Abstract
Bottom trawls are a globally used fishing gear that physically disturb the seabed and kill non-target organisms, including those that are food for the targeted fish species. There are indications that ensuing changes to the benthic invertebrate community may increase the availability of food and promote growth and even fisheries yield of target fish species. If and how this occurs is the subject of ongoing debate, with evidence both in favour and against. We model the effects of trawling on a simple ecosystem of benthivorous fish and two food populations (benthos), susceptible and resistant to trawling. We show that the ecosystem response to trawling depends on whether the abundance of benthos is top-down or bottom-up controlled. Fishing may result in higher fish abundance, higher (maximum sustainable) yield and increased persistence of fish when the benthos which is the best-quality fish food is also more resistant to trawling. These positive effects occur in bottom-up controlled systems and systems with limited impact of fish feeding on benthos, resembling bottom-up control. Fishing leads to lower yields and fish persistence in all configurations where susceptible benthos are more profitable prey. Our results highlight the importance of mechanistic ecosystem knowledge as a requirement for successful management.
Keywords: bottom trawl fishery, bottom-up control, ecosystem-based fishery management, marine soft-bottom community, maximum sustainable yield, top-down control
1. Introduction
There is global concern about the effects of bottom trawling on aquatic ecosystems [1,2]. Bottom trawl fisheries target demersal fish, crustaceans and shellfish by towing fishing gear over the sea floor, thereby not only manipulating the abundance of the target species, but also physically disturbing the seabed, damaging benthic organisms and potentially changing the functioning of the entire benthic ecosystem [1]. The FAO estimates that bottom trawling accounts for 23% of the global fishery capture [3]. This type of fishery occurs predominantly in soft-bottom habitats on the continental shelf, where certain locations may be trawled as often as several times per year [4,5]. The direct impact of the gear on the seabed is seen as a major impediment to sustainability in trawl fisheries [1]. A wide variety of gear modifications and gear restrictions are in development to reduce the effect of bottom trawls on the seabed [6].
The occurrence and magnitude of mortality from bottom trawling on benthic invertebrates is highly species-dependent. Some, such as large bivalves and crustaceans, suffer high mortality with long recovery times, whereas others, such as certain annelids, are virtually unaffected [7]. Generally, it is thought that hard-bodied and large benthic invertebrates are affected most, and that chronic trawling induces a shift in the benthic community towards smaller and soft-bodied species [8–10]. Smaller species are also often associated with shorter generation times, which could lead to higher resilience after disturbances [11]. The trawling-induced shift to smaller species has been shown in several modelling studies [12,13].
Some benthic invertebrates make up the food for flatfish which are targeted by specific bottom trawl fisheries. A debate is ongoing in the literature as to whether bottom trawling can actually increase the food availability for flatfish, by shifting the benthic community towards the ‘fish food’ species [11,13–15]. Fuelling this debate, certain studies report increased growth rates of benthivorous flatfish, plaice (Pleuronectus platessa) and sole (Solea solea), coinciding with higher trawling intensity [16,17], which could be explained by a trawling-induced shift of productivity towards those benthic invertebrates that the flatfish feed on [18]. Others have argued that bottom trawling has negative effects on the food availability [19], or that these effects are substrate-dependent [20]. However, none of these studies took into account the feedback effect of fish, feeding on benthic invertebrates and the manipulation of fish abundance by fishing.
Here, we study a model of these interactions among two different types of benthic invertebrates, a fish predator and bottom trawling as a source of mortality for both fish and benthic prey. We do this for a top-down controlled system, where abundances of benthic invertebrates are largely controlled by fish predation and for a bottom-up controlled system where resource limitation determines the abundances of benthic invertebrates, which, in turn, determines fish abundance. We study both configurations, because the mode of trophic control governs the occurrence and shape of trophic cascades in response to external manipulation of ecosystems, such as fisheries [21]. Both forms of trophic control occur [22] and many studies indicate the importance of both predation (for review, see Seitz [23]) and competition [24–26] as structuring processes in soft-bottom habitats. It is unclear whether there is a single predominant mode of trophic control in soft-bottom benthic ecosystems [27].
Our model describes the generic food web interactions between functional groups (not particular species) and the effects of varying trawling mortality on these groups. Our results apply to benthic ecosystems and bottom trawl fisheries in general. We show that the effects of trawling intensity on the abundances of benthic invertebrates, fish and fisheries yield, depend on the mode of trophic control of the community. Indirect positive effects of trawling on fish abundance and fisheries yield occur in a bottom-up controlled system, when resistant invertebrates are a more profitable prey for fish. The same positive effects may occur in top-down controlled systems when fish have a limited predation impact on benthos. The difference in trawling impact between top-down and bottom-up controlled benthic systems highlight that a mechanistic understanding of benthic community functioning is a prerequisite for a successful management of trawled fish stocks and to conserve the benthic community.
2. Method
(a). Model description
We formulated and analysed two different models, dependent on the mode of trophic control, with fish and two benthic invertebrate prey species (hereafter: benthos). The prey differ in vulnerability to trawling and in their profitability to fish.
Benthos follows in both models semi-chemostat dynamics in absence of predation, with turnover rate r and maximum density Bmax. Interspecific competition is modelled as a dependence of the maximum abundance of each benthos group on the density of the other, implicitly assuming competition for a shared, constant resource, such as space. Both competition for space and food have been observed in field studies in soft-bottom environments [24–26]. Explicit modelling of resource competition between benthos using a dynamic resource would lead to competitive exclusion of one of the benthos groups [28].
(b). Top-down controlled benthic system
The dynamics of both susceptible (BS) and resistant (BR) benthos and fish (S) in a top-down controlled system are described by the following ordinary differential equations:
| 2.1 |
| 2.2 |
| 2.3 |
Predation mortality on the benthos follows a linear functional response, with fish attack rate α. The change in fish biomass depends on attack rate α, and on the abundance and conversion efficiencies gBs and gBr of the prey species. The mortality rate of fish consists of the trawling intensity f (for fish trawling intensity equals mortality) and natural mortality μ. Benthos is subjected to the same trawling intensity f, but scaled by a factor m. The parameter σ represents the asymmetric trawling vulnerability between the benthos groups. As long as σ > 1, trawling mortality is always higher for the susceptible than for the resistant benthos, but note that mortality on each benthos group can be both higher or lower than on fish, dependent on m. The fish attack rate α is used to vary the strength of fish predation in a top-down controlled benthic system. At high α, there is a strong impact of fish predation on the abundance of benthos, whereas at low α, the numerical impact of fish feeding on benthos remain small.
(c). Bottom-up controlled benthic system
Benthos is entirely controlled by their resources in a bottom-up controlled system. The problem with such a system is that there is no non-trivial fish equilibrium. Because fish density is unregulated, it either goes extinct, or to infinity. One way to overcome this is by assuming that fish biomass is an instantaneous function of its environment, in terms of food and mortality. This approximation of equilibrium fish biomass is achieved by setting equation (2.3) equal to zero and solving for S (equation (2.6)). The bottom-up regulated system is then described by:
| 2.4 |
| 2.5 |
| 2.6 |
where equations (2.4) and (2.5) are equal to (2.1) and (2.2) minus the effect of fish feeding on benthos.
(d). Asymmetry in prey profitability
Because benthic species differ in energetic content, defence mechanisms against predation (shells, for example) and vertical position in the seabed, asymmetry in benthos edibility to fish may be expected. This asymmetry has been implemented in our model using the conversion efficiency g. This reflects our choice to keep the model as simple and generic as possible. Both higher conversion efficiencies of the resistant benthos group (gBs < gBr) and higher efficiencies of the susceptible benthos group (gBs > gBr) have been studied. Besides using the conversion efficiency, we have studied two alternative types of asymmetry (difference in productivity and in prey-specific edibility), and find no qualitative difference with our results (see electronic supplementary material, appendix S1).
(e). Parametrization
We used semi-chemostat dynamics to describe invertebrate growth, which means that no predator–prey cycles occur [29]. Parameters r and Bmax can be chosen arbitrarily without affecting the qualitative behaviour of the model [29]. We assumed a 40-fold difference (σ = 40) in trawling vulnerability between susceptible and resistant benthos groups. This is in line with direct beam trawl mortality estimates of 20–30% for susceptible and less than 0.5% for resistant species, particularly annelids [30]. However, trawling vulnerability between susceptible and resistant benthos groups may vary dependent on type of trawl and habitat [7]. For that reason, the sensitivity of our model outcome is tested in the Results section for a range of σ values. In general, our results are robust to substantial variation in σ.
Piet et al. [31] estimated the mean annual trawling mortalities for the most susceptible macrobenthic species in the Dutch sector of the North Sea at 31–44%. This is comparable with the mean annual fishing mortality for plaice and sole, 49% and 45% respectively, in the North Sea during the same period [32]. Hence, we set the trawling mortality of susceptible benthos equal to that of fish, whereas resistant benthos have mortalities 40 times as low (1/σ). We used fish natural mortality μ = 0.1 per year, which is also used in stock assessments of plaice and sole [32]. Parameter values are summarized in table 1. Although our models described changes in biomass, we used biomass and abundance interchangeably throughout the manuscript.
Table 1.
Model parameters and their values. y, year; V, unit of volume; m, unit of mass.
| description | symbol | default value | unit |
|---|---|---|---|
| benthic growth rate | r | 1 | y−1 |
| benthic carrying capacity | Bmax | 2.5 | m V−1 |
| fish attack rate | α | 0–1 | y−1 |
| susceptible benthos conversion efficiency | gBs | 0.3 or 0.6 | m m−1 |
| resistant benthos conversion efficiency | gBr | 0.3 or 0.6 | m m−1 |
| fish natural mortality | μ | 0.1 | y−1 |
| trawling intensity | f | varied | y−1 |
| benthos asymmetric vulnerability to trawling | σ | 40 | — |
| scaled gear impact of trawling on benthos | m | 0–4 | — |
(f). Analysis
We showed the long-term effects of trawling on benthos and fish by numerical continuation of equilibrium biomass densities of the system with changing parameter values, using the software package Content [33]. Trawling intensity f, attack rate α, conversion efficiencies gBs and gBr, benthos asymmetric vulnerability to trawling σ and the scaled trawling impact on benthos m were all varied.
3. Results
(a). Model dynamics of benthos and fish
In a bottom-up controlled system benthos biomass remains at carrying capacity (Bmax), independent of fish attack rate, whereas the net biomass production remains at zero. Fish biomass increases linearly with increasing attack rate (figure 1, dashed lines).
Figure 1.
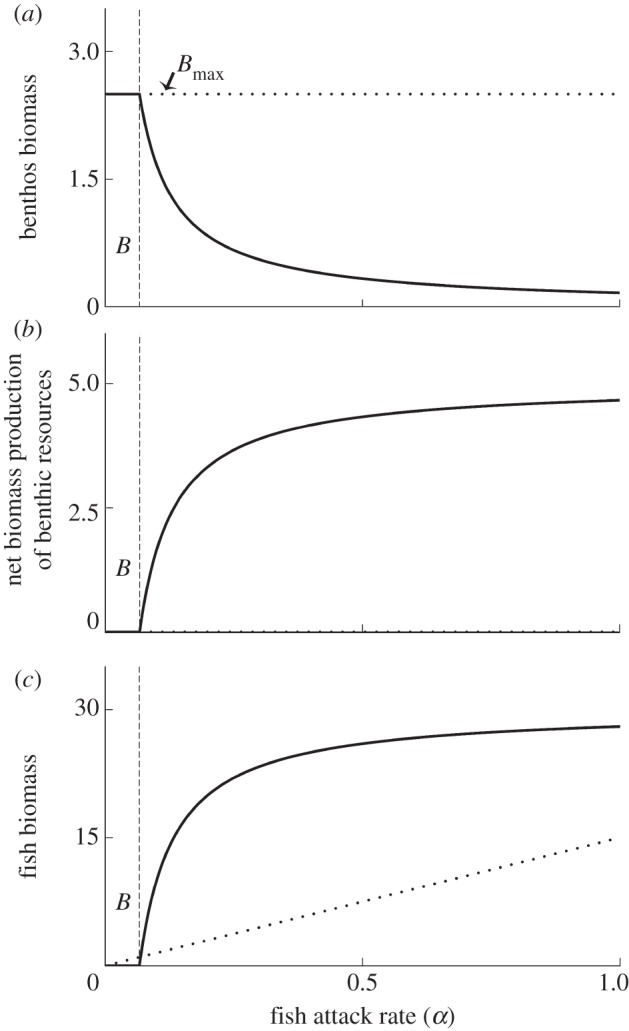
(a) Benthos biomass, (b) net biomass production of benthic resources r(Bmax−(BS + BR)) + r(Bmax−(BR + BS)) and (c) fish biomass as function of the fish attack rate α (both (a) and (b) are the sum of BS and BR). The solid lines show model results when system is top-down controlled, whereas dotted lines show model outcome when system is bottom-up controlled (dotted line in (b) is at zero). At low values of α, fish cannot persist in a top-down controlled system (to the left of the vertical dashed lines). Higher values of α increase fish predation and this results in coexistence between both benthos and fish. B, persistence of benthos without fish. f = 0, m = 0, gBs and gBr are both 0.6, all other parameters have default values.
By contrast, in a top-down controlled system, there is a minimum fish attack rate (α) below which fish cannot persist even in the absence of fishery and where benthos abundances equal carrying capacity (figure 1, solid lines). Close to this persistence threshold, fish equilibrium abundance is low and its effect on the benthos equilibrium densities limited. The presence of fish induces increased net biomass production of both benthos groups. This effect becomes more pronounced at higher attack rates as feeding by fish reduces competition among the benthos (figure 1b). The increased net production leads to strongly increased fish equilibrium abundance (figure 1c).
(b). Impact of bottom trawl fishery on benthos and fish
Bottom trawling increases the mortality of fish and potentially of benthos, and changes the predation pressure on, and the competitive interactions between the two benthos prey groups. The ecosystem effects of trawling depend on whether the system is bottom-up or top-down controlled (figure 2).
Figure 2.
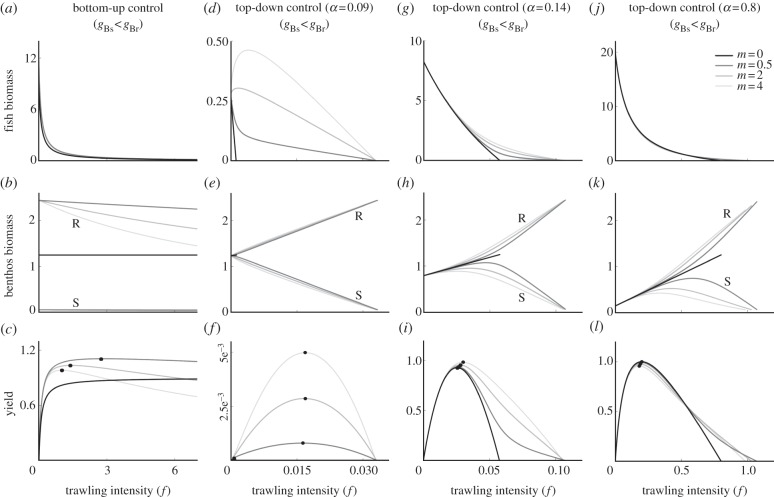
Impact of bottom trawling (by varying f) on fish biomass, benthos biomass and yield, the product of the trawling intensity (f) and fish density, in a bottom-up controlled system (a–c) and three systems with various strengths of top-down control (d–f, α = 0.09; g–i, α = 0.14; j–l, α = 0.8). Resistant benthos are more profitable food for fish (gBs < gBr). The different grey coloured lines present different scaled trawling mortalities on benthos (m). Susceptible and resistant benthos (in b, e, h and k) have the same biomass levels at m = 0. All lines above this black line (m = 0) show biomass levels of resistant benthos (marked with R), whereas all lines below this black line show biomass levels of susceptible benthos (marked with S). The dots (in c, f, i and l) represent maximum sustainable yield levels. gBs = 0.3, gBr = 0.6, all other parameters have default values.
In a bottom-up controlled system, fish predation has no effect on benthos. Therefore, when fishing only affects fish (m = 0), trawling simply reduces fish density (figure 2a). When trawling does affect benthos (m > 0), both benthos and fish respond to trawling. For any degree of asymmetry (σ > 1) between the susceptible and resistant benthos, trawling causes susceptible benthos to lose competition with resistant benthos and this reduces susceptible benthos to very low abundance, whereas resistant benthos abundance strongly increases (these initial changes occur at low f and are not visible in figure 2b). Further increasing trawling intensity leads to a gradual reduction of the resistant benthos (figure 2b). When the resistant is also the more profitable benthos (gBs < gBr), trawling increases the quality of the available prey for fish and can cause a positive relationship between fishing intensity and fish abundance. This positive relationship between trawling intensity and fish abundance occurs up to a certain maximum trawling intensity (f) (not visible in figure 2a as it occurs at very low levels of f), which decreases with the strength of the direct effect of trawling on benthos (m), but increases with σ, the degree of asymmetry of this effect on benthos (figure 3a,b).
Figure 3.
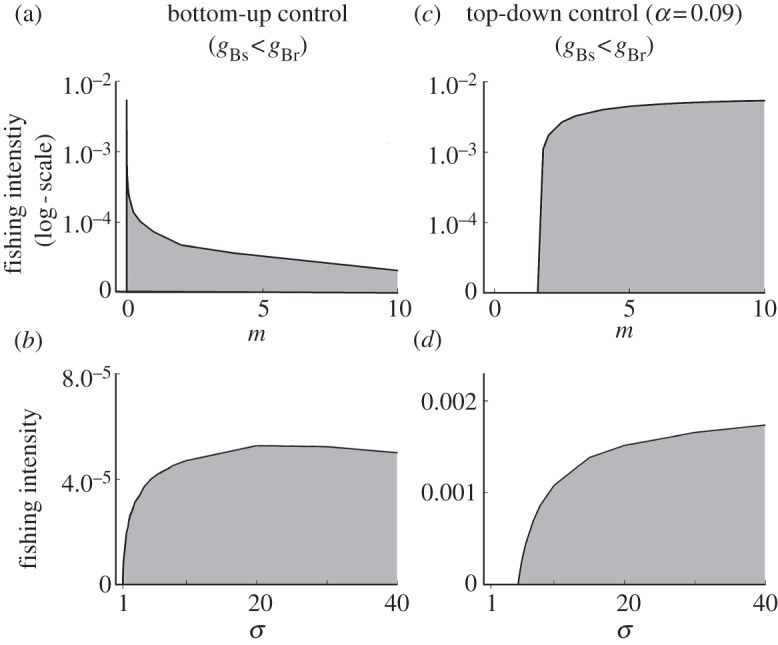
The increase in fish biomass (occurring inside the grey area) as a result of bottom trawling, in relation to the trawling intensity (f) and the scaled fishing mortality on benthos (m) (a,c) or the relative impact of bottom trawling on resistant compared to susceptible benthos (σ) (b,d) in a bottom-up controlled system (a,b) and a system with weak top-down control (α = 0.09, c,d). gBs = 0.3, gBr = 0.6, m = 2 for (b,d), all other parameters have default values.
If, in a bottom-up controlled system, susceptible benthos is the more profitable prey (gBs > gBr), then the decline in fish abundance with trawling intensity is accelerated at higher m, because the quality of the available prey is reduced (figure 4a). This results in less fish and lower fishery yields (figure 4b).
Figure 4.
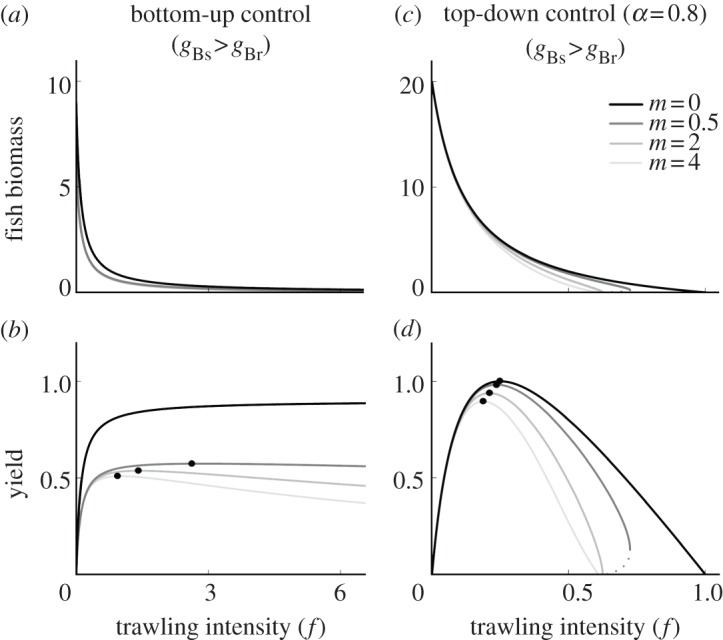
Impact of bottom trawling (by varying f) on fish biomass and yield, the product of the trawling intensity (f) and fish density, in both a system which is bottom-up controlled (a,b) and a strong top-down controlled system (c,d, α = 0.8). Susceptible benthos are more profitable food for fish (gBs > gBr). Solid lines correspond to stable equilibria, dashed lines to unstable equilibria. gBs = 0.6, gBr = 0.3, all else is similar to figure 2.
Under both strong and intermediate top-down control (α = 0.8 and 0.14), trawling reduces the abundance of fish (figure 2g,j). Initially, this leads to higher biomass of both benthos groups, as they suffer reduced predation mortality (figure 2h,k). When there is a direct effect of trawling on benthos (m > 0), the positive effect on the susceptible benthos is reduced, whereas that on the resistant benthos is reinforced by reduced competition. When this divergence is stronger, the larger the effect of trawling on benthos. At high trawling intensity, the upward trend is reversed in the susceptible species as direct trawling mortality outweighs reduced predation mortality.
With any degree of top-down control, trawling intensity drives fish to extinction at the point where fish intake can no longer compensate for mortality (figure 2d,g,j). Generally, this occurs at higher trawling intensity as the fish attack rate increases (compare figure 2f,i,l). When the resistant benthos group is also the more valuable fish food (gBr > gBs), a direct effect of trawling on benthos (m > 0) extends the range of trawling intensity under which fish can persist. This happens because trawling incurs a food quality subsidy which makes up for part of the mortality it imposes on fish. Furthermore, under a range of trawling intensity, a direct effect of trawling on benthos increases both fish biomass (figure 2d,g,j) and fishery yield (figure 2f,i,l). The opposite occurs when susceptible benthos are more profitable prey (figure 4c,d): the stronger the direct effect of trawling on benthos (m), the lower the fish abundance and yield and the earlier fish go extinct.
A weakly top-down controlled system (α = 0.09) behaves somewhat similar to a bottom-up controlled system, because fish have only a limited impact on benthos. It shows both increasing yield with higher m and a positive relationship between trawling and fish biomass (figure 2d,f). However, this positive relationship only occurs when both the trawling effect on benthos and the asymmetry in trawl susceptibility between benthos groups are large enough (figure 3c above m ∼ 1.6 and figure 3d above σ ∼ 5).
(c). Impact of bottom trawl fishery on maximum sustainable yield
In a bottom-up controlled system, maximum sustainable yield (MSY) is generally higher and occurs at higher trawling intensity, the smaller the effect of trawling on benthos. When there is no effect on benthos at all (m = 0), maximum yield occurs at infinitely high trawling intensity and can hardly be classified as sustainable, because it occurs at infinitely low fish biomass (figure 2a,c).
As trawling intensity increases and fish abundance is reduced, any top-down regulated system behaves more ‘bottom-up’, as fish are decreasingly able to control benthos biomass. At weak and intermediate top-down control, this shift occurs relatively early and leads to an increased MSY with higher m (figure 2f,i). The same shift occurs in a strongly top-down controlled system but at fishing intensities higher than MSY, in that case it reduces MSY with a stronger trawling effect on benthos (figure 2l). The same decrease in MSY with increasing m occurs when the susceptible prey is the more profitable (figure 4b,d).
4. Discussion
We have shown that direct mortality of trawl fishery on non-target benthic organisms can lead to persistence of fish up to higher trawling intensities, increased fish biomass and (maximum sustainable) fisheries yield, and a positive relationship between fish abundance and trawling intensity. The presence of these indirect effects depends on the mode and degree of trophic control (top-down or bottom-up) of the benthic organisms, and on the relative susceptibility of the most important benthic fish prey to trawling.
When the benthos which is the best-quality fish food is also more resistant to trawling than the lower-quality benthos, trawl gear that incurs higher mortality on the benthos can lead to higher fish biomass than more selective gear with less effect on the benthos. This result is independent of the mode of trophic regulation. In top-down controlled systems, this also extends the maximum trawling intensity at which fish can persist. The increase in fish biomass leads to higher fishery yield under both top-down and bottom-up control, and to higher MSY under all but the strongest top-down control. When susceptible benthos are the most profitable prey, trawling reduces fish abundance, yield and persistence of fish in all situations.
Under bottom-up and weak top-down control, a positive relationship between trawling intensity and fish biomass emerges as fishing increases the quality of the available prey to such an extent that it more than offsets the direct mortality it imposes on fish. This occurs over a wide range of non-target effects of trawling, but only at low trawling intensity (figure 3). The relevance is hence only expected for irregular fished stocks, where it could lead to a peak in fish abundance. By contrast, increased (maximum sustainable) yield and persistence of fish occur at higher trawling intensity, and so are more relevant to the management of highly exploited ecosystems.
Our analysis shows that in both top-down and bottom-up controlled systems, the abundance of the resistant benthos is positively related to trawling intensity, as a result of either reduced fish predation or interspecific competition. This corresponds with empirical observations of increased abundance of annelids and polychaetes, generally considered resistant to trawling, in heavily trawled areas [8–10]. However, such observational data do not allow us to distinguish between the two possible mechanisms (reduced predation or competition).
Fishing generally leads to reduced abundance of susceptible benthos in our model. This is also found in a number of field studies that have shown a higher abundance of susceptible invertebrates, such as large bivalves and spatangoids, in areas with lower trawling intensities [9] or areas closed for bottom trawling [10,34]. Our model shows that the impact of bottom trawling on susceptible benthos can be mitigated by reducing the mortality imposed by trawling. This may be accomplished by either reducing fishing effort, or through technical adaptations reducing the direct impact of the trawl on the seabed. Development of such less destructive trawls is an active field of research [6,35]. Our results indicate that this may actually, dependent on the mode of trophic control and asymmetry in prey profitability, lead to reduced fish abundance and yield.
The trawling-induced effects on the food availability for fish affect MSY differently in top-down or bottom-up controlled systems (figure 2, yield). A positive effect of trawling on resistant benthos leads to an increased fish abundance and MSY in a bottom-up controlled system (figure 2a,c). This phenomenon occurs at trawling impacts on benthos which reflect those found in the North Sea. There are indications that in the North Sea, the more profitable prey (in particular polychaetes) are also more resistant to trawling [18], which is the configuration for which we find a positive effect of benthic trawling mortality on fish abundance and yield.
Several studies have found faster growth of benthivorous fish with higher trawling intensity [16,17,20]. By contrast, Hiddink et al. [15] found, in the most comprehensive study on this interaction, a negative relation between the condition of plaice individuals and trawling frequency in a field study in the Irish Sea, whereas no effect of trawling was found on the condition of Dab (Limanda limanda). They hypothesized that trawling could indirectly affect growth of target species, resulting in lower fisheries yield. This result is compatible with the model configuration with strong top-down control and/or the susceptible benthos being the most profitable prey, in that case there is no trawling-induced increase in fish resources and the highest MSY occurs when there is no impact of trawling on benthos at all (m = 0 in figures 2l and 4b,d). In this case, the use of fishing gear that minimizes benthic mortality would lead to higher abundance, catches and increased persistence of fish. To the best of our knowledge, there are no studies measuring the long-term consequences of the indirect effects of trawling on fish abundance and MSY.
The response of our modelled community to trawling depends strongly on the asymmetry between the two benthos groups not only in their vulnerability to trawling, but also in their role in the food web. We have used the conversion efficiency parameter as a generic way to impose such asymmetry, but alternative mechanisms are easily conceivable. One alternative is a difference in edibility (or preference) of the benthos groups to fish. We have shown that the results of our analysis are qualitatively identical under this assumption (see electronic supplementary material, appendix S1). Another possibility is that one group has a higher intrinsic growth rate (is more r-selected [36]) and can more efficiently recolonize the ‘free space’ created by trawling. Assuming that such fast-growing species would generally be smaller, Jennings et al. [14] hypothesized that increasing trawling intensity would coincide with smaller benthic invertebrates. Because fish are gape limited [37], a shift to smaller individuals in the benthic community means that a larger proportion is edible to fish. Jennings et al. [14] did not find this effect in field data, but they have no information on fish presence or feeding in their study area, which may have confounded their results. Asymmetry in productivity between susceptible and resistant benthos (see electronic supplementary material, appendix S1) does not qualitatively change our results. The addition of size structure and size-dependent predation in the benthos community is beyond the scope of this study, but could profoundly affect community dynamics and response to trawling [29,38].
Besides a shift towards more profitable prey, other mechanisms by which trawling may increase the food availability for benthivorous fish have also been suggested. The physical disturbance of the seabed and resulting resuspension of nutrients may have increased the primary productivity [5,39]. This higher productivity could then lead to increased benthic productivity. Others have suggested that food subsidies owing to discards and killed organisms in the trawl path can also positively affect the food availability for fish, by delivering easy prey for (scavenging) fish [40,41], but the effect of these food subsidies is considered relatively small on scavenger population levels in the southern North Sea [41].
The response of the benthic component in our model to trawling is consistent with other modelling studies [12,13], which did not incorporate fish predation. However, the interaction between fish and its benthic prey in our model has substantially increased the complexity of the response to trawling. To assess the importance of predation in marine benthic communities, caging experiments are still seen as the most valid method [42], but we know of no predator exclusion experiments conducted at the feeding grounds of commercially important benthivorous fish species.
In this study, we have examined a bottom-up controlled system and three systems with various strengths of top-down regulation. However, the mode of regulation is not a fixed property of natural systems, but depends on the state and history of the system. This is illustrated in our model, where top-down controlled benthic systems behave more bottom-up controlled as the fish population is depleted by fishery (figure 2g,l). Our results show that an assessment of the degree to which a system is bottom-up or top-down controlled should be central to any strategy of adaptive and ecosystem-based management of exploited fish stocks, because it is a key determinant of how the system responds to exploitation. For the North Sea, Heath [43] suggested that macrobenthic species were predominantly top-down controlled. If this is correct, then our results imply that a positive effect of trawling on the food availability of benthivorous fish is expected only at high trawling intensities. It also implies that gear adaptations minimizing damage to benthos may result in higher abundance of susceptible benthos.
Our work highlights that the relative importance of bottom-up and top-down processes is crucial for understanding the dynamics of benthic communities. We also show that incorrect assumptions regarding trophic control of the ecosystem can lead to remarkable failure of management of exploited benthivorous fish and the conservation of benthos. Unfortunately, little is known about the trophic regulation of marine benthic ecosystems worldwide, but our work highlights that further study is urgently required in the light of the recent worldwide push for ecosystem-based marine management [44].
Acknowledgements
We thank André M. de Roos and an anonymous reviewer for their helpful comments on earlier versions of this manuscript.
Funding statement
This research was partially supported through the policy support research programme (BO) of the Dutch Ministry of Economic Affairs to T.vK. and P.D.vD., through the Strategic Research Programme on Sustainable Spatial Development of Ecosystems, Landscapes, Seas and Regions to A.D.R. and the FP7 project BENTHIS (312088) to T.vK. and A.D.R. The article does not necessarily reflect the views of the European Commission and does not anticipate the Commission's future policy in this area.
References
- 1.Kaiser MJ, Collie JS, Hall SJ, Jennings S, Poiner IR. 2002. Modification of marine habitats by trawling activities: prognosis and solutions. Fish Fish. 3, 114–136 (doi:10.1046/j.1467-2979.2002.00079.x) [Google Scholar]
- 2.Puig P, Canals M, Company JB, Martin J, Amblas D, Lastras G, Palanques A, Calafat AM. 2012. Ploughing the deep sea floor. Nature 489, 286–289 (doi:10.1038/nature11410) [DOI] [PubMed] [Google Scholar]
- 3.FAO 2009. The state of world fisheries and aquaculture — 2008 (SOFIA), p. 176 Rome, Italy: FAO [Google Scholar]
- 4.Rijnsdorp AD, Buys AM, Storbeck F, Visser EG. 1998. Micro-scale distribution of beam trawl effort in the southern North Sea between 1993 and 1996 in relation to the trawling frequency of the sea bed and the impact on benthic organisms. ICES J. Mar. Sci. 55, 403–419 (doi:10.1006/jmsc.1997.0326) [Google Scholar]
- 5.Pilskaln CH, Churchill JH, Mayer LM. 1998. Resuspension of sediment by bottom trawling in the Gulf of Maine and potential geochemical consequences. Conserv. Biol. 12, 1223–1229 (doi:10.1046/j.1523-1739.1998.0120061223.x) [Google Scholar]
- 6.Valdemarsen JW, Jorgensen T, Engas A. 2007. Options to mitigate bottom habitat impacts of dragged gear, p. 29 Rome, Italy: FAO Fisheries. Technical paper no. 506 [Google Scholar]
- 7.Kaiser MJ, Clarke KR, Hinz H, Austen MCV, Somerfield PJ, Karakassis I. 2006. Global analysis of response and recovery of benthic biota to fishing. Mar. Ecol. Prog. Ser. 311, 1–14 (doi:10.3354/meps311001) [Google Scholar]
- 8.Engel J, Kvitek R. 1998. Effects of otter trawling on a benthic community in Monterey Bay National Marine Sanctuary. Conserv. Biol. 12, 1204–1214 (doi:10.1046/j.1523-1739.1998.0120061204.x) [Google Scholar]
- 9.Kaiser MJ, Ramsay K, Richardson CA, Spence FE, Brand AR. 2000. Chronic fishing disturbance has changed shelf sea benthic community structure. J. Anim. Ecol. 69, 494–503 (doi:10.1046/j.1365-2656.2000.00412.x) [Google Scholar]
- 10.Duineveld GCA, Bergman MJN, Lavaleye MSS. 2007. Effects of an area closed to fisheries on the composition of the benthic fauna in the southern North Sea. ICES J. Mar. Sci. 64, 899–908 (doi:10.1093/icesjms/fsm029) [Google Scholar]
- 11.Jennings S, Dinmore TA, Duplisea DE, Warr KJ, Lancaster JE. 2001. Trawling disturbance can modify benthic production processes. J. Anim. Ecol. 70, 459–475 (doi:10.1046/j.1365-2656.2001.00504.x) [Google Scholar]
- 12.Duplisea DE, Jennings S, Warr KJ, Dinmore TA. 2002. A size-based model of the impacts of bottom trawling on benthic community structure. Can. J. Fish. Aquat. Sci. 59, 1785–1795 (doi:10.1139/F02-148) [Google Scholar]
- 13.Hiddink JG, Rijnsdorp AD, Piet G. 2008. Can bottom trawling disturbance increase food production for a commercial fish species? Can. J. Fish. Aquat. Sci. 65, 1393–1401 (doi:10.1139/F08-064) [Google Scholar]
- 14.Jennings S, Nicholson MD, Dinmore TA, Lancaster JE. 2002. Effects of chronic trawling disturbance on the production of infaunal communities. Mar. Ecol. Prog. Ser. 243, 251–260 (doi:10.3354/meps243251) [Google Scholar]
- 15.Hiddink JG, Johnson AF, Kingham R, Hinz H. 2011. Could our fisheries be more productive? Indirect negative effects of bottom trawl fisheries on fish condition. J. Appl. Ecol. 48, 1441–1449 (doi:10.1111/j.1365-2664.2011.02036.x) [Google Scholar]
- 16.Rijnsdorp AD, van Leeuwen PI. 1996. Changes in growth of North Sea plaice since 1950 in relation to density, eutrophication, beam-trawl effort, and temperature. ICES J. Mar. Sci. 53, 1199–1213 (doi:10.1006/jmsc.1996.0145) [Google Scholar]
- 17.Millner RS, Whiting CL. 1996. Long-term changes in growth and population abundance of sole in the North Sea from 1940 to the present. ICES J. Mar. Sci. 53, 1185–1195 (doi:10.1006/jmsc.1996.0143) [Google Scholar]
- 18.Rijnsdorp AD, Vingerhoed B. 2001. Feeding of plaice Pleuronectes platessa L. and sole Solea solea (L.) in relation to the effects of bottom trawling. J. Sea Res. 45, 219–229 (doi:10.1016/S1385-1101(01)00047-8) [Google Scholar]
- 19.Hinz H, Prieto V, Kaiser MJ. 2009. Trawl disturbance on benthic communities: chronic effects and experimental predictions. Ecol. Appl. 19, 761–773 (doi:10.1890/08-0351.1) [DOI] [PubMed] [Google Scholar]
- 20.Shephard S, Brophy D, Reid D. 2010. Can bottom trawling indirectly diminish carrying capacity in a marine ecosystem? Mar. Biol. 157, 2375–2381 (doi:10.1007/s00227-010-1502-9) [Google Scholar]
- 21.Pace ML, Cole JJ, Carpenter SR, Kitchell JF. 1999. Trophic cascades revealed in diverse ecosystems. Trends Ecol. Evol. 14, 483–488 (doi:10.1016/S0169-5347(99)01723-1) [DOI] [PubMed] [Google Scholar]
- 22.Menge BA. 2000. Top-down and bottom-up community regulation in marine rocky intertidal habitats. J. Exp. Mar. Biol. Ecol. 250, 257–289 (doi:10.1016/S0022-0981(00)00200-8) [DOI] [PubMed] [Google Scholar]
- 23.Seitz RD. 1998. Incorporation of soft-sediment systems into a model of marine benthic community regulation. Mar. Freshw. Res. 49, 817–826 (doi:10.1071/MF97100) [Google Scholar]
- 24.Peterson CH, Andre SV. 1980. An experimental analysis of interspecific competition among marine filter feeders in a soft-sediment environment. Ecology 61, 129–139 (doi:10.2307/1937163) [Google Scholar]
- 25.Peterson CH. 1982. The importance of predation and intra- and interspecific competition in the population biology of two infaunal suspension-feeding bivalves, Protothaca staminea and Chione undatella. Ecol. Monogr. 52, 437–475 (doi:10.2307/2937354) [Google Scholar]
- 26.Nascimento F, Karlson A, Näslund J, Elmgren R. 2011. Diversity of larger consumers enhances interference competition effects on smaller competitors. Oecologia 166, 337–347 (doi:10.1007/s00442-010-1865-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson WH. 1991. Competition and predation in marine soft-sediment communities. Annu. Rev. Ecol. Syst. 21, 221–241 (doi:10.1146/annurev.es.21.110190.001253) [Google Scholar]
- 28.Hardin G. 1960. The competitive exclusion principle. Science 131, 1292–1297 (doi:10.1126/science.131.3409.1292) [DOI] [PubMed] [Google Scholar]
- 29.De Roos AM, Schellekens T, van Kooten T, van de Wolfshaar KE, Claessen D, Persson L. 2008. Simplifying a physiologically structured population model to a stage-structured biomass model. Theor. Popul. Biol. 73, 47–62 (doi:10.1016/j.tpb.2007.09.004) [DOI] [PubMed] [Google Scholar]
- 30.Bergman MJN, van Santbrink JW. 2000. Mortality in megafaunal benthic populations caused by trawl fisheries on the Dutch continental shelf in the North Sea in 1994. ICES J. Mar. Sci. 57, 1321–1331 (doi:10.1006/jmsc.2000.0917) [Google Scholar]
- 31.Piet GJ, Rijnsdorp AD, Bergman MJN, van Santbrink JW, Craeymeersch J, Buijs J. 2000. A quantitative evaluation of the impact of beam trawling on benthic fauna in the southern North Sea. ICES J. Mar. Sci. 57, 1332–1339 (doi:10.1006/jmsc.2000.0915) [Google Scholar]
- 32.ICES. 2012. Report of the working group on the assessment of demersal stocks in the North Sea and Skagerrak (WGNSSK). ICES document CM 2012/ACOM:13.1346 pp.
- 33.Kuznetsov YA, Levitin VV, Skovoroda AR. 1996. Continuation of stationary solutions to evolution problems in content. Amsterdam, The Netherlands: Centre for Mathematics and Computer Science [Google Scholar]
- 34.Murawski SA, Brown R, Lai HL, Rago PJ, Hendrickson L. 2000. Large-scale closed areas as a fishery-management tool in temperate marine systems: the Georges Bank experience. Bull. Mar. Sci. 66, 775–798 [Google Scholar]
- 35.Soetaert M, Decostere A, Polet H, Verschueren B, Chiers K. 2013. Electrotrawling: a promising alternative fishing technique warranting further exploration. Fish Fish. (doi:10.1111/faf.12047) [Google Scholar]
- 36.MacArthur RH, Wilson EO. 1967. The theory of island biogeography, (2001 reprint edn) Princeton, NJ: Princeton University Press [Google Scholar]
- 37.Piet GJ, Pfisterer AB, Rijnsdorp AD. 1998. On factors structuring the flatfish assemblage in the southern North Sea. J. Sea Res. 40, 143–152 (doi:10.1016/S1385-1101(98)00008-2) [Google Scholar]
- 38.Van Kooten T, De Roos AM, Persson L. 2005. Bistability and an Allee effect as emergent consequences of stage-specific predation. J. Theor. Biol. 237, 67–74 (doi:10.1016/j.jtbi.2005.03.032) [DOI] [PubMed] [Google Scholar]
- 39.Riemann B, Hoffmann E. 1991. Ecological consequences of dredging and bottom trawling in the Limfjord, Denmark. Mar. Ecol. Prog. Ser. 69, 171–178 (doi:10.3354/meps069171) [Google Scholar]
- 40.Kaiser MJ, Spencer BE. 1994. Fish scavenging behaviour in recently trawled areas. Mar. Ecol. Prog. Ser. 112, 41–49 (doi:10.3354/meps112041) [Google Scholar]
- 41.Groenewold S, Fonds M. 2000. Effects on benthic scavengers of discards and damaged benthos produced by the beam-trawl fishery in the southern North Sea. ICES J. Mar. Sci. 57, 1395–1406 (doi:10.1006/jmsc.2000.0914) [Google Scholar]
- 42.Hall SJ, Raffaelli D, Turrell WR. 1990. Predator-caging experiments in marine systems: a reexamination of their value. Am. Nat. 136, 657–672 (doi:10.2307/2462227) [Google Scholar]
- 43.Heath MR. 2005. Changes in the structure and function of the North Sea fish foodweb, 1973–2000, and the impacts of fishing and climate. ICES J. Mar. Sci. 62, 847–868 (doi:10.1016/j.icesjms.2005.01.023) [Google Scholar]
- 44.Pikitch EK, et al. 2004. Ecosystem-based fishery management. Science 305, 346–347 (doi:10.1126/science.1098222) [DOI] [PubMed] [Google Scholar]