-1085(19970630)11:8<825::AID-HYP509>3.0.CO;2-G) [Google Scholar]
- 42.Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH. 1998. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 8, 559–568 (doi:10.2307/2641247) [Google Scholar]