Abstract
Trade of ornamental aquatic species is a multi-billion dollar industry responsible for the introduction of myriad fishes into novel ecosystems. Although aquarium invaders have the potential to alter ecosystem function, regulation of the trade is minimal and little is known about the ecosystem-level consequences of invasion for all but a small number of aquarium species. Here, we demonstrate how ecological stoichiometry can be used as a framework to identify aquarium invaders with the potential to modify ecosystem processes. We show that explosive growth of an introduced population of stoichiometrically unique, phosphorus (P)-rich catfish in a river in southern Mexico significantly transformed stream nutrient dynamics by altering nutrient storage and remineralization rates. Notably, changes varied between elements; the P-rich fish acted as net sinks of P and net remineralizers of nitrogen. Results from this study suggest species-specific stoichiometry may be insightful for understanding how invasive species modify nutrient dynamics when their population densities and elemental composition differ substantially from native organisms. Risk analysis for potential aquarium imports should consider species traits such as body stoichiometry, which may increase the likelihood that an invasion will alter the structure and function of ecosystems.
Keywords: aquarium trade, Loricariidae, nutrient remineralization, invasive species, phosphorus
1. Introduction
Global trade of live organisms is a multi-billion-dollar industry that supports economies throughout the world; yet species invasions are often the inadvertent consequences of commerce. The costs invasions impose frequently outweigh the economic benefits derived from the industry [1]. For example, while sales of nursery and greenhouse-grown plants grossed almost $16 billion in 2004 in the USA [2], the economic losses suffered after ornamental plant introduction and spread were estimated to be more than double this value [3]. This paradox is universal as policy-makers attempt to stimulate economic activities, while minimizing the threats imposed by species invasion [1,2]. More than one billion wild-caught and captive-bred fishes were traded through more than 100 countries in 2000 [4]. Of the freshwater fishes, approximately 90% of the entire trade volume comprises a relatively small number of species [5]. The most popular fishes sold in the aquarium trade are the most likely to become introduced and established in freshwater habitats [6]. Yet little is known about the potential effects of these aquarium invaders on ecosystem processes in freshwater systems. By identifying traits, such as body and dietary stoichiometry, that characterize invaders with the potential to modify ecosystem processes, policy-makers may design species-specific import restrictions for organisms posing threats to ecosystem function.
Fishes can play important roles in freshwater ecosystem function via nutrient sequestration in body tissues [7,8] and nutrient remineralization via excretion and egestion [9–11]. The strength of these interactions is often regulated by the amount and ratio of elements stored in and cycled through fishes [12]. Ecological stoichiometry, or the ratio of elements in ecological processes [13], is a useful framework to employ when evaluating the potential effects of non-native, invasive species on nutrient dynamics in invaded freshwater ecosystems. There is a wide range of body and diet stoichiometries among freshwater fishes [11,12,14], which mediates the amount of food consumed and the waste products produced by a species, thereby influencing ecosystem-wide resource availability and nutrient dynamics [12]. The effect of organismal stoichiometry on nutrient dynamics may be nutrient-specific [15]. Therefore, an invader with high requirements for one element may selectively sequester that element in body tissues relative to other elements. In other words, a stoichiometrically unique invader may function as a net nutrient sink for one element, while functioning as a net remineralizer for other elements, thereby altering nutrient cycling patterns in invaded habitats.
To understand and mitigate the effects of introduced species on ecosystem function, it is imperative to document changes in ecosystem processes after invasion. We argue that ecological stoichiometry could be employed to help predict which species have the potential to exert strong influences on ecosystem structure and function, and allow policy-makers to initiate targeted actions to restrict the import of specific organisms. Such activities may be especially important in developing economies where human populations are often directly dependent upon native biodiversity for their food sources and income [16,17], and where the primary (and sometimes only) action taken to control invasive species is to ban potentially harmful species prior to import [18].
Here, we examine the influence of the sailfin catfish (Loricariidae: Pterygoplichthys) on nutrient storage and cycling after introduction to a nutrient-limited river system in southern Mexico. Sailfin catfish and other loricariids are bottom-dwelling fishes native to South America, Panama and Costa Rica [19,20]. Relative to other fish families, loricariids are phosphorus (P)-rich due to bony-plated armour covering their bodies [11,12,21]. Loricariids are among the most popular freshwater fishes sold in the aquarium trade, where they are marketed as ‘plecos’ or ‘algae eaters’, and have become established in freshwater bodies throughout the globe [22,23]. Non-native Pterygoplichthys were first documented in Chiapas, Mexico in 2004, where they have been linked to the collapse of small-scale fisheries [24,25].
Employing stoichiometric theory, we predicted the high population density of non-native loricariids coupled with their unique stoichiometry would have significant effects on nutrient remineralization and storage in a nutrient-limited system. We began by documenting the density and biomass of loricariids, and measuring their body carbon (C), nitrogen (N) and P content to estimate nutrient storage rates. Second, we estimated the nutrient remineralization rates of loricariids and dominant native fish species and compared them with in-stream nutrient uptake rates. Finally, we used both storage and remineralization rates to estimate whether loricariids were functioning as net sinks or net remineralizers of nutrients. We predicted high densities of loricariids would function as a net remineralizer of N, but would act as a net sink of P relative to their remineralization rates within the invaded system because of their high P demand.
2. Methods
(a). Study site
The fieldwork for this study was conducted in the Chacamax River (17°29′047″ N, 91°58′430″ W) in Chiapas, Mexico during the dry season months of March–May 2008–2010. The native fish assemblage found in the Chacamax River during the study period was similar to species assemblages found in uninvaded streams in the region [26]. Ambient nutrient concentrations in the study reaches were moderate to low (average values: NH4+–N, 10 µg l−1; NO3−–N, 353 µg l−1; total dissolved nitrogen, 387 µg l−1; soluble reactive P, less than 2 µg l−1; total dissolved phosphorus, 3 µg l−1). Stream discharge averaged approximately 1600 l s−1 throughout the study. We employed nutrient diffusing substrata using methods outlined by Capps et al. [27] in 2008 and 2009, to estimate nutrient limitation of primary producers. We determined that primary producers in the river were P-limited.
(b). Fish biomass and remineralization
To estimate the density and areal biomass of Pterygoplichthys and native fishes in the study site, we counted fishes along transects in a 550 m reach of stream using methods modified from Thurow [28]. Loricariids were identified as small (less than 15 cm standard length (SL)), medium (15–25 cm SL) or large (more than 25 cm SL). Five whole individual fishes from each species were collected for C, N, P analysis using standard electroshocking (ABP-3-600 Electrofishing Backpack System, Electrofishing, LLC, Verona, WI) and seining techniques [29,30], and fish were euthanized using an overdose of MS-222. To compare the density and estimate the biomass of Pterygoplichthys with native fishes, we conducted two snorkelling surveys on two dates (10 March 2010 and 5 May 2010) using the aforementioned methods. Carbon, N and P storage rates were calculated as the change in the product of the areal biomass estimates and percentage element in the fish tissue samples between 2008 and 2010.
Fish nutrient recycling rates were estimated based on the difference in dissolved N and P concentrations between plastic tubs incubated with and without fishes using standard methods that accounted for matrix effects [10,11,31]. After collection, we immediately incubated five individuals from the seven most common native fish genera (Astyanax, Monopterus, Poecillia, Rhamdia, Theraps, Thorichthys and Vieja) and five Pterygoplichthys in 10 l plastic tubs with 2–7 l of stream water for 1 h. At the end of the incubation, we collected water samples for NH4+ and soluble reactive phosphorus (SRP) analysis. Areal excretion estimates were calculated as the product of the areal biomass estimates and the mass-based excretion rates for each species [10]. Water samples were filtered through glass-fibre filters (Gelman A/E) to remove faeces and other particles. Water samples collected for P analysis were acidified with 2N H2SO4 (less than pH 2) for preservation and shipped to the USA for analysis. We employed standard colorimetric methods to analyse P samples [32] using a Lachat QuickChem 8000 (Lachat Instruments, Loveland, CO). All NH4+ samples were refrigerated and analysed in the field using the fluorometric methods outlined by Taylor et al. [31].
(i). Nutrient dynamics
Nutrient demand of primary producers and the microbial community was estimated using nutrient additions in 2010 after methods outlined by Hall & Tank [33]. Briefly, we measured NH4+ and SRP uptake in the river in April 2010 by conducting two additions of NH4Cl and two additions of KH2PO4 using NaBr as a conservative tracer. Uptake was calculated using the formula: ln Nx = ln No – ax, where No and Nx were the nutrient concentrations at the addition site and x m downstream from the addition site, and a was the uptake rate per m2 [34]. We also calculated excretion turnover distance, the distance required for excretion to completely turn over the ambient nutrient pool, using methods outlined by Benstead et al. [35]. To estimate whether loricariids were acting as net sinks or net remineralizers of nutrients, we subtracted the amount of nutrients produced via areal nutrient remineralization from the amount of nutrients stored in loricariid tissues through growth between 2008 and 2010.
(ii). Statistical analysis
We performed linear regressions with and without Pterygoplichthys to estimate the change in cross-species relationships between nutrient excretion and body mass, and nutrient excretion and body nutrient content after Pterygoplichthys invasion (see electronic supplementary material, table S1). We analysed the effect of taxon on fish body nutrient concentration and fish excretion rate (per gram of fish) using a generalized linear model (PROC GLM), with Tukey's adjustment for multiple comparisons. All data were log10-transformed to address non-uniform variance. Fish body and excretion data were analysed using SAS v. 9.2 (SAS Institute, 2010).
3. Results
Loricariids attained a high areal biomass (230 g Pterygoplichthys m−2), at least two orders of magnitude greater than the native fish biomass (1.42 g native fishes m−2; figures 1 and 2). Pterygoplichthys biomass increased by approximately 100 g m−2 per year between 2008 and 2010 (figure 2). Loricariids had significantly lower body C (p < 0.0001, F7,32 = 10.54; electronic supplementary material, figure S1A) and N concentrations (p < 0.0001, F7,32 = 27.55; electronic supplementary material, figure S1B) relative to the other fishes we examined (C mean: 32% versus 41% and N mean: 8% versus 11%, respectively). As predicted, Pterygoplichthys were almost twice as rich in P (mean: 5.7%) relative to the other fishes (mean: 3.3%) sampled (p < 0.0001, F7,32 = 12.01; electronic supplementary material, figure S1C), which yielded significantly lower molar C : P (p < 0.0001, F7,32 = 12.63; electronic supplementary material, figure S1E) and N : P (p < 0.0001, F7,32 = 19.59; electronic supplementary material, figure S1F) than the native fish species. Thus, in 2010, loricariids sequestered approximately 11.5 g m−2 P, whereas native fishes sequestered an average of 0.05 g m−2 P in the Chacamax.
Figure 1.

Pterygoplichthys in the Chacamax River (17°29′047″ N, 91°58′430″ W). (a) Individual Pterygoplichthys, (b) underwater close-up of part of a Pterygoplichthys aggregation, (c) surface photo of Pterygoplichthys aggregations denoted by the black dotted lines. (Online version in colour.)
Figure 2.
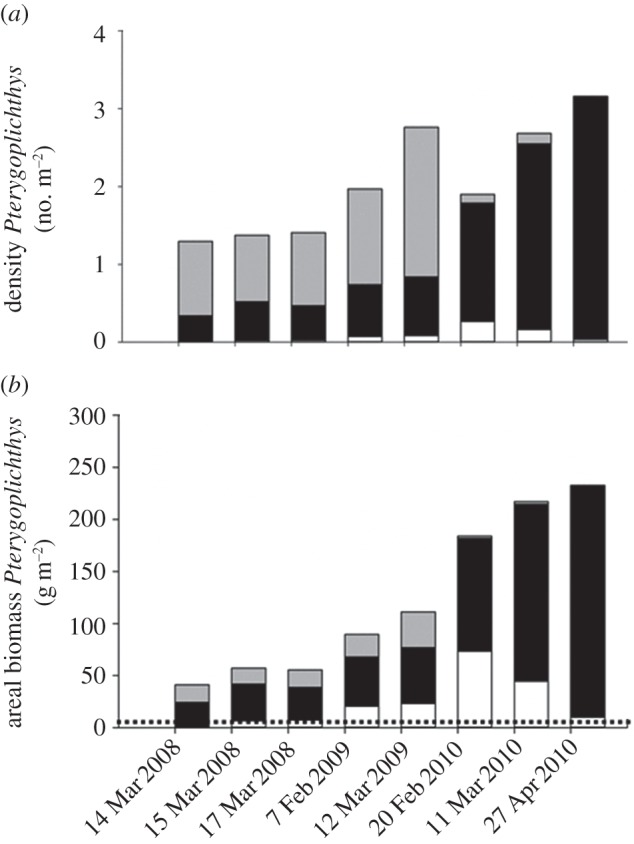
Pterygoplichthys density and biomass in the Chacamax River (17°29′047″ N, 91°58′430″ W). (a) Density of Pterygoplichthys. (b) Areal biomass of Pterygoplichthys. Small fish (less than 15 cm SL) are represented in grey, medium fish (15–25 cm SL) in black, and large fish (more than 25 cm SL) in white. The dashed line denotes native fish biomass in 2010 (1.42 g m−2).
Pterygoplichthys excreted significantly less N per gram of body mass compared with all other genera measured except the swamp eel, Synbranchidae: Monopterus sp. (p < 0.0001, F7,32 = 20.83; electronic supplementary material, figure S2). Phosphorus excretion per gram of Pterygoplichthys was less than the characid, Astyanax aeneus, the molly, Poecilia mexicana and the native pimelodid catfish, Rhamdia guatemalensis, but did not significantly differ from the native cichlids (Vieja sp., Cichlasoma sp., Theraps sp.) or the swamp eel (Monopterus sp.) we sampled (p < 0.0001, F7,32 = 23.50). Despite differences in body stoichiometry, nutrient remineralization ratios of Pterygoplichthys differed only from two species sampled (p < 0.0001, F7,32 = 23.50), whereby the N : P excretion ratio for Pterygoplichthys was significantly less than the cichlid, Theraps sp., and significantly greater than Astyanax aeneus (see electronic supplementary material, figure S2). Areal excretion estimates showed that loricariids excreted approximately 25 times the amount of N (191 versus 7.5 µmol N m−2 h−1) and P (4.5 versus 0.18 µmol P m−2 h−1) than native fishes.
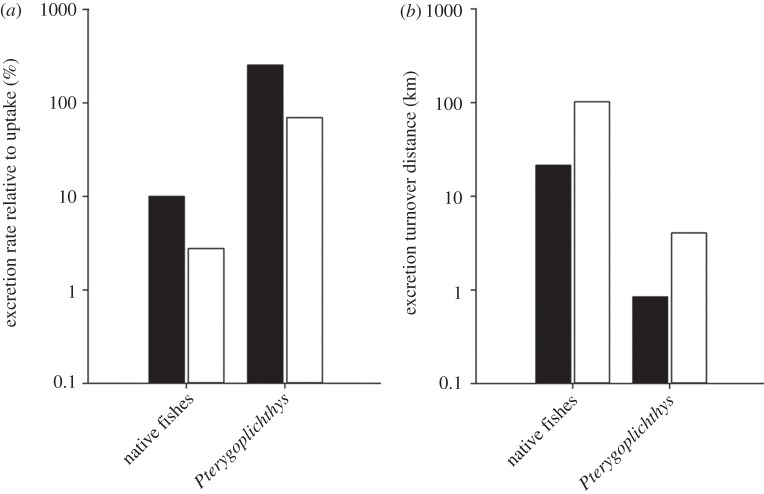
Nutrient uptake rates in the Chacamax were approximately 75 µmol NH4+–N m−2 h−1 and 7 µmol PO4−3–P m−2 h−1. Consequently, loricariid excretion was equivalent to approximately 255% of the NH4+ and 70% of the P demand in the Chacamax. By contrast, excretion by native fishes was equivalent to approximately 10% of the NH4+ and 3% of the P demand (figure 3a). Moreover, loricariids dramatically reduced the fish excretion turnover distance of NH4+ and P from 21 (native fishes) to 0.8 km (loricariids) for NH4+ and 102 (native fishes) to 4.06 km (loricariids) for P (figure 3b). To estimate the net effects of loricariids on nutrient dynamics, we subtracted the nutrients loricariids remineralized (see electronic supplementary material, figure S2; 191 µmol N m−2 h−1 and 7.5 µmol P m−2 h−1) from the nutrients sequestered in loricariid tissues through growth between 2008 and 2010 (figure 2; 60 µmol N m−2 h−1 and 20 µmol P m−2 h−1). The net effect of loricariids on nutrient dynamics was element-dependent; loricariids were net remineralizers of N (131 µmol N m−2 h−1), but at the same time sequestered P through growth (12.5 µmol P m−2 h−1; figure 4).
Figure 3.
(a) Nutrient excretion rate relative to stream nutrient uptake (%) over a 100 m reach in the Chacamax River. (b) Excretion turnover distance (in kilometres) by loricariids and native fishes in the Chacamax River. Note the y-axes are on log scales. Black bars denote NH4+ and white bars denote SRP.
Figure 4.
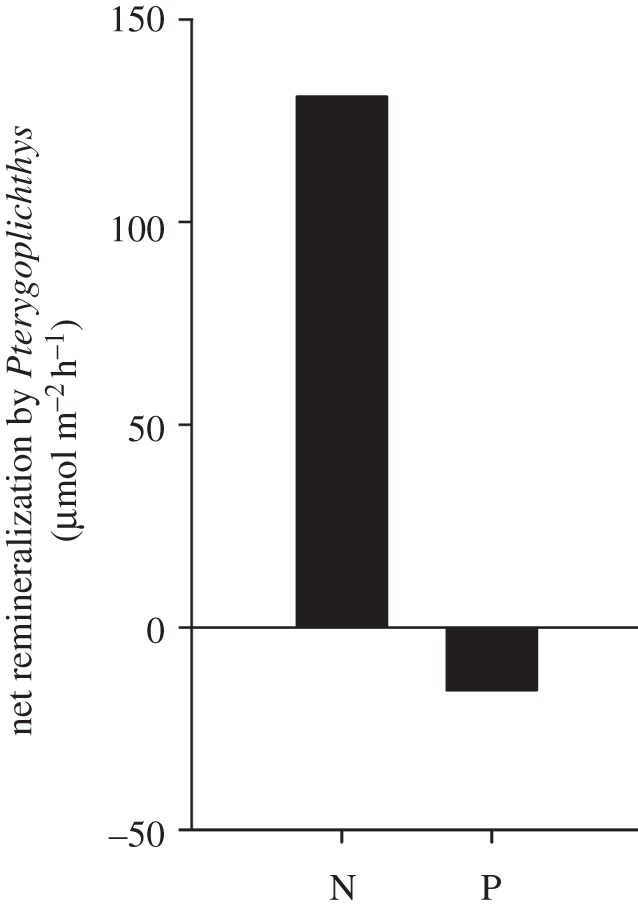
Nutrient remineralization estimates of nutrients by loricariids in the Chacamax River. The values were obtained by subtracting areal rate of nutrient sequestration by loricariids from the areal nutrient recycling rates of loricariids in the Chacamax River. Positive values indicate that loricariids are acting as a recycler of nutrients via remineralization and negative values indicate that loricariids are acting as net sink of nutrients through sequestration in body tissue.
4. Discussion
Our results indicate that stoichiometrically unique invaders can exert strong impacts on nutrient dynamics and have the ability to alter the functional role of fishes in aquatic ecosystems, especially when they attain high population densities. Relative to the contribution from native fishes, loricariid invasion converted the upper Chacamax River to a system where fishes formed an important pool of stored nutrients, and where fish remineralization had the potential to meet most of the ammonium and P demand. As we predicted, the high population density and unique body stoichiometry of loricariids influenced their effect on nutrient dynamics; they produced a net sink of P relative to their remineralization rates. These results illustrate the utility of ecological stoichiometry as a predictive framework for understanding the potential effects of non-native fishes on nutrient dynamics in freshwater ecosystems. Moreover, this study highlights the importance of estimating both elemental storage and remineralization rates of invaders to elucidate net ecosystem effects of invaders on biogeochemical processes.
(a). Predicting consequences of non-native fishes using ecological stoichiometry
The elemental requisites of all species observe the law of conservation of mass [15]; therefore, the general principles of ecological stoichiometry may provide additional insights into understanding the ability of introduced organisms to flourish in novel ecosystems [36]. Body stoichiometry is diverse among aquatic species and displays phylogenetic and size-based interactions [11,12]. Stoichiometric differences can be used to predict the influence of aquatic organisms on nutrient dynamics [10,11,37,38]; thus, stoichiometrically unique aquarium invaders would be expected to alter nutrient dynamics after invasion if they attain high biomass relative to other species and/or if they modify the flux of limiting nutrients in invaded ecosystems [39,40]. Loricariids are P-rich compared with the majority of freshwater fishes that have been studied [12,21]. In the Chacamax River, P-rich loricariids invaded a P-limited system and strongly influenced P storage and remineralization. Availability of N and P often constrains other ecosystem processes, such as organic matter decomposition and primary productivity [12]; consequently, dense populations of loricariids may influence trophic interactions by altering the availability and nutrient content of basal food resources in invaded systems [41].
Fishes are often the most nutrient-rich species in freshwater ecosystems, and, when abundant, can form the dominant pool of nutrients in streams and lakes [7,12]. Hence, invading fishes attaining high biomass may significantly shift where and for how long nutrients are stored in an ecosystem. In less than a decade [24,25], loricariids attained a biomass two orders of magnitude larger than native fishes, and substantially altered the amount of N and P stored in fish tissues in the Chacamax River. The areal biomass of loricariids increased steadily throughout the study period, growing at a rate of approximately 100 g Pterygoplichthys m−2 yr−1 (figure 2), sequestering approximately 8 g N m−2 yr−1 and 6 g P m−2 yr−1. Although there are no published estimates of native fish biomass prior to loricariid invasion in the Chacamax, we are confident that the current biomass of invasive catfish is much greater than would have been observed for native fishes prior to invasion. Biomass of native fishes in similar reaches of uninvaded streams in the region never approach the biomass attained by loricariids in the upper Chacamax, and both ichthyologists and local fishers who witnessed the invasion corroborate the explosive increase in fish biomass associated with loricariids (A. A. Pease 2010, personal communication).
Nutrient remineralization by fishes can also influence nutrient dynamics in aquatic environments [10,14,42], and recent work has demonstrated that the aggregating behaviour of non-native loricariids can create areas of enhanced biogeochemical activity or hotspots, in stream ecosystems [43]. In our study, loricariid excretion was equivalent to approximately 255% of the NH4+ demand and 70% of the P demand, compared with 10% of the NH4+ demand and 3% of the P demand met by native fish excretion (figure 3a). Daytime areal excretion estimates indicated that loricariids excreted approximately 25 times the amount of N and P than was excreted by the native fish community. This led to a 95% reduction in the distance required for fish excretion to turn over the ambient pools of NH4+ and SRP relative to the turnover distance of the native fish community (figure 3b). Loricariids recycled nutrients via excretion and sequestered nutrients through growth in the Chacamax River; however, they influenced N and P dynamics differently. When combined, recycling and sequestration estimates suggest that the loricariid population may act as a net remineralizer of N (131 µmol N m−2 h−1), but a net sink of P (12.5 µmol P m−2 h−1) (figure 4). Few studies have simultaneously examined elemental storage and remineralization of fish invaders [44,45]; yet both measurements are needed to elucidate the net effects of an invader on biogeochemical cycling. Our findings demonstrate that body stoichiometry is a useful predictor of how invaders can influence nutrient storage and cycling among elements, and suggest that organisms can simultaneously function as a net remineralizer of one nutrient, while functioning as a net sink of another in the same system. It is most likely that the effects of loricariids on nutrient storage and remineralization take on great importance in the Chacamax River due to the combined effects of the high biomass and unique body stoichiometry of Pterygoplichthys.
Invaders that are stoichiometrically imbalanced with their food would also be expected to have greater effects than organisms consuming food items that are stoichiometrically similar to their own body chemistry [12]. Low-trophic-position fishes, such as herbivores and detritivores, are often stoichiometrically imbalanced with their food [11,12]. They compensate for this disparity by consuming large quantities of plant matter and detritus, and remineralizing nutrients via excretion and egestion [13]. Barring other life-history limitations to invasion, grazing fishes, such as Pterygoplichthys, are predicted to be successful invaders, because they are rarely food-limited [46,47]. Coupled with a lack of natural predators, this characteristic may also enable loricariids to attain such high population densities. Some of the most popular aquarium fishes, such as guppies (Poecilia reticulata), neon tetras (Paracheirodon innesi), goldfish (Carassius auratus), swordtails (Xiphophorus maculatus) [48] and armoured catfish (Pterygoplichthys sp., Hypostomus sp.) [23,49], can maintain herbivorous and detritivorous diets [50]. Stoichiometric theory indicates that aquarium invaders with the aforementioned trophic ecology have the potential to restructure the chemical environment of an ecosystem by altering nutrient storage, cycling and demand via consumption, excretion and egestion.
(b). Global implications for aquarium fish invasions
At present, global trade of aquarium fishes is minimally regulated [51], though many studies have documented the risk the aquarium trade poses to freshwater fish faunas [52]. Up to 5300 species of fishes have been sold in the aquarium trade [51], and many of the commonly sold species will probably be released into ecosystems outside their native range [6]. While the cost of eradicating invaders and managing their effects can be high, the challenge for policy-makers to bolster economic activities while mitigating negative environmental impacts from introduced species is often more acute in developing countries [17,53]. This is likely to be due to the dependence of many developing economies on natural and agricultural resources [16] and the minimal financial resources available to document, monitor and manage species invasions [16,18,54]. For instance, in Mexico, loricariid invasion has decimated freshwater fisheries in several states. Loricariids now make up 70–85% of the fish biomass harvested by commercial and subsistence fishers in fisheries in the Infernillo Dam in the state of Michoacán (see electronic supplementary material, figure S3) [55]. Currently, no commerce has developed around invasive loricariids in Mexico; therefore, the fisheries have collapsed and thousands of fishers are out of work because of an aquarium invader [49,55].
The argument that import restrictions on selected species put too much financial burden on those involved with the live animal trade is a limited viewpoint that puts freshwater ecosystems at risk. This argument does not take into account the costs levied on local populations by invaders that modify ecosystem processes [17]. As demonstrated in this study, popular aquarium species can have profound effects on ecosystem processes, and ecological stoichiometry can be used to predict potential changes in ecosystem function after invasion. Therefore, risk analysis for potential aquarium imports should not be limited to the probability of establishment of the species and the identification of actions to manage or reduce risks that reflect potential socioeconomic or cultural consequences. Rather, risk analyses should strive to identify and examine traits, such as body and dietary stoichiometry, that may increase the likelihood that an aquarium species would alter the function of ecosystems.
Acknowledgements
We thank Rocío Rodiles-Hernández, Jessica Strickland, Daniel Rodríguez, Alfonso González-Díaz, Rodrigo Acinorev, Luis Gasca and Allison Pease for help with fieldwork and laboratory support in Mexico. We would also like to thank Amber Ulseth for her support in the field. Finally, we would like to thank Bob Hall for his suggestions designing field methods and Christopher Dalton, Stuart Findlay, Christy Goodale and Nelson Hairston for providing feedback on early versions of this manuscript. Organisms were harvested in Mexico using Mexican collection permit no. DGOPA.07525.25706.3233 and fishes were handled using methods outlined in the IACUC protocol 2006-0169 from Cornell University.
Funding statement
This work was supported by the National Science Foundation (Doctoral Dissertation Enhancement Program grant no. (183-8371); Integrated Graduate Education and Research in Biogeochemistry and Environmental Biocomplexity (0221658) Small Grant Program at Cornell University), the Fulbright-Hays Doctoral Dissertation Research Abroad Program and the Margaret Paul Graduate Fellowship in the Life Sciences at Cornell University.
References
- 1.Perrings C. 2010. Exotic effects of capital accumulation. Proc. Natl Acad. Sci. USA 107, 12 063–12 064 (doi:10.1073/pnas.1007335107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jerardo A. 2005. Floriculture and nursery crops yearbook. Washington, DC: US Department of Agriculture [Google Scholar]
- 3.Pimentel D, Zuniga R, Morrison D. 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 52, 273–288 (doi:10.1016/j.ecolecon.2004.10.002) [Google Scholar]
- 4.Whittington RJ, Chong R. 2007. Global trade in ornamental fish from an Australian perspective: the case for revised import risk analysis and management strategies. Prev. Vet. Med. 81, 92–116 (doi:10.1016/j.prevetmed.2007.04.007) [DOI] [PubMed] [Google Scholar]
- 5.Gerstner CL, Ortega H, Sanchez H, Graham DL. 2006. Effects of the freshwater aquarium trade on wild fish populations in differentially-fished areas of the Peruvian Amazon. J. Fish Biol. 68, 862–875 (doi:10.1111/j.1095.8649.2006.00978.x) [Google Scholar]
- 6.Duggan IC, Rixon CAM, MacIsaac HJ. 2006. Popularity and propagule pressure: determinants of introduction and establishment of aquarium fish. Biol. Invasions 8, 377–382 (doi:10.1007/s10530-004-2310-2) [Google Scholar]
- 7.Griffiths D. 2006. The direct contribution of fish to lake phosphorus cycles. Ecol. Freshwater Fish 15, 86–95 (doi:10.1111/j.1600-0633.2006.00125.x) [Google Scholar]
- 8.Sereda JM, Hudson JJ, Taylor WD, Demers E. 2008. Fish as sources and sinks of nutrients in lakes. Freshwater Biol. 53, 278–289 (doi:10.1111/j.1365-2427.2007.01891.x) [Google Scholar]
- 9.Vander Zanden MJ, Vadeboncoeur Y. 2002. Fishes as integrators of benthic and pelagic food webs in lakes. Ecology 83, 2152–2161 (doi:10.1890/0012-9658(2002)083[2152:faioba]2.0.co;2) [Google Scholar]
- 10.McIntyre PB, Flecker AS, Vanni MJ, Hood JM, Taylor BW, Thomas SA. 2008. Fish distributions and nutrient cycling in streams: can fish create biogeochemical hotspots? Ecology 89, 2335–2346 (doi:10.1890/07-1552.1) [DOI] [PubMed] [Google Scholar]
- 11.Vanni MJ, Flecker AS, Hood JM, Headworth JL. 2002. Stoichiometry of nutrient recycling by vertebrates in a tropical stream: linking species identity and ecosystem processes. Ecol. Lett. 5, 285–293 (doi:10.1046/j.1461-0248.2002.00314.x) [Google Scholar]
- 12.McIntyre PB, Flecker AS. 2010. Ecological stoichiometry as an integrative framework in stream fish ecology. In Communtiy ecology of stream fishes: concepts, approaches, and techniques (eds Jackson DA, Gido KB.), pp. 539–558 Bethesda, MD: American Fisheries Society [Google Scholar]
- 13.Elser J. 2006. Biological stoichiometry: a chemical bridge between ecosystem ecology and evolutionary biology. Am. Nat. 168, S25–S35 (doi:10.1086/509048) [DOI] [PubMed] [Google Scholar]
- 14.Small GE, Pringle CM, Pyron M, Duff JH. 2011. Role of the fish Astyanax aeneus (Characidae) as a keystone nutrient recycler in low-nutrient Neotropical streams. Ecology 92, 386–397 (doi:10.1890/10-0081.1) [DOI] [PubMed] [Google Scholar]
- 15.Sterner RW, Elser JJ. 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton, NJ: Princeton University Press [Google Scholar]
- 16.GISP 2007. Invasive species and poverty: exploring the links. Cape Town: Global Invasive Species Program [Google Scholar]
- 17.Khuroo AA, Reshi ZA, Rashid I, Dar GH. 2011. Towards an integrated research framework and policy agenda on biological invasions in the developing world: a case-study of India. Environ. Res. 111, 999–1006 (doi:10.1016/j.envres.2011.02.011) [DOI] [PubMed] [Google Scholar]
- 18.Lin W, Cheng XY, Xu RM. 2011. Impact of different economic factors on biological invasions on the global scale. PLoS ONE 6, e18797 (doi:10.1371/journal.pone.0018797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber C. 1991. New taxa in Pterygoplichthys S I (Pisces, Siluriformes, Loricariidae). Rev. Suisse Zool. 98, 637–643 [Google Scholar]
- 20.Nico LG, Martin RT. 2001. The South American suckermouth armored catfish, Pterygoplichthys anisitsi (Pisces: Loricariidae), in Texas, with comments on foreign fish introductions in the American southwest. Southwestern Nat. 46, 98–104 (doi:10.2307/3672381) [Google Scholar]
- 21.Hood JM, Vanni MJ, Flecker AS. 2005. Nutrient recycling by two phosphorus-rich grazing catfish: the potential for phosphorus-limitation of fish growth. Oecologia 146, 247–257 (doi:10.1007/s00442-005-0202-5) [DOI] [PubMed] [Google Scholar]
- 22.Capps KA, Nico LG, Mendoza-Carranza M, Arevalo-Frias W, Ropicki AJ, Heilpern SA, Rodiles-Hernandez R. 2011. Salinity tolerance of non-native suckermouth armoured catfish (Loricariidae: Pterygoplichthys) in south-eastern Mexico: implications for invasion and dispersal. Aquat. Conserv. Mar. Freshwater Ecosyst. 21, 528–540 (doi:10.1002/aqc.1210) [Google Scholar]
- 23.Nico LG, Jelks HL, Tuten T. 2009. Non-native suckermouth armored catfishes in Florida: description of nest burrows and burrow colonies with assessment of shoreline conditions. Aqu. Nuisance Species Res. Program 9, 1–30 [Google Scholar]
- 24.Wakida-Kusunoki AT, del Angel LEA. 2008. New records of the sailfish catfishes Pterygoplichthys pardalis (Castelnau 1855) and P. disjunctivus (Weber 1991) (Siluriformes: Loricariidae) in southeastern Mexico. Hidrobiológica 18, 251–255 [Google Scholar]
- 25.Wakida-Kusunoki AT, Ruiz-Carus R, Amador-del-Angel E. 2007. Amazon sailfin catfish, Pterygoplichthys pardalis (Castelnau, 1855) (Loricariidae), another exotic species established in southeastern Mexico. Southwestern Nat. 52, 141–144 (doi:10.1894/0038-4909(2007)52[141:ASCPPC]2.0.CO;2) [Google Scholar]
- 26.Pease AA, Gonzalez-Diaz AA, Rodiles-Hernandez R, Winemiller KO. 2012. Functional diversity and trait-environment relationships of stream fish assemblages in a large tropical catchment. Freshwater Biol. 57, 1060–1075 (doi:10.1111/j.1365-2427.2012.02768.x) [Google Scholar]
- 27.Capps KA, Booth MT, Collins SM, Davison MA, Moslemi JM, El-Sabaawi RW, Simonis JL, Flecker AS. 2011. Nutrient diffusing substrata: a field comparison of commonly used methods to assess nutrient limitation. J. N. Am. Benthol. Soc. 30, 522–532 (doi:10.1899/10-146.1) [Google Scholar]
- 28.Thurow RF. 1994. Underwater methods for study of salmonids in the Intermountain West. Ogden, UT: US Department of Agriculture Forest Service [Google Scholar]
- 29.Hicks BJ. 2003. Distribution and abundance of fish and crayfish in a Waikato stream in relation to basin area. N. Z. J. Zool. 30, 149–160 (doi:10.1080/03014223.2003.9518333) [Google Scholar]
- 30.Hauer FR, Lamberti GA. 2006. Methods in stream ecology, 2nd edn Boston, MA: Academic Press [Google Scholar]
- 31.Taylor BW, Keep CF, Hall RO, Koch BJ, Tronstad LM, Flecker AS, Ulseth AJ. 2007. Improving the fluorometric ammonium method: matrix effects, background fluorescence, and standard additions. J. N. Am. Benthol. Soc. 26, 167–177 (doi:10.1899/0887-3593(2007)26[167:ITFAMM]2.0.CO;2) [Google Scholar]
- 32.APHA 1999. Standard methods for the examination of water and waste water, 20th edn, p. 1268 Washington, DC: American Public Health Association, American Water Works Association and Water Environment Federation [Google Scholar]
- 33.Hall RO, Tank JL. 2003. Ecosystem metabolism controls nitrogen uptake in streams in Grand Teton National Park, Wyoming. Limnol. Oceanogr. 48, 1120–1128 (doi:10.4319/lo.2003.48.3.1120) [Google Scholar]
- 34.Newbold JD, Elwood JW, O'Neil RV, Van Winkle W. 1981. Measuring nutrient spiralling in streams. Can. J. Fish. Aqu. Sci. 38, 860–863 (doi:10.1139/f81-114) [Google Scholar]
- 35.Benstead JP, Cross WF, March JG, McDowell WH, Ramirez A, Covich AP. 2010. Biotic and abiotic controls on the ecosystem significance of consumer excretion in two contrasting tropical streams. Freshwater Biol. 55, 2047–2061 (doi:10.1111/j.1365-2427.2010.02461.x) [Google Scholar]
- 36.Gonzalez AL, Kominoski JS, Danger M, Ishida S, Iwai N, Rubach A. 2010. Can ecological stoichiometry help explain patterns of biological invasions? Oikos 119, 779–790 (doi:10.1111/j.1600-0706.2009.18549.x) [Google Scholar]
- 37.McIntyre PB, Jones LE, Flecker AS, Vanni MJ. 2007. Fish extinctions alter nutrient recycling in tropical freshwaters. Proc. Natl Acad. Sci. USA 104, 4461–4466 (doi:10.1073/pnas.0608148104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knoll LB, McIntyre PB, Vanni MJ, Flecker AS. 2009. Feedbacks of consumer nutrient recycling on producer biomass and stoichiometry: separating direct and indirect effects. Oikos 118, 1732–1742 (doi:10.1111/j.1600-0706.2009.17367.x) [Google Scholar]
- 39.Strayer DL. 2012. Eight questions about invasions and ecosystem functioning. Ecol. Lett. 15, 1199–1210. (doi:10.1111/j.1461-0248.2012.01817.x) [DOI] [PubMed] [Google Scholar]
- 40.Johnson PTJ, Olden JD, Solomon CT, Vander Zanden MJ. 2009. Interactions among invaders: community and ecosystem effects of multiple invasive species in an experimental aquatic system. Oecologia 159, 161–170 (doi:10.1007/s00442-008-1176-x) [DOI] [PubMed] [Google Scholar]
- 41.Capps KA. 2012. Changes in community structure and ecosystem processes in response to armored catfish (Silurformes: Loricariidae) invasion. Ithaca, NY: Cornell University [Google Scholar]
- 42.Reisinger AJ, Presuma DL, Gido KB, Dodds WK. 2011. Direct and indirect effects of central stoneroller (Campostoma anomalum) on mesocosm recovery following a flood: can macroconsumers affect denitrification? J. N. Am. Benthol. Soc. 30, 840–852 (doi:10.1899/10-169.1) [Google Scholar]
- 43.Capps KA, Flecker AS. 2013. Invasive fishes generate biogeochemical hotspots in a nutrient-limited system. PLoS ONE 8, e54093 (doi:10.1371/journal.pone.0054093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kraft CE. 1993. Phosphorus regeneration by Lake Michigan alewives in the mid-1970s. Trans. Am. Fisheries Soc. 122, 749–755 (doi:10.1577/1548-8659(1993)122<0749:PRBLMA>2.3.CO;2) [Google Scholar]
- 45.Vanni MJ, Boros G, McIntyre PB. In press. When are fish sources versus sinks of nutrients in lake ecosystems? Ecology. (doi:10.1890/12-1559.1) [DOI] [PubMed] [Google Scholar]
- 46.Moyle PB, Light T. 1996. Biological invasions of fresh water: empirical rules and assembly theory. Biol. Conserv. 78, 149–161 (doi:10.1016/0006-3207(96)00024-9) [Google Scholar]
- 47.Gido KB, Franssen NR. 2007. Invasion of stream fishes into low trophic positions. Ecol. Freshwater Fish 16, 457–464 (doi:10.1111/j.1600-0633.2007.00235.x) [Google Scholar]
- 48.Chapman FA, FitzCoy SA, Thunberg EM, Adams CM. 1997. United States of America trade in ornamental fish. J. World Aquacult. Soc. 28, 1–10 (doi:10.1111/j.1749-7345.1997.tb00955.x) [Google Scholar]
- 49.Mendoza RE, et al. 2009. Trinational risk assessment guidelines for aquatic alien invasive species. CEC Project Report, Commission for Environmental Cooperation, Montréal, Canada
- 50.Froese R, Pauly D.2011. FishBase. See http://www.fishbase.org.
- 51.Collins RA, Armstrong KF, Meier R, Yi YG, Brown SDJ, Cruickshank RH, Keeling S, Johnston C. 2012. Barcoding and border biosecurity: identifying cyprinid fishes in the aquarium trade. PLoS ONE 7, e28381 (doi:10.1371/journal.pone.0028381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDowall RM. 2004. Shoot first, and then ask questions: a look at aquarium fish imports and invasiveness in New Zealand. N. Z. J. Mar. Freshwater Res. 38, 503–510 (doi:10.1080/00288330.2004.9517256) [Google Scholar]
- 53.Nuñez MA, Pauchard A. 2010. Biological invasions in developing and developed countries: does one model fit all? Biol. Invasions 12, 707–714 (doi:10.1007/s10530-009-9517-1) [Google Scholar]
- 54.Pimentel D, et al. 2001. Economic and environmental threats of alien plant, animal, and microbe invasions. Agric. Ecosyst. Environ. 84, 1–20 (doi:10.1016/S0167-8809(00)00178-X) [Google Scholar]
- 55.Mendoza R, Contreras S, Ramirez C, Koleff P, Alvarez P, Aguilar V. 2007. Los peces diablo. Biodiversitas 70, 1–5 [Google Scholar]