Abstract
Endemic, low-virulence parasitic infections are common in nature. Such infections may deplete host resources, which in turn could affect the reproduction of other parasites during co-infection. We aimed to determine whether the reproduction, and therefore transmission potential, of an epidemic parasite was limited by energy costs imposed on the host by an endemic infection. Total lipids, triacylglycerols (TAG) and polar lipids were measured in cockroaches (Blattella germanica) that were fed ad libitum, starved or infected with an endemic parasite, Gregarina blattarum. Reproductive output of an epidemic parasite, Steinernema carpocapsae, was then assessed by counting the number of infective stages emerging from these three host groups. We found both starvation and gregarine infection reduced cockroach lipids, mainly through depletion of TAG. Further, both starvation and G. blattarum infection resulted in reduced emergence of nematode transmission stages. This is, to our knowledge, the first study to demonstrate directly that host resource depletion caused by endemic infection could affect epidemic disease transmission. In view of the ubiquity of endemic infections in nature, future studies of epidemic transmission should take greater account of endemic co-infections.
Keywords: Blattella germanica, Gregarina blattarum, Steinernema carpocapsae, lipids, triacylglycerols
1. Introduction
Endemic parasites are abundant in nature [1,2]. Such infections are often characterized by relatively low virulence, persistence in the host population and an extended evolutionary association between the parasite and its host [3,4]. Conversely, epidemic diseases tend to produce more acute and severe infections and have high reproductive rates within the host [3]. Owing to the severity of diseases caused by epidemic infections, much research is focused on determining those factors which influence epidemic disease transmission. However, the ubiquity of endemic disease means that many epidemic parasite species will encounter hosts already infected with endemic parasites [5], yet very little research takes into account the potential effects of such co-infection upon epidemic transmission.
The transmission rate of a parasite is fundamentally linked to its reproductive capacity, as parasites that are capable of producing more progeny are more likely to encounter and infect new hosts [6]. Parasite reproductive capacity is dependent on the availability of resources within the host, which provide the nutrients for parasite growth and reproduction [7]. In turn, parasites reduce the amount and may alter the composition of host energy stores [8–10]. As parasites can alter host resource levels, infection with one parasite species may alter the quality of the host environment for another parasite, potentially resulting in changes to the reproductive capacity of the second species. There is some evidence of this phenomenon for closely related parasites; for example, competition between Schistosoma mansoni and Schistosoma rodhaini in the same snail host Biomphalaria glabrata led to reduced cercarial output for both parasite species [11]. Different taxa (protozoa and fungi) have also been shown to influence one another's transmission potential; for example co-infection of Daphnia galeata with the intestinal protozoan, Caullerya mesnili, and fungi, Metschnikowia sp., results in reduced output of transmission stages of both species [12]. While resource competition has been postulated as the mechanism of the interactions in these studies, a direct link between the depletion of host energy resources and the interaction between the co-infecting parasites has not been demonstrated. Further, the effect of low-virulence endemic infections on epidemic parasite species has, to our knowledge, never been tested empirically.
A suitable host system in which to explore endemic–epidemic parasite interactions is the German cockroach, Blattella germanica. This cockroach species is host to a range of parasites, including the endemic, gastrointestinal, apicomplexan parasite, Gregarina blattarum and the entomopathogenic nematode, Steinernema carpocapsae. Infection with the gregarine results in relatively low virulence, whereas the nematode kills its host within 72 h of infection [13]. Lipids are the main energy store of the cockroach host and are also important for both parasite species, and therefore this is also an ideal model system in which to examine the potential role of resource competition in mediating parasite interspecific interactions.
The majority of the cockroach hosts' lipids are stored in the fat body and are predominantly comprised triacylglycerols (TAG) [14]. Other classes of lipids, e.g. polar lipids, are also important for the host, particularly in cellular activities such as nutrient transport and cell membrane permeability [15,16]. The lipid classes can be further characterized by a fatty acid profile from which the lipids are formed. Changes in both the quantity of lipids and the fatty acid profiles can indicate changes in physiological processes within the host [17–19]. Therefore, it is important when considering the effects of parasites upon host resources to consider both gross changes in lipid levels and the potential for finer scale alteration in fatty acid profiles.
Although information on the role of lipids is largely lacking for gregarines, they are clearly important for these apicomplexans, as electron microscopy on a number of gregarine species has revealed the presence of large intracellular lipid droplets [20–22]. Infection by G. blattarum occurs when oocysts are ingested by the host, from which sporozoites emerge within the mid-gut and attach to the cell membrane [23]. The sporozoites develop into trophozoites, which attach to the mid-gut lumen by means of an epimerite, with the remaining body structure protruding into the lumen of the gut. Although the exact mechanism of resource acquisition is not known for G. blattarum, it is at this stage that the parasite is likely to interact with the host, that is by fusing with the epithelial cells that line the host mid-gut in the same way as other intestinal gregarines [24]. Such attachment is also associated with epithelial cell damage, which may reduce uptake of nutrients by the host [25]. Gregarines may therefore deplete host resources through direct utilization and by reducing the host's ability to absorb nutrients. When fully grown, the trophozoites detach from the host cells and pair-up to form reproductive units (gametocysts) that are excreted by the host into the environment. Within the gametocysts, several reproductive stages occur to yield oocysts, which complete the infection cycle when ingested by the next host [23].
Lipids are important throughout the life cycle of entomopathogenic nematodes. Infection occurs when nematode larvae invade the host through openings in the host cuticle (spiracles, mouth or anus). Once within the host haemocoel, the nematodes release symbiotic bacteria (Xenorhabdus nematophila) that colonize the host and metabolize stored host energy reserves, including lipids [13]. The nematodes are then able to mature and reproduce (passing through several life cycles within the host), using both host energy reserves directly and by feeding on the X. nematophila. When host resources are close to depletion, the nematode life cycle switches to the production of non-feeding transmission stages (infective juveniles) [13], which ingest (but do not digest) their symbiotic bacteria [26]. The infective juveniles then sheath themselves, become non-feeding and exit the host. At this stage, the lipid reserves within the nematodes are essential for survival in the environment [27], while they await a new host to infect.
In this study, we used our cockroach host–two parasite model system to investigate whether an endemic parasite infection alters the transmission potential of an epidemic parasite species. We hypothesized that competition for host lipid resources would result in reduced emergence of infective nematode larvae (and hence reduced transmission potential) from hosts co-infected with the endemic gregarine parasite. Further, we examined whether endemic infection in this system led to absolute or qualitative changes in lipid levels and fatty acid profiles, which are important indicators of specific processes (e.g. immune response) during infection.
2. Material and methods
(a). Host and gregarine cultures
Cockroaches (B. germanica) were originally obtained from a laboratory supply company (Blades Biologicals Ltd). A subsample of these cultures was found to contain G. blattarum, a pathogen specific to this cockroach species [23]. From these original stocks, parasite-free cultures were founded by oothecae sterilization (in accordance with [28]). All cockroach stocks were reared in 19 l plastic boxes (Really Useful Box Co.) lined with Fluon (Blades Biologicals Ltd) to prevent escape. Each colony was provided with a cardboard egg-box refugia (4 × sheets of 20 cm2), ad libitum food (Tesco Value Complete Dog Food) and dechlorinated water. All cockroaches were maintained at 25±1°C, 30±2% relative humidity, with a 12 L : 12 D photoperiod. Experiments were conducted under the same temperature and light regimes.
(b). Nematode cultures
Steinernema carpocapsae were obtained from Becker Underwood Ltd, and subsequently maintained by passage through cockroach hosts in infection arena comprising a 275 ml plastic pot (Cater For You Ltd; 275 ml volume, 11.5 cm diameter × 7.5 cm height) lined with Fluon, with a dampened sterile sand base initially inoculated with 50 nematodes cm−2 and with an initial 20 cockroaches placed in the arena. Infected cockroaches die within 72 h, and subsequently release new infective larvae onto the sand base. Infection was maintained by weekly seeding of the pots with live gregarine-uninfected cockroaches. Periodically, recently dead cockroaches were placed on White's traps [29] containing 30 ml sterile distilled water to isolate infective juvenile nematodes. For experimental infections, nematodes were collected from the White's traps after 14 days, transferred to 50 ml falcon tubes, topped up to 50 ml using sterile distilled water and stored at 5°C for up to 10 days. The number of emerged larval nematodes was estimated using a Sedgewick–Rafter counting cell under a compound microscope (×40 magnification; Olympus UCC/BY 501).
(c). Food and parasite effects on host lipid levels
(i). Host starvation
The effect of starvation on host lipid levels was assessed in adult female cockroaches over a period of 28 days. Only adult females were chosen for this initial experiment, in order to minimize size variation between individuals (as males and females are sexually dimorphic). Cockroaches (n = 120) were collected from uninfected colonies and placed in groups of five in circular plastic containers (275 ml) lined with Fluon. Half of the cockroaches (n = 60) were fed on ground dog food ad libitum and provided with distilled water, whereas the other half (n = 60) were given distilled water only. At 0, 7, 14, 21 and 28 days, two containers from each treatment were collected and cockroaches found alive at that time point were used for lipid analysis (§2e(i)). Cockroaches that died during the experimental period were excluded from the lipid analysis. A further two containers from each treatment were taken on day 14 and used in the S. carpocapsae emergence experiment (§2d(i)). Only females without oothecae at the beginning of the treatment period were included in the experiment to minimize potential influence of oviposition, which is known to affect lipid levels in cockroaches [16].
(ii). Gregarina blattarum infection
The effect of G. blattarum parasitism on host energy reserves (lipids) was measured in hosts that were taken directly from uninfected (n = 40 females and n = 25 males) and infected colonies (n = 72 females and n = 61 males). More cockroaches were taken from the infected colonies as G. blattarum shows an aggregated distribution in the cockroaches (data not shown). All individuals were anaesthetized with CO2, weighed, and dissected to assess female sexual status (i.e. females with no eggs present, eggs inside the body or oothecae present) and parasite load in the mid-gut. The total lipids were subsequently extracted (§2e(i)). A further group of female cockroaches were collected (n = 15 uninfected and n = 15 infected; both without oothecae) and used to examine the relative abundance of TAG, polar lipids and the fatty acid profiles (§2e(ii)). As the analysis of TAG, polar lipids and fatty acid profiles was conducted on a relatively small sample of cockroaches, only females were used, in order to minimize variation in lipids which was unrelated to the gregarine infection.
(d). The effect of an endemic parasite on Steinernema carpocapsae transmission potential
(i). Host starvation and nematode emergence
To establish the baseline effect of host condition on nematode reproduction, the emergence of infective juvenile nematodes from the fed and starved cockroach groups was determined. Cockroaches collected during the lipid assessment experiment (n = 10 fed and n = 10 starved; §2c(i)) were transferred to a nematode infection arena. The infection arenas were prepared by pipetting a solution of infective stage S. carpocapsae juveniles (5 ml, 50 nematodes cm−2) onto filter paper (90 mm diameter Whatmann) within a Fluon-lined pot. Hosts were exposed to nematodes for 72 h, during which time all the hosts died. At 72 h, the cockroaches were removed from the infection arena and transferred to separate White's traps. The total number of infective juvenile nematodes that emerged from each host were collected after 14 days and counted as described above (§2b).
(ii). Co-infection
To determine the effect of endemic infection on nematode reproduction, female cockroaches (oothecae absent) were collected (n = 24 uninfected and n = 24 G. blattarum infected) and added in groups of six to one of eight nematode infection arenas as described above (§2d(i)). If oothecae started to develop during the experiment, this was noted. After 72 h (in which time all cockroaches had died), the cadavers were transferred to White's traps. Thereafter, every 72 h, for 21 days (after which nematodes cease to emerge from the cadavers), nematodes were collected and counted using a Sedgewick–Rafter counting cell. After each collection, the White's traps were refilled with distilled water.
(e). Lipid analysis
(i). Total lipid extraction
Cockroaches collected for lipid analysis (see §2c(i) and 2c(ii)) were anaesthetized with CO2, weighed and dissected in order to count and remove gregarines from the cockroach gut (see §2c(ii)). The gut was then replaced and total lipid levels were assessed from the whole cockroach following extraction using the method of Marden [30] (see the electronic supplementary material, S1).
(ii). Triacylglycerols, polar lipids and fatty acid profiles
Lipids were extracted from host samples after assessment for parasite load in the mid-gut (n = 15 control and n = 15 infected) and from samples of host diet (n = 3 g × 1 g). Lipids were extracted by a modified Folch method [31] and then separated by one-dimensional thin layer chromatography (see the electronic supplementary materials, S2a). Fatty acid methyl esters (FAMEs) were prepared from total lipids, TAG and polar lipids by transmethylation with 2.5% H2SO4 in dry methanol/toluene (2 : 1, by volume) in accordance with Bychek et al. [32]. The FAMEs were separated using a Clarus 500 gas chromatograph (Perkin–Elmer, Norwalk, CT, USA) fitted with a 30 m × 0.25 mm i.d. capillary column (Elite 225, Perkin Elmer). The amounts of each lipid class were estimated on the basis of fatty acid content using the internal standard. For full details of the lipid extraction and fatty acid profiling, see the electronic supplementary material, S2b.
(f). Statistical analysis
All analyses were conducted in the R statistical package v. 2.15.0 [33]. A list of the six datasets used in these analyses is provided in the electronic supplementary material, S3, all of which are available to view as .csv files in the digital data repository datadryad.org (doi:10.5061/dryad.h37n2). A series of general linear models (GLMs) were used to assess the effects of feeding regime (i.e. fed or starved) and gregarine infection on cockroach lipids and on cockroach fresh weight. Time and host status (i.e. male, female without eggs, female with internal eggs and female with oothecae) were included as independent variables when these were a component of the experimental design. The number of nematode infective stages emerging from host cadavers was assessed by bootstrapping the mean difference between treatments. The bootstrapping method resampled with replacement from each treatment group (i.e. fed, starved, gregarine uninfected or gregarine infected) and produced a mean from that resampled data. The difference between the means of the relevant groups was then calculated (i.e. fed mean minus starved mean; uninfected mean minus infected mean), this procedure was then repeated 10 000 times. Finally, the overall mean and 95% confidence intervals (CIs) of these 10 000 mean differences were calculated. The bootstrapping method was undertaken because preliminary GLM analysis gave rise to non-normal residuals which neither dependent variable transformations nor available error distributions and link functions were able to normalize. Nematode emergence through time from gregarine infected and uninfected hosts was assessed using a generalized linear mixed model (GLMM) using the ASReml-R package (v. 3; VSN International Ltd.). A full description of all starting models is presented in the electronic supplementary material, S4. GLMs were refined using standard stepwise deletions. Stepwise deletion was also used to refine the GLMM by likelihood ratio tests for the random model and the Wald test and the conditional F-statistic for the fixed model.
3. Results
(a). The effect of host food and gregarines on host lipid levels
(i). Host starvation
Total lipid levels of adult female cockroaches were significantly reduced during starvation in interaction with time (figure 1; F1,80 = 15.20, p < 0.001; see the electronic supplementary material, S5 for a full list of coefficients from the models reported here and throughout the results). Mean lipid levels were reduced to less than half of their starting levels (0.22–0.08 mg) in starved cockroaches, whereas in fed groups mean lipid levels more than doubled (0.24–0.55 mg) over the same 28 day period. Similarly, the wet body mass of hosts showed a significant increase in fed groups and a decrease in starved individuals, with the same time-dependent pattern as for lipids (F1,80 = 14.49, p < 0.001; data not shown).
Figure 1.
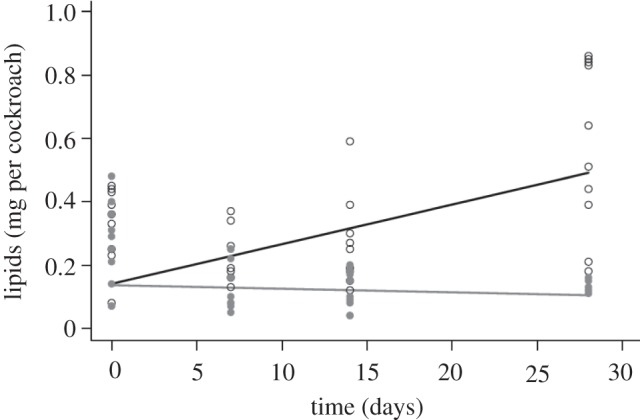
Lipid levels (mg per cockroach) from adult female Blattella germanica, through time, from fed (black line and open circles) and starved (grey line and filled grey circles) treatment groups. Lines and circles denote GLM predictions and raw data, respectively.
(ii). Gregarina blattarum infection
Infection with G. blattarum significantly reduced host lipid levels in interaction with host status (figure 2; F1,175 = 6.97, p < 0.001). Cockroaches of different sexual status (i.e. males, females without eggs, females with internal eggs and females with ootheca) differed in their lipid mass (mg per cockroach) in both infected and uninfected groups, with males having the least lipids and females with internal eggs having the greatest lipid content. However, all cockroaches from infected colonies had significantly lower lipids than their uninfected counterparts for all host sexual status groups. This reduction was greatest in females with oothecae and lowest in females with eggs internally (figure 2).
Figure 2.
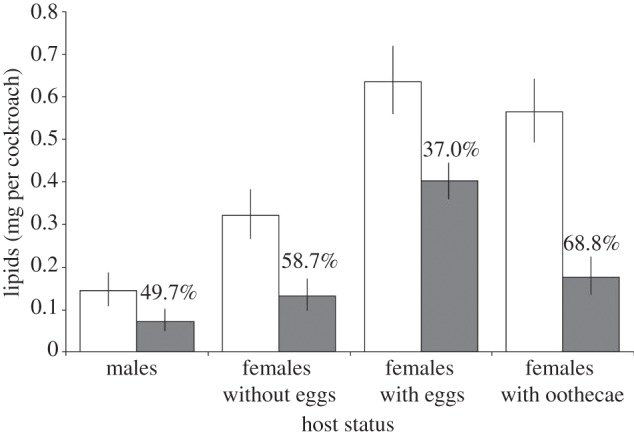
GLM predicted lipid levels (mg) from Gregarina blattarum uninfected (open bars) and infected (filled bars) colonies, for each host status group. Error bars denote 95% confidence intervals (CIs) of the predictions. Values above the bars represent the percentage reduction in lipids between hosts from the infected and uninfected colonies.
Not all cockroaches from gregarine-infected colonies had current infections with G. blattarum. Of the 133 individuals taken from these colonies, 82 (62%) were found to have gregarine trophozoites in the mid-gut. The intensity of infection (counts in infected cockroaches only) was significantly and negatively associated with host lipid levels (see the electronic supplementary material, S6; F1,77 = 16.25, p < 0.001). Host sexual status was also important in this model (electronic supplementary material, S6; F3,77 = 67.91, p < 0.001) but did not interact with the trophozoite count, and therefore the relationship between infection intensity and lipid levels remains constant across sexual status groups. However, mean gregarine intensity did differ between host status groups (geometric mean trophozoite intensity: males = 21.9, female without eggs = 34.5, females with internal eggs = 30.4 and females with oothecae = 25.0), which accounts for the absolute differences in lipids observed between the status groups (figure 2).
(iii). Quantification of triacylglycerols, polar lipids and fatty acid profiles
Both the quantity of TAG and polar lipids were lower in infected hosts. Levels of TAG were reduced by 33% (F1,28 = 5.87, p < 0.05) and polar lipids by 25% compared with uninfected controls (F1,28 = 8.62, p < 0.01). Cockroach wet body mass was also significantly lower in the infected group (F1,28 = 4.22, p < 0.05). However, despite the reduction in the amounts of TAG and polar lipids, the fatty acid profiles of the cockroach lipid groups were unchanged by infection. Eight major fatty acids were detected in TAG (figure 3a), the most abundant of which were oleate C18 : 1n9 (48.2%), palmitate C16 : 0 (30.1%) and linoleate C18 : 2n6 (9.5%). While eight fatty acids were detected in the polar groups, the profiles differed with respect to the identity and quantity of the fatty acids (figure 3b), notably with less palmitate and relatively more linoleate. All the main fatty acids present in the host diet (figure 3c) were found in the cockroach lipid fractions, with the exception of a C20 : ln9 group. The cockroach diet contained two fatty acids that were absent from the host TAG and polar lipid profiles (C18 : ln7 and C20 : ln9), while arachidonic acid (C20 : 0) present in polar lipids was not present in the host diet.
Figure 3.
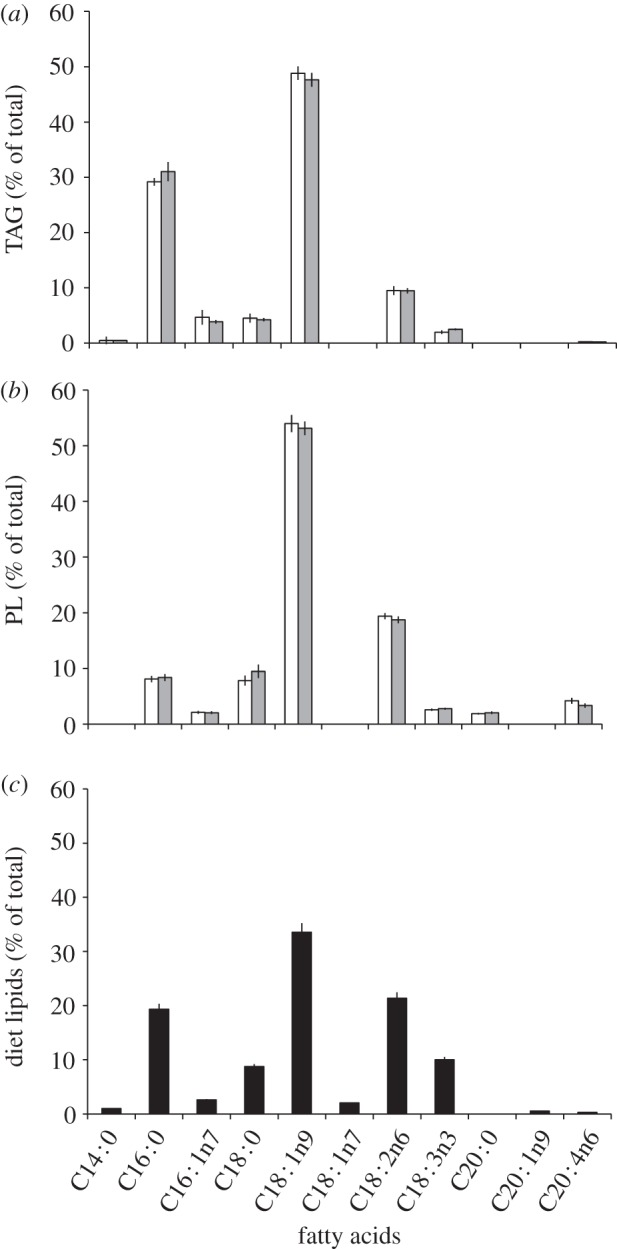
The mean fatty acid profiles of (a) TAG and (b) polar lipids (PL) from G. blattarum uninfected (open bars) and infected (grey bars), and (c) the total fatty acid profile from host diet (black columns). Error bars denote 95% CIs. Shorthand nomenclature for the fatty acids is shown with the number before the colon indicating the number of carbons and the figure after representing the number of double bonds. The position of the first double bond is also shown as the number after the n.
(b). Gregarina blattarum infection reduces reproductive output of Steinernema carpocapsae
The bootstrapped mean difference in nematode emergence between fed and starved hosts was 10 259 (upper 95% CI = 22 025 and lower 95% CI = 75). Infective juvenile emergence was 76% lower from hosts that were starved prior to nematode infection, compared with hosts that had been fed ad libitum.
Nematode emergence was significantly and substantially lower from G. blattarum infected hosts compared with hosts without the endemic co-infection (reduction in cumulative emergence 50.25%). This relationship was nonlinear and dependent on time (i.e. in interaction with the spline fit of day; likelihood ratio: p < 0.001 on 1 d.f.). Nematodes began to emerge after the third day of infection, increasing to a peak at 10 days (gregarine-uninfected peak = approx. 11 000 nematodes ml−1, gregarine co-infected peak approx. 5200 nematodes ml−1) and then declined, with cessation of emergence by day 21. There were only subtle differences in the time-specific pattern of nematode emergence between gregarine-free and gregarine-infected hosts with no visible change in peak emergence (figure 4). The code for arena and individual host identification were retained in the random model to control for pseudo-replication.
Figure 4.
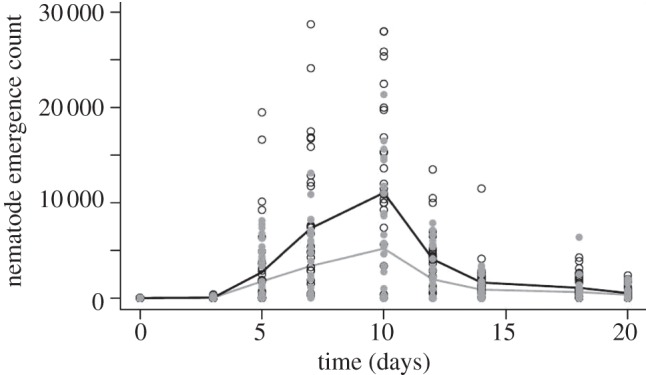
Number of Steinernema carpocapsae infective juveniles emerging from gregarine-free (black line and open circles) and gregarine-infected (grey line and filled grey circles) female cockroaches through time. Lines represent GLM predicted values and circles represent raw data.
4. Discussion
Parasites, particularly low-virulence, endemic gastrointestinal worms and protozoa, are extremely common in nature [1,2]. As a result, hosts already infected with endemic infections are likely to be present during outbreaks of any epidemic disease. Much current research aims to determine how different parasitic taxa interact during co-infections in natural systems to bring about changes to host fitness and population dynamics [34–36]. It is expected that many of the interactions are driven by resource–competition in the host. Here, we have demonstrated, to our knowledge, for the first time that exploitation of stored energy resources by an endemic parasite has a negative effect on the reproduction, and therefore transmission capacity of an epidemic parasite. The endemic parasite G. blattarum caused a substantial reduction in host total lipids (37–68.8%), which was a consequence of the reduction in both TAG and polar lipids, with the variation in lipid reduction related to the sex and reproductive status of the host cockroach. Gregarine infection was further associated with a substantial reduction in the emergence of infective larvae of the epidemic parasitic nematode, S. carpocapsae (50.25%). The timing of emergence did not differ markedly between gregarine-infected and -uninfected hosts, although the significance of the nonlinear interaction with time (spline fit of day) demonstrates that there is a subtle difference in the shape of the nematode emergence curves between the two groups (figure 4). Given that it is the depletion of host resources that stimulates the nematodes to change their reproduction over to the production of the infective larval stage, this lack of difference is perhaps surprising. However, it is possible that because of the timing of our larval sampling (with each sample a minimum of 2 days apart), we could have missed a subtle shift in the beginning of emergence, which occurred sometimes between day 3 and 5. Similarly, there was no measurement taken between day 7 and 10 and the peak of larval emergence, which appears to occur at day 10, could have been slightly earlier for one or both groups of cockroach. However, as larvae accumulated in the samples between time points, the difference in the counts of larvae emerging remains unaffected by this potential sampling bias.
The reduction in host lipid levels associated with G. blattarum infection may occur as a result of processes common to gastrointestinal parasites. For example, the occurrence of tapeworms in ruminants can lead to anorexia and weight loss as a result of decreased food consumption [37,38]. German cockroach females with oothecae have been shown to reduce movement and food intake [39], hence during this period, the costs of gregarine parasitism are likely to be exacerbated by the host's inability to replace lost lipids. The costs of gregarine infection were comparable to an extended period of starvation, where lipids were reduced by 65.6% after 28 days (figure 1). However, in other work (not shown), we found cockroaches did not alter their food consumption rates when infected with gregarines and so the inappetance sometimes associated with gastrointestinal infections does not seem to play a direct role here. Alternatively, the presence of parasites in the gut may prevent the passage of nutrients and decrease absorption by microvilli in the intestinal cell membrane. Takahashi et al. [25] observed that attachment of the gregarine parasite Cephaloidophora pacifica to the host intestinal cells, was associated with damage to microvilli in the epithelium of its krill host Euphausia superba, which might also affect lipid absorption from the gut. Given that gregarines are large parasites, which are just visible with the naked eye upon dissection of the mid-gut (approx. 170 × 80 µM [23]) and that we have observed them in aggregations that can completely fill the gut lumen, it is plausible that reduced fatty acid absorption may restrict the assimilation and storage of lipids in the fat body.
A reduction in host lipids might also occur as a result of metabolic activities associated with the host–parasite interaction. Gregarines attach to the cell membrane, which is expected to provide a stable anchor for growth and a mechanism for obtaining host nutrients [23,24]. Yet, little is known about precisely which resources are taken from the host, or whether the parasites may also obtain resources that are taken directly from the food available in the host's mid-gut lumen. Alternatively, lipid reduction in gregarine parasitized hosts may be an indirect consequence of increased host metabolism resulting from such mechanisms either as a raised immune response [40] or tissue repair [25]. Although we observed a total reduction in lipid levels within gregarine-infected hosts, we observed no difference in the fatty acid profiles compared with uninfected controls. A change in fatty acid profile could have indicated either differential use of lipids by the parasite, a shift in resource allocation by the host, or a fatty acid-specific reduced absorption from the mid-gut, none of which seem to have occurred here. On the other hand, the fatty acid profile of the diet did differ from the profile of the cockroach, highlighting biosynthesis of fatty acids by the insect when those acids are absent from the diet (e.g. arachidonic acid).
Lipid reduction in gregarine-infected hosts has the potential to affect the nematodes beyond the simple reduction in the number of infective juveniles; resource provisioning of the emerging infective larvae might also be affected. In the latter stages of infection, host resources reach low levels within the cockroach as they are used by the nematodes. As the nutrient levels begin to fall, there is a shift in the development of the nematode offspring to transmissible third stage (L3) infective juveniles. These juveniles are non-feeding and so are dependent on the lipids that they acquire as L1 and L2 stage larvae prior to development into L3. Nematodes, particularly ambush (‘sit and wait’) parasites like S. carpocapsae, may have to survive for long periods in the environment [13,41]. Indeed, lipid levels were shown to reduce to just 10% of their original levels in S. carpocapsae maintained at 25°C for 60 days [42]. Nematode transmission from hosts that are infected with the endemic infection could therefore be reduced not only because of the substantial drop in emerging transmission stages but also potentially by the fitness of those larvae whose ability to store resources may be constrained by gregarine infection; this warrants further investigation.
A major branch of epidemiological research focuses on trying to predict the likely transmission dynamics of epidemic parasites. Here, we have demonstrated that a within-host interaction between endemic and epidemic parasite species can have substantial effects on the epidemic parasite's transmission potential. Further, we have demonstrated a resource-mediated mechanism for this interaction. Given the ubiquity of endemic infections in natural systems and subsequent likelihood of epidemic–endemic co-infections, our results indicate that endemic infection must be a consideration when assessing the risk and predicting the dynamics of epidemic spread.
Acknowledgements
We thank Becker Underwood for the consistent supply of nematodes for the study.
Funding statement
This work was financially supported by a Natural Environmental Research Council (NERC; NE/G523420) UK studentship to J.R.
References
- 1.Shaw DJ, Dobson AP. 1995. Patterns of macroparasite abundance and aggregation in wildlife populations: a quantitative review. Parasitology 111, S111–S133 (doi:10.1017/S0031182000075855) [DOI] [PubMed] [Google Scholar]
- 2.Wang E, Haolin N, Xu R, Barrett AD, Watowich SJ, Gubler DJ, Weaver SC. 2000. Evolutionary relationships of endemic/epidemic and sylvatic dengue viruses. J. Virol. 74, 3227–3234 (doi:10.1128/JVI.74.7.3227-3234.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson RM, May RM. 1979. Population biology of infectious diseases. I. Nature 280, 361–367 (doi:10.1038/280361a0) [DOI] [PubMed] [Google Scholar]
- 4.Viney M, Cable J. 2011. Macroparasite life histories. Curr. Biol. 21, R767–R774 (doi:10.1016/j.cub.2011.07.023) [DOI] [PubMed] [Google Scholar]
- 5.Fenton A. 2008. Worms and germs: the population dynamic consequences of microparasite-macroparasite co-infection. Parasitology 135, 1545–1560 (doi:10.1017/S003118200700025X) [DOI] [PubMed] [Google Scholar]
- 6.Heffernan JM, Smith RJ, Wahl LM. 2005. Perspectives on the basic reproductive ratio. J. R. Soc. Interface 2, 281–293 (doi:10.1098/rsif.2005.0042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seppälä O, Liljeroos K, Karvonen A, Jokela J. 2008. Host condition as a constraint for parasite reproduction. Oikos 117, 749–753 (doi:10.1111/j.0030-1299.2008.16396.x) [Google Scholar]
- 8.Tocque K. 1993. The relationship between parasite burden and host resources in the desert toad (Scaphiopus couchii), under environmental conditions. J. Anim. Ecol. 62, 683–693 (doi:10.2307/5388) [Google Scholar]
- 9.Booth DT, Clayton DH, Block BA. 1993. Experimental demonstration of the energetic cost of parasitism in free-ranging hosts. Proc. R. Soc. Lond. B 253, 125–129 (doi:10.1098/rspb.1993.0091) [Google Scholar]
- 10.Tocque K, Tinsley RC. 1994. The relationship between Pseudodiplorchis americanus (Monogenea) density and host resources under controlled environmental conditions. Parasitology 108, 175–183 (doi:10.1017/S003118200006827X) [DOI] [PubMed] [Google Scholar]
- 11.Norton A, Rollinson D, Richards L, Webster J. 2008. Simultaneous infection of Schistosoma mansoni and S. rodhaini in Biomphalaria glabrata: impact on chronobiology and cercarial behaviour . Parasites Vectors 1, 43–52 (doi:10.1186/1756-3305-1-43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohr JN, Mingbo Y, Wolinska J. 2010. Prior residency does not always pay off: co-infections in Daphnia. Parasitology 137, 1493–1500 (doi:10.1017/S0031182010000296) [DOI] [PubMed] [Google Scholar]
- 13.Adams B, Nguyen K. 2001. Taxonomy and systematics. In Entomopathogenic nematology (ed. Gaugler R.), pp. 2–34 Wallingford, UK: CABI Publishing [Google Scholar]
- 14.Nelson DR, Terranova AC, Sukkestad DR. 1967. Fatty acid composition of glyceride and free fatty acid fractions of fat body and hemolymph of cockroach Periplaneta americana (L). Comp. Biochem. Physiol. 20, 907–917 (doi:10.1016/0010-406X(67)90062-X) [Google Scholar]
- 15.Schal C, Sevala VL, Young HP, Bachmann JAS. 1998. Sites of synthesis and transport pathways of insect hydrocarbons: cuticle and ovary as target tissues. Am. Zool. 38, 382–393 (doi:10.1093/icb/38.2.382) [Google Scholar]
- 16.Ross M, Mullins D. 1995. Biology of the German cockroach. In Understanding and controlling the German cockroach (ed. Rust M.), pp. 21–41 Oxford, UK: Oxford University Press [Google Scholar]
- 17.Baldus TJ, Mutchmor JA. 1988. The effects of temperature acclimation on the fatty acid composition of the nerve cord and fat body of the American cockroach, Periplaneta americana. Comp. Biochem. Physiol. 89, 141–147 (doi:10.1016/0300-9629(88)91071-7) [Google Scholar]
- 18.Zhao ZW, Zera AJ. 2002. Differential lipid biosynthesis underlies a tradeoff between reproduction and flight capability in a wing-polymorphic cricket. Proc. Natl Acad. Sci. USA 99, 16 829–16 834 (doi:10.1073/pnas.262533999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yajima M, Takada M, Takahashi N, Kikuchi H, Natori S, Oshima Y, Kurata S. 2003. A newly established in vitro culture using transgenic Drosophila reveals functional coupling between the phospholipase A(2)-generated fatty acid cascade and lipopolysaccharide-dependent activation of the immune deficiency (imd) pathway in insect immunity. Biochem. J. 371, 205–210 (doi:10.1042/BJ20021603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciancio A, Scippa S, Cammarano M. 2001. Ultrastructure of trophozoites of the gregarine Lankesteria ascidiae (Apicomplexa: Eugregarinida) parasitic in the ascidian Ciona intestinalis (Protochordata). Eur. J. Protistol. 37, 327–336 (doi:10.1078/0932-4739-00829) [Google Scholar]
- 21.Simdyanov TG. 1996. The morphology and ultrastructure of the gregarine Loxomorpha harmothoe from the White Sea. Parazitologiya (St Petersburg) 30, 174–177 [Google Scholar]
- 22.Desai RN, Nadkarni VB. 1987. Studies on steroidogenic potential of the gregarine Stylocephalus conoides Devdhar, 1962 (Protozoa: Sporozoa). A cytochemical study. Arch. Protistenkd. 133, 301–308 (doi:10.1016/S0003-9365(87)80063-5) [Google Scholar]
- 23.Clopton RE, Gold RE. 1996. Host specificity of Gregarina blattarum von Siebold, 1839 (Apicomplexa: Eugregarinida) among five species of domiciliary cockroaches. J. Invertebr. Pathol. 67, 219–223 (doi:10.1006/jipa.1996.0036) [DOI] [PubMed] [Google Scholar]
- 24.Simdyanov TG, Kuvardina ON. 2007. Fine structure and putative feeding mechanism of the archigregarine Selenidium orientale (Apicomplexa: Gregarinomorpha). Eur. J. Protistol. 43, 17–25 (doi:10.1016/j.ejop.2006.09.003) [DOI] [PubMed] [Google Scholar]
- 25.Takahashi KT, Kawaguchi S, Toda T. 2009. Observation by electron microscopy of a gregarine parasite of Antarctic krill: its histological aspects and ecological explanations. Polar Biol. 32, 637–644 (doi:10.1007/s00300-008-0563-4) [Google Scholar]
- 26.Cowles CE, Goodrich-Blair H. 2008. The Xenorhabdus nematophila nilABC genes confer the ability of Xenorhabdus spp. to colonize Steinernema carpocapsae nematodes. J. Bacteriol. 190, 4121–4128 (doi:10.1128/JB.00123-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andaló V, Moino A, Jr, Maximiniano C, Campos CP, Mendonça LA. 2011. Influence of temperature and duration of storage on the lipid reserves of entomopathogenic nematodes. Rev. Colomb. Entomol. 37, 203–209 [Google Scholar]
- 28.Müller-Graf CDM, Jobet E, Cloarec A, Rivault C, van Baalen M, Morand S. 2001. Population dynamics of host-parasite interactions in a cockroach-oxyuroid system. Oikos 95, 431–440 (doi:10.1034/j.1600-0706.2001.950308.x) [Google Scholar]
- 29.White G. 1927. A method for obtaining infective nematode larvae from cultures. Science 66, 302–303 (doi:10.1126/science.66.1709.302-a) [DOI] [PubMed] [Google Scholar]
- 30.Marden JH. 1989. Bodybuilding dragonflies: costs and benefits of maximizing flight muscle. Physiol. Zool. 62, 505–521 [Google Scholar]
- 31.Garbus J, Deluca HF, Loomans ME, Strong FM. 1963. Rapid incorporation of phosphate into mitochondrial lipids. J. Biol. Chem. 238, 59–63 [PubMed] [Google Scholar]
- 32.Bychek EA, Dobson GA, Harwood JL, Guschina IA. 2005. Daphnia magna can tolerate short-term starvation without major changes in lipid metabolism. Lipids 40, 599–608 (doi:10.1007/s11745-005-1421-1) [DOI] [PubMed] [Google Scholar]
- 33.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/ [Google Scholar]
- 34.Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M. 2010. Species interactions in a parasite community drive infection risk in a wildlife population. Science 330, 243–246 (doi:10.1126/science.1190333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cattadori IM, Boag B, Hudson PJ. 2008. Parasite co-infection and interaction as drivers of host heterogeneity. Int. J. Parasitol. 38, 371–380 (doi:10.1016/j.ijpara.2007.08.004) [DOI] [PubMed] [Google Scholar]
- 36.Lello J, Boag B, Fenton A, Stevenson IR, Hudson PJ. 2004. Competition and mutualism among the gut helminths of a mammalian host. Nature 428, 840–844 (doi:10.1038/nature02490) [DOI] [PubMed] [Google Scholar]
- 37.Coop RL, Holmes PH. 1996. Nutrition and parasite interaction. Int. J. Parasitol. 26, 951–962 (doi:10.1016/S0020-7519(96)80070-1) [DOI] [PubMed] [Google Scholar]
- 38.Stien A, Irvine RJ, Ropstad E, Halvorsen O, Langvatn R, Albon SD. 2002. The impact of gastrointestinal nematodes on wild reindeer: experimental and cross-sectional studies. J. Anim. Ecol. 71, 937–945 (doi:10.1046/j.1365-2656.2002.00659.x) [Google Scholar]
- 39.Lee HJ, Wu YL. 1994. Mating effects on the feeding and locomotion of the German cockroach, Blattella germanica. Physiol. Entomol. 19, 39–45 (doi:10.1111/j.1365-3032.1994.tb01071.x) [Google Scholar]
- 40.Lafferty K, Kuris A. 2005. Parasitism and environmental disturbances. In Parasitism and ecosystems (eds Thomas F, et al.), pp. 113–123 Oxford, UK: Oxford University Press [Google Scholar]
- 41.Selvan S, Gaugler R, Lewis EE. 1993. Biochemical energy reserves of entomopathogenic nematodes. J. Parasitol. 79, 167–172 (doi:10.2307/3283503) [Google Scholar]
- 42.Wright DJ, Grewal PS, Stolinski M. 1997. Relative importance of neutral lipids and glycogen as energy stores in dauer larvae of two entomopathogenic nematodes, Steinernema carpocapsae and Steinernema feltiae. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 118, 269–273 (doi:10.1016/S0305-0491(97)00165-X) [DOI] [PubMed] [Google Scholar]


