Abstract
During embryonic development, endogenous signals, for example steroid hormones, and exogenous signals, for example endocrine disrupting chemicals (EDCs), have the capacity to produce phenotypic effects that persist into adulthood. As the actions of steroids are mediated through the binding of steroid receptors, most studies of EDCs have assumed that they too elicit their effects by binding steroid receptors. We tested an alternative hypothesis, namely that EDCs elicit their effects during embryonic development by disrupting the metabolism of maternally derived steroids, thereby allowing maternally derived steroids to bind steroid receptors and elicit effects. Specifically, we examined the ability of the EDC, bisphenol-A (BPA) to inhibit the normal metabolism of oestradiol during the first nine days of embryonic development in the red-eared slider turtle (Trachemys scripta). We found that, when BPA was present, oestrogen metabolism was inhibited when compared to control eggs. In particular, the formation of oestrone sulfate was blocked in BPA-treated eggs. We postulate that the oestrogenic effects of EDCs may be driven, at least in part, by inappropriate oestrogen signalling. The retention of oestrogens at points of development when they would normally be metabolized to inactive forms might also help explain low-dose effects frequently reported for EDCs.
Keywords: oestradiol, oestrone sulfate, steroid metabolism
1. Introduction
It has long been recognized that the endocrine environment experienced by developing vertebrate embryos can produce long-lasting phenotypic effects (reviewed in [1]). Many of these effects are unique to the embryonic period such that similar endocrine signals later in life are not capable of producing the same effect. The heightened sensitivity of embryos to the endocrine environment has led to a great deal of research focused on understanding how early endocrine signals influence development. During embryonic development, vertebrate embryos are exposed to steroids of embryonic and maternal origin. Because one of the main functions of oestradiol is to regulate reproduction in females [2], there is an inherent link between maternal oestradiol and offspring development. For many vertebrates, maternal oestradiol levels increase during reproduction [2]. In placental vertebrates, this results in embryos developing within a maternal environment that contains elevated oestradiol concentrations [3], whereas in oviparous vertebrates, this results in the presence of oestradiol, and other lipophilic steroids, in the yolk at oviposition [4].
Research on the early endocrine environment has taken on additional importance in light of the fact that a large number of environmental and man-made chemicals often interfere with normal endocrine signalling, and produce long-lasting phenotypic effects, including effects similar to those observed following oestrogen exposure [5]. These endocrine disrupting chemicals (EDCs) are comprised of a structurally diverse group of compounds that are also known as ‘xenoestrogens’ [6], ‘endocrine disrupting chemicals with oestrogenic activity (EEDCs)’ [7], ‘oestrogen mimics’ [8] and ‘environmental oestrogens’ [9]. A major focus of ongoing research is to determine how such a structurally diverse group of chemicals could produce effects similar to such a tightly regulated ligand, for example oestradiol, with an emphasis on the ability of EDCs to bind to steroid receptors as a potential mechanism for producing steroid-like effects [5].
Previous research has found that a variety of EDCs can bind to oestrogen receptors (ERs) [10,11], suggesting that these EDCs may elicit oestrogenic effects by direct ER activation, thereby increasing ER-dependent gene transcription [11,12]. This model of EDC action via ER binding assumes a promiscuous ER-binding site owing to the extreme variation in EDC structure [13]. While it is clear that EDCs can bind ERs, when binding does occur, only weak, partial agonistic actions are noted [14], such that the activity of an EDC is often significantly weaker than that of the natural ligand [15,16]. Together, the evidence suggests that while ER activation by EDCs may play a role in producing oestrogenic endpoints, it is not likely the sole mechanism for oestrogenic effects in all tissues. Other possible mechanisms for the oestrogenic effects of EDCs include the induction of oestradiol production [17] and the inhibition of oestradiol metabolism [18,19]. Both of these mechanisms would result in elevated oestradiol levels and do not require a direct interaction between the EDC and an ER.
Understanding how EDCs influence steroid metabolism in developing embryos could be especially important because of the high sensitivity of the embryo to steroid signals paired with the elevated levels of maternal oestrogens. A key metabolic pathway in the metabolism of maternal steroids, including oestradiol, is sulfonation [20] because the primary conjugative pathway found in adults (glucuronidation) does not fully develop until after birth [21]. The sulfonation of steroids is carried out by a family of sulfotransferase enzymes that transfer a sulfonate group to the acceptor steroid and use a universal sulfonate donor (3′-phosphoadenosine 5′-phosphosulfate or PAPS) to carry out this reaction [22]. The sulfonation of steroids typically reduces or eliminates the ability of the steroid to bind its respective steroid receptor [23], while also decreasing the hydrophobicity of the steroid to facilitate clearance [21]. Recent work in reptiles [24–27] and birds [28–30] has demonstrated that in ovo metabolism of maternal steroids occurs. Characterization of this metabolism in the red-eared slider turtle (Trachemys scripta) demonstrates that oestradiol is converted to a variety of oestrogen sulfates (primarily oestrone sulfate) [24,26,27,31] through sulfonation. Interestingly, sulfotransferase enzymes not only can be subjected to inhibition by EDCs [32,33], but are also vital to the metabolism and clearance of exogenous chemicals such as those that act as EDCs [34], creating a situation where there is a shared metabolic fate for both maternal steroids and EDCs. Given that both endogenous steroids and EDCs use sulfonation for clearance, it is possible that the presence of an EDC might affect the normal clearance of oestradiol.
Here, we investigate the effect of the EDC, bisphenol-A (BPA), on the metabolism of maternal oestradiol in T. scripta. BPA is a commonly used plasticizer that is one of the most mass-produced chemicals in the world with an essentially ubiquitous presence in the environment ([35], reviewed in [36]). It has been found around the world in human [36,37] and non-human animals alike, and is known to have oestrogenic effects on developing organisms across vertebrate and invertebrate taxa (reviewed in [38–41]). Although BPA has been shown to bind to both ERα [15,42] and ERβ [42], the binding affinity for BPA is up to 12 500-fold lower than for oestradiol [43]. Importantly, it has been shown that BPA is subject to sulfonation [44,45] and can also inhibit the sulfonation of oestradiol in vitro [18]. These characteristics make BPA an ideal compound for investigating the potential for EDCs to produce oestrogenic effects by inhibiting the natural metabolism of maternal oestradiol.
2. Material and methods
(a). Egg collection
In this species, there is consistent variation in levels of maternal oestradiol with concentrations in the eggs from a female's second clutch of the nesting season being approximately 10× higher than those from her first clutch of the season [25]. In both first and second clutches, these concentrations decline to undetectable levels early in development [25]. For this study, eggs were collected at Banner Marsh State Fish and Wildlife Area in Fulton Co., IL, USA [24,25] either from freshly laid nests in the field or from gravid females that were caught in baited hoop traps and transported to Illinois State University where egg laying was induced by injection of oxytocin [46,47]. All adult trapping, handling and egg collection methods were approved by the Illinois Department of Natural Resources Illinois (IDNR permit NH11.2084) and the Illinois State University Institutional Animal Care and Use Committee (04-2010).
(b). Effect of bisphenol-A on in ovo oestradiol metabolism
To determine the effect of BPA on the early in ovo decline of oestradiol in T. scripta, seven clutches were collected and eggs from each clutch were randomly allocated to one of two treatments: control eggs = 0.1 µg oestradiol in 5 µl vehicle (70% ethanol), or BPA-treated eggs = 0.1 µg oestradiol plus 40 µg BPA in 5 µl vehicle. For oestradiol, the dose was selected to elevate levels of oestradiol in the early season clutches to levels found in late season clutches [25]. For BPA, we performed a pilot study (data not shown), basing our initial doses on those used in Stoker et al. [48]; we determined that 40 µg of BPA, given on day 0 of incubation, did not result in significant egg mortality in T. scripta. Treatment occurred within 24 h of the start of chalking, a sign that the egg contains a viable embryo that has begun to develop. For this study, only clutches (n = 5) in which all eggs began chalking within the first 24 h after laying were used to control timing of treatment. Within each treatment, eggs from each clutch were randomly assigned to a sampling day either 1, 3, 6 or 9 days post-treatment. Eggs (n = 4–5 per sampling day and treatment) were incubated at 27°C in moist vermiculite (approx. −150 kPa) until sampling, at which point the eggs were frozen at −20°C until analysis. For analysis, each egg was dissected and components (yolk and albumen/fluid) were separated and weighed to the nearest 0.01 g.
Levels of oestrogens in the yolk were measured using radioimmunoassay (RIA) (modified from [49,50]). Oestradiol and oestrone were measured in independent assays with slightly modified extraction techniques. For oestradiol, 50 mg of yolk was diluted and homogenized in 1 ml distilled water. Two thousand cpm of tritiated oestradiol (Perkin Elmer, Waltham, MA, USA) was added to each sample to allow for the calculation of per cent recovery. Steroids were extracted with ether (30 : 70, petroleum ether : diethyl ether) producing two phases: an aqueous phase and an organic phase, which contain the water-soluble and lipid-soluble molecules, respectively. The organic phase, containing any free steroids present in the sample, was then dried down under nitrogen gas, resuspended in 1 ml of 90% ethanol, vortexed and held at −20°C overnight. The samples were then centrifuged at 2000 r.p.m. for 5 min at 0°C to remove neutral lipids and proteins. The supernatant was poured off, dried down under nitrogen gas and resuspended in 10% ethyl acetate in isooctane and loaded onto celite-packed columns. For oestrone, 100 mg of yolk was diluted and homogenized in 200 µl of distilled water and 2000 cpm of tritiated oestone (Perkin Elmer) was added to calculate recoveries. To precipitate proteins and neutral lipids, 1 ml of methanol was added and samples were held at −20°C overnight. The samples were centrifuged at 2000 r.p.m. for 15 min and the supernatant was decanted. An additional 1 ml of methanol was added, and the samples were centrifuged again, and the supernatants were combined and dried under nitrogen. The dried sample was resuspended in 1 ml of distilled water and 2 ml of diethyl ether was added, producing an aqueous phase and an organic phase, respectively. The extraction was repeated, the organic phases combined and dried under nitrogen gas, and resuspended in 10% ethyl acetate in isooctane loaded onto celite-packed columns.
Both steroids were eluted with solutions containing progressively higher concentrations of ethyl acetate in isooctane (0, 10, 20 and 40%). Oestrone was isolated with the 20% fraction, whereas oestradiol was isolated with the 40% fraction. Duplicate aliquots of each steroid were subjected to RIA using specific antibodies (oestrone antibody E3135, Sigma, USA; oestradiol antibody 7010, Biogenesis, UK). To quantify endogenous oestrogens, a standard curve (range: 1.95–500 pg) was used for comparison. Oestrone was analysed in a single assay, with an intra-assay coefficient of variation (CV) of 4%. Oestradiol was analysed across two assays, with intra-assay CVs of 13 and 20%, and an inter-assay CV of 23%.
Oestrone sulfate, oestriol sulfate and oestradiol sulfate were quantified using liquid chromatography–mass spectrometry (LC/MS/MS). Samples were prepared by vortexing 1 ml of albumen with 3 ml of methanol (HPLC grade), frozen overnight and centrifuged (2000 r.p.m.) at 0°C for 20 min. The supernatant was poured off and kept at −20°C. A second methanol extraction was performed on the pellet with 2 ml of methanol following the same protocol. Supernatants from both rounds were consolidated, dried down under nitrogen gas and resuspended in 5 ml nano-pure water. Water-soluble metabolites were extracted using Sep-pak cartridges (C18 plus short cartridge, 55–105 µm particle size; Waters, Milford, MA, USA). For each sample, two Sep-pak cartridges were stacked and charged with 5 ml methanol followed by 5 ml nano-pure water. The samples were then loaded onto the cartridges. Lipid-soluble molecules were eluted with 5 ml ether followed by elution of the water-soluble metabolites with 5 ml methanol. The methanol eluate was then dried down under nitrogen gas and metabolites characterized using LC/MS/MS.
The LC/MS/MS analysis was performed in the Metabolomics Center at University of Illinois at Urbana–Champaign with a 5500 QTRAP mass spectrometer (AB Sciex, Foster City, CA, USA), that is equipped with a 1200 Agilent HPLC. Analyst (v. 1.5.1, Applied Biosystems) was used for data acquisition and processing. The HPLC flow rate was set at 0.35 ml min−1. HPLC mobile phases consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The autosampler was kept at 5°C. The injection volume was 5 µl. An Agilent Zorbax SB-Aq column (3.5 µm, 50 × 4.6 mm) was used for the separation with the following gradient: 0–2 min, 80% A; 6–10 min, 25% A; 11–16.5 min, 80% A. Mass spectrometer was operated under negative mode electrospray ionization. The electrospray voltage was set to −4500 V, the heater was set at 600°C, the curtain gas was 35, and GS1 and GS2 were 50, 65, respectively. Quantitative analysis was performed via MRM where m/z 349.1 → m/z 269.1 (3-OL-17-one; oestrone sulfate); m/z 351.1 → m/z 271.1 (17-β oestradiol; oestradiol sulfate) and m/z 367.1 → m/z 287.1 (16-α-17-β-oestriol; oestriol sulfate) were monitored.
(c). Statistical analyses
Levels of oestrone, oestradiol and oestrone sulfate were analysed by ANOVA in PROC GLM (SAS 9.3 SAS Institute, Cary, NC, USA). For the ANOVAs, treatment (control or BPA treated), sampling day (1, 3, 6 or 9) and their interaction were included as fixed factors in the model. Clutch of origin was included as a random factor. We ran contrasts to probe any significant interactions. Levels of oestradiol sulfate were low to undetectable, while oestriol sulfate levels were below detection limits in all samples; neither was included in the final analyses. To meet normality assumptions of ANOVA, all steroids were log(10) transformed prior to analysis.
3. Results
Mean levels of oestradiol, oestrone and oestrone sulfate in eggs (n = 1 per clutch) sampled prior to any treatment were 2.7, 0.10 and 5.6 ng g−1, respectively. Levels of oestradiol in the yolk were significantly affected by treatment, with eggs from the BPA treatment group having elevated levels of yolk oestradiol relative to those from the control treatment (F1,1 = 11.10, p = 0.003; figure 1a). There were no significant effects of either day (F1,3 = 2.50, p = 0.085), or the interaction of treatment × day (F1,3 = 0.19, p = 0.90) on yolk oestradiol. However, we did detect a significant clutch effect on yolk oestradiol levels (F1,4 = 18.16, p < 0.0001). Levels of oestrone in the yolk were also significantly affected by treatment, with eggs from the BPA treatment group having elevated levels of yolk oestrone relative to those from the control treatment (F1,1 = 6.86, p = 0.015; figure 1b). Yolk oestrone levels varied by sampling day (F1,3 = 12.06, p < 0.0001), but there was no significant interaction detected (F1,3 = 0.09, p = 0.97). Levels of oestrone sulfate in the albumen varied significantly by treatment group (F1,1 = 19.30, p = 0.0002) and by day (F1,3 = 54.55, p < 0.0001). There was a significant treatment × day interaction (F1,3 = 4.94, p = 0.0086; figure 1c). Probing this interaction, we found that the treatment groups varied on day 1 (p < 0.0001) and day 6 (p = 0.006), but not on days 3 and 9 (p > 0.05). There were no clutch effects detected for either oestrone or oestrone sulfate. Data used in these analyses are available as the electronic supplementary material.
Figure 1.
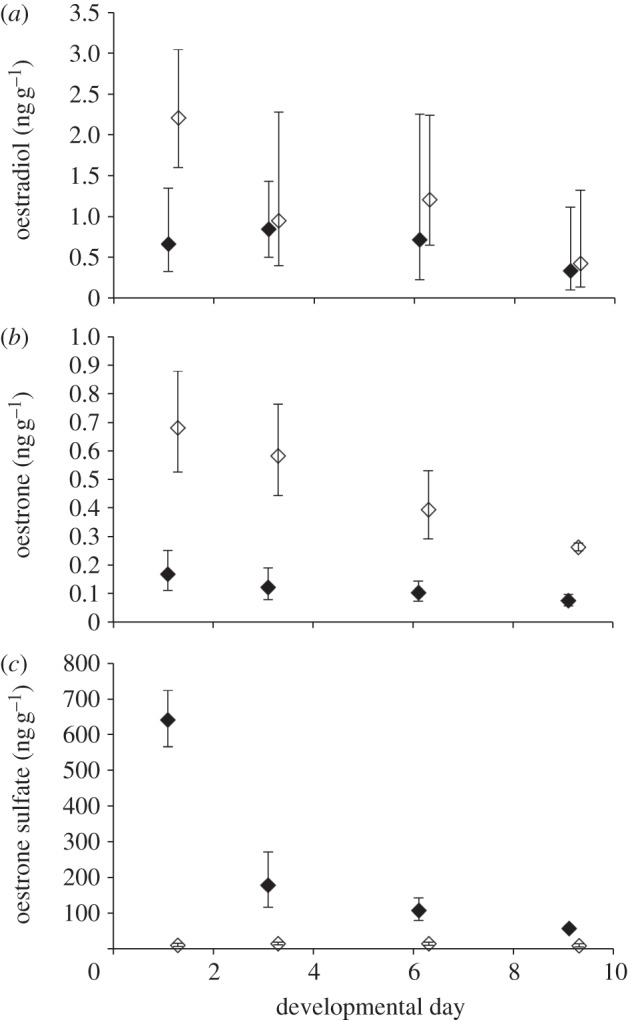
Levels of (a) oestradiol, (b) oestrone and (c) oestrone sulfate in T. scripta eggs during early embryonic development. Control eggs (0.1 µg oestradiol, filled symbols, n = 17) had lower levels of oestradiol and oestrone, but higher levels of oestone sulfate than eggs treated with bisphenol-A (0.1 µg oestradiol + 40 µg BPA, open symbols, n = 18). Data represent back-transformed means of log-transformed data ±s.e.
4. Discussion
Here, we demonstrate that BPA alters the metabolism of oestrogens in ovo. We found that levels of both oestradiol and oestrone were higher, whereas levels of oestrone sulfate were lower, in eggs treated with BPA when compared with the control eggs. The low levels of oestrone sulfate paired with the elevated levels of free oestrogens indicate that oestrogen sulfotransferase activity is altered in the presence of BPA in T. scripta (figure 2). Because, however, there are several metabolic conversions required to produce oestrone sulfate from oestradiol, it is also possible that BPA could be affecting other steps in this process. For example, BPA could alter the conversion of oestradiol to oestrone instead of affecting the sulfonation of oestrone itself, thus producing the lower oestrone sulfate levels observed in the BPA-treated group. If the conversion from oestradiol to oestrone was the main pathway inhibited, we would have expected to see decreased levels of oestrone in the BPA-treated eggs. In fact, we found just the opposite pattern, indicating that BPA is inhibiting the conversion of oestrone to oestrone sulfate. Our data support the hypothesis that EDCs, for instance BPA, can produce their effects by increasing the bioavailability of active steroids during critical periods of development by effectively interfering with the metabolism of maternal steroids.
Figure 2.
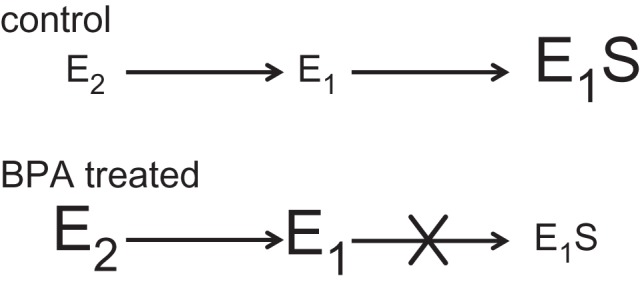
Schematic of how steroid metabolism is altered during the first nine days of embryonic development in T. scripta when eggs are exposed to the endocrine disruptor bisphenol-A. In the control eggs, oestradiol (E2) is converted to oestrone (E1), and then to oestrone sulfate (E1S) which is present at high levels in the egg. Eggs treated with BPA maintained higher levels of both E2 and E1, with little to no E1S being formed. Our data indicate that BPA interferes with the metabolic conversion of E1 to E1S in ovo.
The ability of EDCs to inhibit the sulfonation of oestrogens has been demonstrated in a number of in vitro systems ([18,19], reviewed in [51]), but to our knowledge, this is the first time it has been shown that the sulfonation of maternal oestrogens is subject to inhibition by an EDC in ovo. In both placental and oviparous vertebrates, the sulfonation of steroids is thought to serve as a buffer that protects the embryo from being exposed to the high levels of maternal oestrogens [20,24]. The presence of increased levels of free oestradiol and oestrone in the yolks of BPA-treated eggs suggests that inhibiting sulfotranferase activity may lead to increased levels of active steroid that is potentially available for signalling within the egg.
Demonstrating that the sulfonation of maternal oestrogens is subject to inhibition by BPA has important implications for developing vertebrate embryos. Previous work has demonstrated that EDCs can be metabolized through the same enzymatic pathways as steroids, including sulfonation [34], and our data indicate that the presence of BPA results in the inhibition of oestrogen metabolism. At present, it is unclear if the observed inhibition is the result of competitive inhibition of BPA for access to the sulfotransferase enzyme, PAPS depletion, or if non-competitive or allosteric inhibition may be taking place. Regardless, if embryos are simultaneously exposed to both maternal oestrogens and exogenous compounds that can be metabolized through the sulfotransferase pathway, it appears that they may not be able to adequately remove all active compounds in a timely manner, ultimately producing effects on the offspring's phenotype.
(a). EDC effects in vertebrates
EDCs have been shown to elicit a wide variety of effects from the ‘classic’ feminizing effects of DDT on gull embryos [52] to more recent work demonstrating transgenerational epigenetic effects (reviewed in [53]). Interestingly, many effects are observed when embryos are exposed to low doses of the compounds, and the effects of the EDC can be influenced by endogenous steroid levels. For example, fetal mice with higher levels of endogenous oestradiol responded to exposure to a low dose of BPA in utero, whereas those with low endogenous oestradiol levels did not [54]. It is possible, then, that even EDC exposures considered to be ‘low doses’, may be high enough relative to the available enzyme to produce altered steroid metabolism, resulting in the inhibition of sulfotransferase activity toward endogenous and exogenous compounds alike, which may account for the low-dose effects often observed (see [41] for discussion of dose–response curves for BPA).
This is the first study, to our knowledge, that demonstrates alteration of steroid metabolism by an EDC in ovo. While in vitro data provides evidence for the potential of an EDC to inhibit steroid metabolism, the in vitro approach may lack the feedback and compensatory mechanisms that may otherwise prevent alteration in vivo. By demonstrating alteration in a closed and complete developmental environment, we provide support for altered steroid metabolism as a mechanism of endocrine disruption in the whole organism. This altered steroid metabolism could produce inappropriate signalling by endogenous compounds, potentially producing or contributing to the known effects of EDCs on tissue differentiation. Although we have only discussed oestrogens here, the implications of this research may extend to any endogenous signalling molecules that use similarly promiscuous conjugation pathways. The relative promiscuity of conjugative enzymes as compared to steroid receptors suggests that altered steroid metabolism has the potential to be a more universal mechanism across structurally varied EDCs and to better account for low-dose effects than the traditional receptor-mediated mechanism.
Acknowledgements
We thank Zhong Li at the University of Illinois Metabolomics Center for performing the LC/MS/MS analysis, Steve Juliano for statistical advice, the Illinois Department of Natural Resources for access to Banner Marsh, and Laura Zimmerman and Justin Hicke for assistance in the field.
Funding statement
This work was supported by an EPA STAR Fellowship to S.G.C., and NSF IOS-0952840 to R.M.B.
References
- 1.Arnold AP. 2009. The organizational–activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 55, 570–578 (doi:10.1016/j.yhbeh.2009.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norris DO. 2007. Vertebrate endocrinology, 4th edn San Diego, CA: Academic Press [Google Scholar]
- 3.Conley AJ, Mason JI. 1990. Placental steroid-hormones. Baillière's Clin. Endocrinol. Metab. 4, 249–272 (doi:10.1016/s0950-351x(05)80050-3) [DOI] [PubMed] [Google Scholar]
- 4.Carere C, Blathazart J. 2007. Sexual versus individual differentiation: the controversial role of avian maternal hormones. Trends Endocrinol. Metab. 18, 73–80 (doi:10.1016/j.tem.2007.01.003) [DOI] [PubMed] [Google Scholar]
- 5.Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, vom Saal FS. 2012. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology 153, 4097–4110 (doi:10.1210/en.2012-1422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gore AC. 2008. Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front. Neuroendocrinol. 29, 358–374 (doi:10.1016/j.yfrne.2008.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. 2003. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ. Health Perspect. 111, 994–1006 (doi:10.1289/ehp.5494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schug TT, Janesick A, Blumberg B, Heindel JJ. 2011. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 127, 204–215 (doi:10.1016/j.jsbmb.2011.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinmetz R, Brown NG, Allen DL, Bigsby RM, BenJonathan N. 1997. The environmental estrogen bisphenol a stimulates prolactin release in vitro and in vivo. Endocrinology 138, 1780–1786 (doi:10.1210/en.138.5.1780) [DOI] [PubMed] [Google Scholar]
- 10.Kuiper G, Carlson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J. 1997. Comparison of ligand binding specificity and transcript tissues distribution of estrogen receptors α and β. Endocrinology 138, 863–870 (doi:10.1210/en.138.3.863) [DOI] [PubMed] [Google Scholar]
- 11.Kuiper G, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg P, Gustafsson JA. 1998. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139, 4252–4263 (doi:10.1210/en.139.10.4252) [DOI] [PubMed] [Google Scholar]
- 12.Nadal A, Alonso-Magdalena P, Ripoll C, Fuentas E. 2005. Disentangling the molecular mechanisms of action of endogenous and environmental estrogens. Pflugers Arch. 449, 335–343 (doi:10.1007/s00424-004-1343-9) [DOI] [PubMed] [Google Scholar]
- 13.Katzenellenbogen JA. 1995. The structural pervasiveness of estrogenic activity. Environ. Health Perspect. 103, 99–101 (doi:10.2307/3432517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller SO. 2004. Xenoestrogens: mechanisms of action and detection methods. Anal. Bioanal. Chem. 378, 582–587 (doi:10.1007/s00216-003-2238-x) [DOI] [PubMed] [Google Scholar]
- 15.Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, Safe S, McDonnell DP, Gaido KW. 1998. Bisphenol A interacts with the estrogen receptor α in a distinct manner from estradiol. Mol. Cell. Endocrinol. 142, 203–214 (doi:10.1016/S0303-7207(98)00084-7) [DOI] [PubMed] [Google Scholar]
- 16.Matsushima A, Shimohigashi Y. 2008. A strategy to explore the target receptor of endocrine disruptors: estrogen-related receptor γ (ERR γ) as a genuine acceptor of bisphenol A. In Proc. 6th World Congress on Alternatives & Animal Use in the Life Sciences, 21–25 August 2007, Tokyo, Japan. AATEX14, 495–497
- 17.Chung E, Genco MC, Megrelis L, Ruderman JV. 2011. Effects of bisphenol A and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. Proc. Natl Acad. Sci. USA 108, 17 732–17 737 (doi:10.1073/pnas.1115187108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kester MHA, et al. 2002. Potent inhibition of estrogen sulfotransferase by hydroxylated metabolites of polyhalogenated aromatic hydrocarbons reveals alternative mechanism for estrogenic activity of endocrine disrupters. J. Clin. Endocrinol. Metab. 87, 1142–1150 (doi:10.1210/jc.87.3.1142) [DOI] [PubMed] [Google Scholar]
- 19.Harris RM, Wood DM, Bottomley L, Blagg S, Owen K, Hughes PJ, Waring RH, Kirk CJ. 2004. Phytoestrogens are potent inhibitors of estrogen sulfation: implications for breast cancer risk and treatment. J. Clin. Endocrinol. Metab. 89, 1779–1787 (doi:10.1210/jc.2003-031631). [DOI] [PubMed] [Google Scholar]
- 20.Diczfalusy E. 1964. Endocrine functions of human fetoplacental unit. Fed. Proc. 23, 791–798 [PubMed] [Google Scholar]
- 21.Dutton GJ. 1978. Developmental aspects of drug conjugation, with special reference to glucuronidation. Annu. Rev. Pharmacol. Toxicol. 18, 17–35 (doi:10.1146/annurev.pa.18.040178.000313) [DOI] [PubMed] [Google Scholar]
- 22.Strott CA. 2002. Sulfonation and molecular action. Endocr. Rev. 23, 703–732 (doi:10.1210/er.2001.0040) [DOI] [PubMed] [Google Scholar]
- 23.Pasqualini JR, Gelly C, Lecerf F. 1986. Estrogen sulfates: biological and ultrastructural responses and metabolism in MCF-7 human breast cancer cells. Breast Cancer Res. Treat. 8, 233–240 (doi:10.1007/bf01807336) [DOI] [PubMed] [Google Scholar]
- 24.Paitz RT, Bowden RM. 2008. A proposed role of the sulfotransferase/sulfatase pathway in modulating yolk steroid effects. Integr. Comp. Biol. 48, 419–427 (doi:10.1093/icb/icn034) [DOI] [PubMed] [Google Scholar]
- 25.Paitz RT, Bowden RM. 2009. Rapid decline in the concentrations of three yolk steroids during development: is it embryonic regulation? Gen. Comp. Endocrinol. 161, 246–251 (doi:10.1016/j.ygcen.2009.01.018) [DOI] [PubMed] [Google Scholar]
- 26.Paitz RT, Bowden RM. 2011. Biological activity of oestradiol sulphate in an oviparous amniote: implications for maternal steroid effects. Proc. R. Soc. B 278, 2005–2010 (doi:10.1098/rspb.2010.2128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paitz RT, Sawa AR, Bowden RM. 2012. Characterizing the metabolism and movement of yolk oestradiol during embryonic development in the red-eared slider (Trachemys scripta). Gen. Comp. Endocrinol. 176, 507–512 (doi:10.1016/j.ygcen.2011.10.009) [DOI] [PubMed] [Google Scholar]
- 28.von Engelhardt N, Henriksen R, Groothuis TGG. 2009. Steroids in chicken egg yolk: metabolism and uptake during early embryonic development. Gen. Comp. Endocrinol. 163, 175–183 (doi:10.1016/j.ygcen.2009.04.004) [DOI] [PubMed] [Google Scholar]
- 29.Paitz RT, Bowden RM, Casto JM. 2011. Embryonic modulation of maternal steroids in European starlings (Sturnus vulgaris). Proc. R. Soc. B 278, 99–106 (doi:10.1098/rspb.2010.0813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paitz RT, Casto JM. 2012. The decline in yolk progesterone concentrations during incubation is dependent on embryonic development in the European starling. Gen. Comp. Endocrinol. 176, 415–419 (doi:10.1016/j.ygcen.2011.12.014) [DOI] [PubMed] [Google Scholar]
- 31.Paitz RT, Bowden RM. 2013. The sulfonation of maternal steroids is a conserved metabolic pathway in vertebrates. Integr. Comp. Biol. (doi:10.1093/icb/ict027) [DOI] [PubMed] [Google Scholar]
- 32.Ekuase EJ, Liu Y, Lehmler HJ, Robertson LW, Duffel MW. 2011. Structure–activity relationships for hydroxylated polychlorinated biphenyls as inhibitors of the sulfation of dehydroepiandrosterone catalyzed by human hydroxysteroid sulfotransferase SULT2A1. Chem. Res. Toxicol. 24, 1720–1728 (doi:10.1021/tx200260h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang LQ, Lehmler HJ, Robertson LW, James MO. 2006. Polychlorobiphenylols are selective inhibitors of human phenol sulfotransferase 1A1 with 4-nitrophenol as a substrate. Chem. Biol. Interact. 159, 235–246 (doi:10.1016/j.cbi.2005.12.004) [DOI] [PubMed] [Google Scholar]
- 34.Xu CJ, Li CYT, Kong ANT. 2005. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 28, 249–268 (doi:10.1007/BF02977789) [DOI] [PubMed] [Google Scholar]
- 35.Talsness CE, Andrade AJM, Kuriyama SN, Taylor JA, vom Saal FS. 2009. Components of plastic: experimental studies in animals and relevance for human health. Phil. Trans. R. Soc. B 364, 2079–2096 (doi:10.1098/rstb.2008.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. 2007. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 24, 139–177 (doi:10.1016/j.reprotox.2007.07.010) [DOI] [PubMed] [Google Scholar]
- 37.Vandenberg LN, Chahoud I, Heindel J, Padmanabhan V, Paumgartten FJR, Schoenfelder G. 2010. Urinary circulating and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 118, 1055–1070 (doi:10.1289/ehp.0901716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLachlan JA. 2001. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr. Rev. 22, 319–341 (doi:10.1210/er.22.3.319) [DOI] [PubMed] [Google Scholar]
- 39.Welshons WV, Nagel SC, vom Saal FS. 2006. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 147, S56–S69 (doi:10.1210/en.2005-1159) [DOI] [PubMed] [Google Scholar]
- 40.Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, LeBlanc GA, Guillette LJ. 2007. An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod. Toxicol. 24, 225–239 (doi:10.1016/j.reprotox.2007.05.008) [DOI] [PubMed] [Google Scholar]
- 41.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. 2009. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr. Rev. 30, 75–95 (doi:10.1210/er.2008-0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matthews JB, Twomey K, Zacharewski TR. 2001. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors α and β. Chem. Res. Toxicol. 14, 149–157 (doi:10.1021/tx0001833) [DOI] [PubMed] [Google Scholar]
- 43.U.S. EPA 2005. A cross-species mode of action information assessment: a case study of bisphenol-A (BPA) (EPA/600/R-50/044F) Washington, DC: US Environmental Protection Agency [Google Scholar]
- 44.Hanoika N, Takeda Y, Tanaka-Kagawa T, Hayashi K, Jinno H, Narimatsu S. 2008. Interaction of bisphenol a with human UDP-glucuronosyltransferase 1a6 enzyme. Environ. Toxicol. 23, 407–412 (doi:10.1002/tox.20345) [DOI] [PubMed] [Google Scholar]
- 45.Fini J-B, Dolo L, Cravedi J-P, Demeneix B, Zalko D. 2009. Metabolism of the endocrine disruptor BPA by Xenopus laevis tadpoles. Ann. NY Acad. Sci. 1163, 394–397 (doi:10.1111/j.1749-6632.2008.03655.x) [DOI] [PubMed] [Google Scholar]
- 46.Ewert MA, Legler JM. 1978. Hormonal induction of oviposition in turtles. Herpetologica 34, 314–318 [Google Scholar]
- 47.Les HL, Paitz RT, Bowden RM. 2007. Experimental test of the effects of fluctuating incubation temperatures on hatchling phenotype. J. Exp. Zool. 307A, 274–280 (doi:10.1002/jez.374) [DOI] [PubMed] [Google Scholar]
- 48.Stoker C, Rodriguez H, Ramos JG, Sirosky P, Larriera A, Luque EH, Muñoz-de-Toro M. 2003. Sex reversal effects on Caiman latirostris exposed to environmentally relevant doses of the xenoestrogen bisphenol A. Gen. Comp. Endocrinol. 133, 287–296 (doi:10.1016/S0016-6480(03)00199-0) [DOI] [PubMed] [Google Scholar]
- 49.Wingfield JC, Farner DS. 1975. The determination of five steroids in avian plasma by radioimmunoassay and competitive protein binding. Steroids 26, 311–327 (doi:10.1016/0039-128X(75)90077-X) [DOI] [PubMed] [Google Scholar]
- 50.Bowden RM, Ewert MA, Nelson CE. 2000. Environmental sex determination in a reptile varies seasonally and with yolk hormones. Proc. R. Soc. Lond. B 267, 1745–1749 (doi:10.1098/rspb.2000.1205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang LQ, James MO. 2006. Inhibition of sulfotransferases by xenobiotics. Curr. Drug Metab. 7, 83–104 (doi:10.2174/138920006774832596) [DOI] [PubMed] [Google Scholar]
- 52.Fry DM, Toone CK. 1981. DDT-induced feminization of gull embryos. Science 213, 922–924 (doi:10.1126/science.7256288) [DOI] [PubMed] [Google Scholar]
- 53.Anway MD, Skinner MK. 2006. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology 147, S43–S49 (doi:10.1210/en.2005-1058) [DOI] [PubMed] [Google Scholar]
- 54.Howdeshell KL, vom Saal FS. 2000. Developmental exposure to bisphenol A: interaction with endogenous estradiol during pregnancy in mice. Am. Zool. 40, 429–437 (doi:10.1668/0003-1569(2000)040[0429:DETBAI]2.0.CO;2) [Google Scholar]


