Abstract
Introduction:
The relevance of tobacco use in opioid addiction (OA) has generated a demand for available and more effective interventions. Thus, further analysis of less explored nicotine–opioid clinical interactions is warranted.
Methods:
A post-hoc analysis of OA participants in a double-blind, randomized very low dose naltrexone (VLNTX) inpatient detoxification trial evaluated measures of opioid withdrawal and tobacco use. Intreatment smokers were compared with nonsmokers, or smokers who were not allowed to smoke.
Results:
A total of 141 (81%) of 174 OA participants were smokers, all nicotine-dependent. Inpatient smoking was a predictor of opioid withdrawal discomfort. Intreatment smokers (n = 96) showed significantly higher opioid craving (F = 3.7, p < .001) and lower detoxification completion rate (χ2 = 7.9, p < .02) compared with smokers who were not allowed to smoke (n = 45) or nonsmokers (n = 33). Smoking during treatment was associated with more elevated cigarette craving during detoxification (F = 4.1, p < .001) and a higher number of cigarettes smoked at follow-up (F = 3.6, p < .02). Among intreatment smokers, VLNTX addition to methadone taper was effective in easing opioid withdrawal and craving more than other treatments, whereas the combination VLNTX–clonidine was associated with significantly reduced cigarette craving and smoking during detoxification.
Conclusions:
Failure to address tobacco use may negatively affect pharmacologically managed opioid discontinuation. Opioid detoxification may offer a window of opportunity to expand smoking cessation treatment, hence improving OA outcomes. The observed effects support testing of VLNTX–clonidine in smoking cessation trials among individuals with or without substance abuse.
INTRODUCTION
Tobacco use in opioid addiction (OA) remains a clinical challenge despite continued progress in treatment for smoking cessation. Smoking prevalence rates of 80%–95% among OA individuals (Clemmey, Brooner, Chutuape, Kidorf, & Stitzer, 1997; Haas et al., 2008; Richter, Gibson, Ahluwalia, & Schmelzle, 2001) are higher than in other addictions (Kalman, Morissette, & George, 2005) and nearly 5 times higher than in the general population (Blazer & Wu, 2012; Centers for Disease Control and Prevention, 2011). The health cost of tobacco use is greater among active or recovering drug abusers: longitudinal studies in OA demonstrate that smoking is more detrimental than alcohol (Hughes et al., 1992), contributing to fourfold rates of excess mortality (Engström, Adamsson, Allebeck, & Rydberg, 1991; Hser, McCarthy, & Anglin, 1994). In comparison, opioid replacement therapy reduces death risk by one half (Clark, Samnaliev, Baxter, & Leung, 2011; Cornish, Macleod, Strang, Vickerman, & Hickman, 2010). There is reasonable concern that tobacco use may offset the benefits of effective interventions for OA. Thus, smoking cessation in OA is crucial to eliminate health disparities and reduce the burden of disease.
OA patients show a considerable interest in quitting smoking (Clarke, Stein, McGarry, & Gogineni, 2001; Frosch, Shoptaw, Jarvik, Rawson, & Ling, 1998) but have limited access to smoking cessation treatment (Richter, Choi, McCool, Harris, & Ahluwalia, 2004) and show 4 times lower cessation rates than the general U.S. population (Okoli et al., 2010; Richter et al., 2001). Tobacco use is often associated with negative opioid agonist treatment outcomes. Smoking increases requests for methadone and its rewarding effects (Frosch, Shoptaw, Nahom, & Jarvik, 2000; Spiga, Martinetti, Meisch, Cowan, & Hursh, 2005; Spiga, Schmitz, & Day, 1998). In turn, the administration of methadone or buprenorphine enhances smoking preferences (Elkader, Brands, Selby, & Sproule, 2009; Mello, Lukas, & Mendelson, 1985; Mutschler, Stephen, Teoh, Mendelson, & Mello, 2002; Richter et al., 2007), and high smoking rates are associated with higher levels of illicit drug use (Frosch, Nahom, & Shoptaw, 2002; Frosch et al., 2000; Harrell, Montoya, Preston, Juliano, & Gorelick, 2011; Shoptaw et al., 2002), which makes smoking cessation even more difficult (Frosch et al., 2002; Stapleton, Keaney, & Sutherland, 2009). These clinical relationships suggest possible functional interactions of opioid and nicotinic pathways in the brain (Berrendero, Robledo, Trigo, Martin-Garcia, & Maldonado, 2010).
Clinicians’ efforts may be facilitated if they had more systematic information on how smoking interacts with opioids under diverse treatment conditions. Many studies have assessed the effects of smoking in methadone- or buprenorphine-treated patients (for a review, see Guydish et al., 2011; Okoli et al., 2010), whereas clinical investigations have not examined how active smoking influences opioid use and withdrawal during detoxification. Although opioid detoxification cannot be considered an effective standalone treatment of OA, it is the primary point of contact with the treatment system for almost 50% of OA patients (Substance Abuse and Mental Health Services Administration, 2010). Reduced smoking has been recorded during heroin, buprenorphine, or methadone taper (Bigelow, Stitzer, Griffiths, & Liebson, 1981; Mello et al., 1985; Mello, Mendelson, Sellers, & Kuehnle, 1980), though cigarette craving and relapse into higher smoking rates have been described shortly after detoxification (Conner, Stein, Longshore, & Stacy, 1999). Tobacco users are less likely to successfully withdraw from opioids (Ziedonis et al., 2009), and some clinicians have argued that an attempt to quit smoking might interfere with a concurrent effort to discontinue other drug use (Campbell, Wander, Stark, & Holbert, 1995; Weinberger, Reutenauer, Vessicchio, & George, 2008). In addition, medications commonly used in detoxification, such as naltrexone and clonidine have been studied in smoking cessation with mixed results (David, Lancaster, Stead, & Evins, 2006; Herman & Sofuoglu, 2010). Thus, the study of smoking behaviors during opioid detoxification warrants further exploration.
We describe a post-hoc analysis of opioid withdrawal symptoms and smoking behaviors during a clinical study of methadone taper, withdrawal, and inpatient medical stabilization with very low dose naltrexone (VLNTX) in OA individuals. The observed attenuation of opioid withdrawal and craving intensity using VLNTX for the duration of detoxification was explained with its ability to reduce mu-opioid receptor activity and dependence levels during opioid agonist treatment (Mannelli, Gottheil, Peoples, Oropeza, & Van Bockstaele, 2004; Mannelli et al., 2009). In this study, intreatment smokers were compared with nonsmokers, and with smokers who were not allowed to smoke.
METHODS
Study Design
This was a 6-day, double-blind, randomized trial evaluating the safety and efficacy of two different oral naltrexone regimens (0.125 and 0.25mg/day) for the treatment of withdrawal in 174 OA subjects during inpatient methadone-based detoxification at two community treatment programs (Mannelli et al., 2009). Smoking cessation was not offered as part of the treatment, though access to smoking was permitted at one study site.
Subjects and Procedures
Participants were nonmethadone-treated OD patients age 18 years or older who were seeking detoxification at community treatment programs. Potential subjects were excluded for any of the following criteria: hypersensitivity to naltrexone, pregnancy, psychiatric or medical conditions rendering participation hazardous, current dependence on alcohol, or current dependence on substances other than opioids or nicotine. Psychiatric and medical examination, with routine laboratory tests including urine testing for drugs of abuse, were performed at screening. The Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; First, Spitzer, Gibbon, & Williams, 2002) was used to formulate psychiatric diagnoses. The Addiction Severity Index (ASI; McLellan et al., 1992) was used to evaluate drug use, social status, and psychological status. The Timeline Followback (TLFB) method (Sobell, Brown, Leo, & Sobell, 1996) was used to gather information on the use of psychotropic medications and pattern of illicit drug use. Patients were asked to participate in a 1-week evaluation following discharge. The screening process, treatment, and evaluation procedures are described in detail elsewhere (Mannelli et al., 2009).
Treatment
Subjects received the standard methadone taper schedule that was in use at the community treatment programs: a single 30-mg dose on Day 1 following baseline assessment, after which methadone was tapered by 5mg/day, with treatment completion and discharge on Day 6. In addition to methadone taper, subjects were randomly assigned to one of three add-on medication groups: placebo, naltrexone 0.125mg daily, or naltrexone 0.250mg daily. Ancillary medications were available as needed. Need for, and use of ancillary medications was determined in routine clinical fashion by the nursing and medical staff (Mannelli et al., 2009).
Clonidine flexible dose (0.1–0.2mg every 6 hr) was routinely used for detoxification only at the site where patients received permission to smoke. However, a nonconsecutive group of participants enrolled at that site did not receive the medication due to factors unrelated to the study or to characteristics of the subjects (e.g., low supply).
Smoking
The diagnosis of nicotine dependence was based on DSM-IV criteria; severity of dependence was rated using the Fagerström Test for Nicotine Dependence (FTND; 0–2 = very low dependence, 3–4 = low dependence, 5 = medium dependence, 6–7 = high dependence, and 8–10 = very high dependence) (Heatherton, Kozlowski, Frecker, & Fagerström, 1991). At one study site, subjects were allowed to smoke in a dedicated area between 10 a.m. and 12 p.m. and between 3 and 5 p.m. Cigarette counts were performed by study staff and every patient was asked to keep a daily smoking diary.
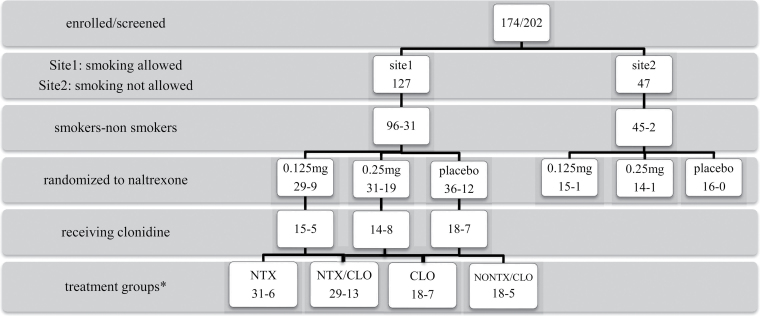
Figure 1 shows the patient flow for the participants in the study, including smoking status and treatment received.
Figure 1.
Flow diagram of clinical trial participants describing smoking and treatment status. CLO = clonidine; NONTX/CLO = no naltrexone–no clonidine; NTX = naltrexone; NTX/CLO = naltrexone + clonidine. *Patients receiving the two naltrexone doses were combined into a single naltrexone treatment condition as reported in Results section.
In-Study Assessments
Daily evaluation of opioid withdrawal and opioid and tobacco cravings was conducted between 9 and 10 a.m., before methadone and naltrexone administration or smoking. Opioid withdrawal was assessed using the Subjective Opiate Withdrawal Scale (SOWS; Handelsman et al., 1987), a self-rating instrument that evaluates the intensity of 16 symptoms on a 5-point Likert scale including a single item-defined craving (Kanof et al., 1992).
Cigarette craving was evaluated using the Brief Ques tionnaire of Smoking Urges (QSU-Brief; Cox, Tiffany, & Christen, 2001). The QSU-Brief is a self-report list of 10 items rated on a 100-point scale. Factor analyses of the items have identified distinct verbal descriptions of craving of the rewarding properties of smoking (“positive craving” five items) or of its capacity of relief from negative affect (“negative craving” five items; Cox et al., 2001). Adverse events were noted as reported by patients and observed during treatment.
Follow-up
Patients were contacted and interviewed by telephone after 1 week using the TLFB method to gather information on use of psychotropic medications, tobacco, or illicit drugs. Subjects were then given an appointment to return to the study site and provide urine drug test.
Statistical Analysis
All analyses were carried out on the intent-to-treat sample. Demographic and clinical characteristics were compared using analysis of variance (ANOVA) for continuous variables and chi-square test for categorical variables. Peak reduction in SOWS scores (ΔSOWS) were used as a measure of withdrawal discomfort and calculated by subtracting the lowest intreatment daily withdrawal scores from baseline values. Hierarchical regression analyses were performed with ΔSOWS as the independent value and naltrexone treatment, nicotine dependence, and smoking during treatment as predictor variables, controlling for effects of demographics and ASI scores. Changes of SOWS and QSU-Brief scores from baseline were analyzed using one-way repeated measure ANOVA with Bonferroni correction to control for experiment-wise errors. The odds ratio approximation of relative probability (RR; Sheskin, 2004) for subjects to smoke less than the daily average was calculated in each treatment group, using the “no naltrexone–no clonidine” condition as the reference group.
RESULTS
Subjects
A total of 141 of the 174 OA participants were smokers (81%), of whom 96 had permission to smoke, whereas 45 were not allowed to smoke during inpatient detoxification (Figure 1). All smokers had medium–high levels of nicotine dependence based on FTND scores of 5–7 (Heatherton et al., 1991). The characteristics of the sample are summarized in Table 1. There were no significant differences in demographic and clinical measures among patients with nicotine dependence who smoked during treatment (NDS), nicotine-dependent individuals who were not allowed to smoke (ND), and non– nicotine-dependent patients (NOND).
Table 1.
Sociodemographic, Substance Use Characteristics, and Detoxification Treatment Status of 174 Opioid-Addicted Patients Undergoing 6-Day Methadone Detoxification
| NDS (n = 96) | ND (n = 45) | NOND (n = 33) | |
|---|---|---|---|
| % or mean (SD) | |||
| Demographics | |||
| Age | 30.9 (5.4) | 32.8 (6.4) | 32.2 (4.9) |
| Male | 70 | 65.4 | 64 |
| African American | 30 | 38.5 | 28 |
| Years of education | 9.6 (3.2) | 9.2 (2.8) | 7.9 (2.7) |
| Married or cohabitant | 27 | 19.2 | 20 |
| Unemployed | 60 | 57.7 | 60.1 |
| Substance use | |||
| Smoking behavior | |||
| Cigarettes/day/30 days | 20.3 (7.1) | 22.8 (6.9) | – |
| Fagerström test score | 5.21 (1.1) | 5.46 (1.2) | – |
| Days of use in last month | |||
| Opioids | 23.6 (2.9) | 19.1 (3.5) | 24.2 (3.5) |
| Alcohol | 7.8 (3.5) | 8.9 (6.7) | 3.9 (4.1) |
| Cannabis | 5.8 (4.2) | 7.9 (5.3) | 6.2 (8.5) |
| Cocaine | 6.7 (6.6) | 10.1 (8.9) | 9.1 (3.1) |
| Tobacco | 26.1 (3.1) | 24.6 (2.1) | – |
| Years of use | |||
| Opioids | 9.3 (3.7) | 7.8 (6.7) | 8.7 (3.9) |
| Alcohol | 7.9 (6.8) | 8.6 (7.4) | 5.9 (4.1) |
| Cannabis | 5.6 (6.2) | 6.1 (3.5) | 7.4 (3.3) |
| Cocaine | 6.8 (3.1) | 5.7 (6.2) | 5.9 (4.1) |
| Tobacco | 10.6 (6.1) | 8.9 (7.7) | 8.2 (6.2) |
| Addiction Severity Index | |||
| Drug Composite Score | .23 (.10) | .29 (.11) | .20 (.12) |
| Alcohol Composite Score | .11 (.04) | .14 (.06) | .08 (.05) |
| Psychiatric Composite Score | .19 (.09) | .17 (.10) | .15 (.12) |
| Other drug test at admission | |||
| Positive cocaine | 43 | 53.4 | 52 |
| Positive cannabis | 46.6 | 42.3 | 56 |
| Positive amphetamine | 3.3 | 7.7 | 4 |
| Positive alcohol | 33.3 | 30.8 | 27 |
| Treatments | |||
| Naltrexone 0.125mg/day | 30.2 | 33.3 | 30.3 |
| Naltrexone 0.25mg/day | 32.3 | 31.1 | 33.3 |
| Naltrexone placebo | 37.5 | 35.5 | 36.4 |
| Clonidine | 48.9 | – | 60.6 |
| Clonidine mg/day | .43 (.11) | – | – |
| Ancillary medications (request) | 59.1 | 57.6 | 59.9 |
Note. All comparisons were nonsignificant (p > .05). ND = smokers who were not allowed to smoke during treatment; NDS = smokers who smoked during treatment; NOND = nonsmoker.
To evaluate treatment effects on smoking, NDS participants were divided into four treatment groups: VLNTX (NTX, n = 31), VLNTX plus clonidine (NTX/CLO, n = 29), clonidine (CLO, n = 18), and no naltrexone/no clonidine (NONTX/CLO, n = 18) (Figure 1, Table 1). There was no significant difference in withdrawal intensity between patients randomized to different VLNTX doses, either administered alone (naltrexone 0.125mg/day = 14, 0.25mg/day = 17) or with clonidine (naltrexone 0.125mg/day = 15, 0.25mg/day = 14; data not shown). Thus, patients receiving the two naltrexone doses were combined into a single treatment condition in the NTX and NTX/CLO groups. There were no significant differences in demographic and clinical measures among the four groups (data not shown). The daily dose of clonidine administered during the 6-day detoxification treatment did not significantly differ between NTX/CLO and CLO groups (0.41 vs. 0.39mg, F = 1.1 [1,51], p < .8).
Nicotine Dependence, Smoking, and Opioid Withdrawal
VLNTX addition was significantly associated with reduced withdrawal severity in the primary detoxification trial (Mannelli et al., 2009). We performed hierarchical regressions to determine the relative contribution of treatment, nicotine dependence, and smoking toward predicting peak withdrawal reduction, a measure of withdrawal discomfort (ΔSOWS, Table 2). Step 1 entered VLNTX treatment that was positively correlated with withdrawal reduction. On Step 2, after nicotine dependence failed to add significance and was excluded, intreatment smoking was found to be negatively correlated with ΔSOWS. Step 3 showed a significant interaction between VLNTX use and smoking during treatment in predicting withdrawal changes. Age, gender, and ASI drug, alcohol, and psychiatric scores entered as a block did not improve ΔSOWS prediction and were excluded from the model. Overall, VLNTX treatment explained 27% of the variance in peak opioid withdrawal reduction; smoking during treatment and a combination of VLNTX and smoking explained an additional almost 17% of the variance.
Table 2.
Hierarchical Regression of ΔSOWS (Peak Subjective Withdrawal Reduction) on Very Low Naltrexone Treatment and Smoking During Opioid Detoxification (N = 174)
| Step | Variable | r | R2 added | Beta | 95% CI | F(3,174) |
|---|---|---|---|---|---|---|
| 1 | NTX treatment (y/n) | .520*** | .270*** | .460 | 441.7 to 478.1 | |
| 2 | Smoking (y/n) | −.401** | −.060* | −.133 | −161.1 to −179.4 | |
| 3 | 1 × 2 | .380** | .108** | .492 | 460.8 to 522.8 | |
| 5.07*** |
Note. *p < .03; **p < .01; ***p < .001; cumulative R 2 = .438; adjusted R 2 = .435.
Smoking and Behavioral Changes
Opioid Withdrawal and Craving
Total subjective withdrawal scores did not differ between intreatment smokers, nonsmokers, and smokers who were not allowed to smoke during opioid detoxification (F = 1.8 [2,173], p < .09). Clonidine is commonly used to treat tobacco and OA (van den Brink, 2012). To investigate whether clonidine use influenced withdrawal expression, we repeated the analysis excluding participants who received clonidine (NDS, n = 47; NOND, n = 20; Figure 1). Subjective withdrawal scores remained nonsignificantly different among groups (F = 2.1 [1,107], p < .10). NDS participants, including those treated with clonidine, reported more elevated craving for opioids compared with the other groups (F = 3.7 [2,171], p < .001; NDS vs. ND, p < .002; NDS vs. NOND, p < .001).
Cigarette Craving
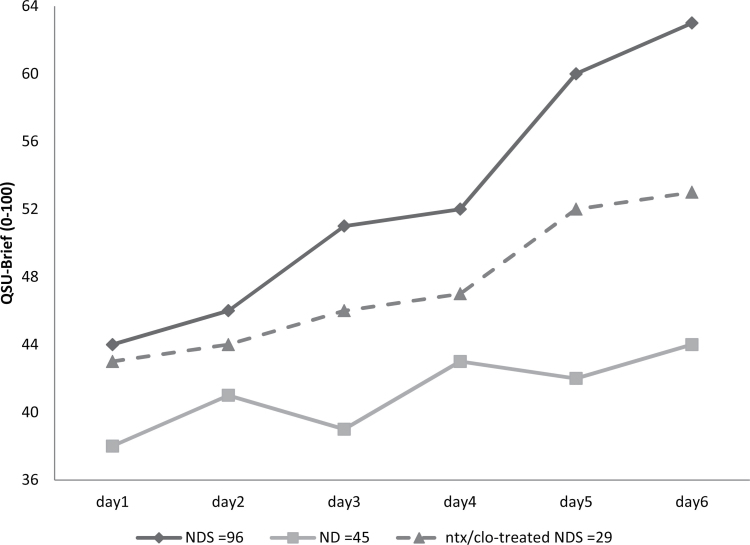
Craving for cigarettes was higher in patients who smoked during treatment (NDS) compared with smokers who were not allowed to smoke (ND; Figure 2; QSU-Brief, F = 4.1 [1,141], p < .001). Clonidine use has shown to reduce craving for tobacco (Gourlay, Stead, & Benowitz, 2004). Nonetheless, even when only smokers treated with clonidine in the NDS group (n = 47) were compared with those in the ND group, craving remained significantly higher among nicotine-dependent patients who smoked during treatment (F = 3.8 [1,94], p < .002). NDS individuals’ scores of “positive craving” items were significantly higher (F = 5.4 [1,141], p < .0001), whereas no significant difference was noted for “negative craving” items (F = 1.4 [1,141], p < .11).
Figure 2.
Brief Questionnaire of Smoking Urges (QSU-Brief) scores rating cigarette craving for intreatment smokers (NDS), smokers who were not allowed to smoke during treatment (ND), and NDS patients treated with very low dose naltrexone–clonidine combination (dashed line). See text for comparisons.
Retention and Follow-up
One-hundred and twenty patients (69%) completed detoxification. Intreatment smokers showed significantly lower completion rates (NDS 59.4%, ND 79%, NOND 78.8%, χ2 = 7.9, df = 2, p < .02; NDS vs. ND, p < .02; NDS vs. NOND, p < .04) and a higher number of cigarettes smoked the week following discharge compared with patients who were not allowed to smoke during detoxification (cigarettes per day: NDS 19.8 [5.6] vs. ND 13.3 [4.3], F = 3.6 [1,85], p < .02). No significant between-group differences were found in use of opioids (χ2 = 1.3), alcohol (χ2 = 2.1), cocaine (χ2 = 0.9), or cannabis (χ2 = 1.3).
Treatment Outcomes of Active Smokers
Opioid Withdrawal and Craving
NDS patients treated with VLNTX reported significantly lower subjective withdrawal intensity compared with clonidine or no naltrexone/no clonidine conditions (SOWS, F = 4.5 [3,101], p < .01; NTX vs. NONTX/CLO, p < .001; NTX vs. CLO, p < .01; NTX vs. NTX/CLO, p < .9). Individuals in the NTX/CLO group reported attenuated withdrawal compared with those in the CLO group (p < .01). There were no significant differences in withdrawal scores between clonidine or no naltrexone/no clonidine groups (CLO vs. NONTX/CLO, p < .18). Opioid craving scores were significantly lower among patients treated with VLNTX compared with clonidine or those receiving neither naltrexone nor clonidine (F = 3.9 [3,96], p < .02; NTX vs. NONTX/CLO, p < .03; NTX vs. CLO, p < .03; NTX vs. NTX/CLO, p < .10; CLO vs. NONTX/CLO, p < .43).
Cigarette Smoking and Craving
All NDS patients smoked daily during treatment, an average 7.6 (4.8) cigarettes/day. Patients treated with VLNTX plus clonidine reported reduced smoking compared with other groups (F = 4.2 [3,96], p < .01; NTX/CLO vs. NONTX/CLO, p < .01; NTX/CLO vs. CLO, p < .01; NTX/CLO vs. NTX, p < .02). Craving for cigarettes was significantly attenuated among intreatment smokers receiving VLNTX plus clonidine (Figure 1; QSU-Brief, F = 4.9 [3,96], p < .003; NTX/CLO vs. NONTX/CLO, p < .001; NTX/CLO vs. CLO, p < .001; NTX/CLO vs. NTX, p < .01; CLO vs. NONTX/CLO, p < .13). Scores of both “positive” and “negative” craving items were significantly lower in the NTX/CLO group compare with NTX (positive: p < .03, negative: p < .01) and CLO (positive: p < .02, negative: p < .002).
The probability of smoking less than the daily average was significantly higher in the NTX/CLO group compared with the NONTX/CLO group (RR = 1.78, 95% CI = 1.03–3.09, p < .04). No significant difference was found in probability of departing from daily smoking average among patients who were administered naltrexone or clonidine compared with those individuals who did not receive either medication (NTX vs. NONTX/CLO, RR = .73, p = .4; CLO vs. NONTX/CLO, RR = 1.2, p = .5).
Use of Ancillary Medications and Adverse Events
All subjects received ancillary medications, including ibuprofen, acetaminophen, loperamide, hydroxyzine, prochlorperazine, and cyclobenzaprine. Medications were requested by subjects in 59.5% of cases, with no significant difference between active smokers, smokers who were not allowed to smoke, and nonsmokers (Table 1). Requests of each medication did not differ significantly between groups (range = 53%–78%, χ2 = 0.15–1.77, p < .21–.78). Among intreatment active smokers, there were no significant differences in amount of each medication administered to the four treatment groups (for Days 1–6, F range = 0.27–1.61 [3]; p < .17–.81).
There were no medication-related adverse events. Of the two adverse events previously described in this sample (Mannelli et al., 2009), one (brief seizure-like episode) was recorded in an actively smoking individual receiving naltrexone placebo and no clonidine, the other (hyperglycemia) was observed in a nonsmoker treated with clonidine and naltrexone placebo. There were no episodes of medication-precipitated withdrawal. There were no significant differences among the four treatment groups in peak heart rate (F = 0.58 [3]; p < .89; total mean [SD] = 70.8 [7.6]) or peak systolic (F = 1.02 [3]; p < .16; total mean [SD] = 100.9 [20.8]) or diastolic blood pressure (F = 0.24 [3]; p < 1.07; total mean [SD] = 57.8 [9.4]).
DISCUSSION
In this inpatient opioid detoxification trial, smoking during treatment was associated with increased opioid withdrawal discomfort. Patients who smoked reported more intense opioid craving and had lower detoxification completion rates compared with smokers who were not allowed to smoke and with nonsmokers. Increased cigarette craving during detoxification and higher number of cigarettes smoked at follow-up distinguished intreatment smokers from those who were not allowed to smoke. Among active smokers, VLNTX addition to methadone taper was more effective than clonidine or naltrexone placebo in easing opioid withdrawal, whereas the combination of VLNTX with clonidine attenuated cigarette craving and reduced intreatment smoking more than each medication alone.
Potential modifiers of withdrawal and craving, including sociodemographic, psychiatric, and drug/alcohol use characteristics, or use of ancillary medications during detoxification, did not differ significantly between groups. Thus, it is unlikely that such variables biased the observed findings.
More than 80% of OA individuals were daily smokers and nicotine-dependent. At one study site, the rate of nicotine-dependent OA patients was 96% (Figure 1). This is consistent with findings in opioid-addicted populations treated at community treatment programs (Reid et al., 2011; Ziedonis et al., 2009).
To our knowledge, this is the first investigation of the effects of cigarette smoking during opioid detoxification. A recent investigation in a large nonsubstance abusing population reported that active smokers treated for chronic pain were less likely to complete methadone taper (Hooten, Townsend, Bruce, & Warner, 2009). Ziedonis et al. (2009) found that smoking was a predictor of negative opioid detoxification outcome in a multisite trial and argued for the need to study the impact of tobacco on opioid withdrawal. We identified an association between active smoking and opioid withdrawal and craving intensity, in combination with lower rates of detoxification completion. These findings were not associated with nicotine dependence alone and may have a biological explanation. At the pharmacokinetic level, the strong induction of hepatic cytochrome P-450 1A2-isoenzymes by polycyclic aromatic hydrocarbons in tobacco smoke may enhance methadone metabolism, resulting in lower methadone levels and increased behavioral discomfort during taper. Although these enzymes have only a secondary role in methadone metabolism (Kapur, Hutson, Chibber, Luk, & Selby, 2011), their concentration changes rapidly in response to smoking (Faber & Fuhr, 2004), and a sudden decrease has been implicated in the onset of methadone toxicity due to smoking cessation (Wahawisan, Kolluru, Nguyen, Molina, & Speake, 2011). Another possibility is a pharmacodynamic interaction. Daily evaluations were performed in the morning before smoking or treatment delivery, when methadone and nicotine were at trough levels. Hence, “abstinence” effects of smoking would be expected to interact with similar effect of methadone, opposite to the positive effects observed when methadone and tobacco are taken together (Elkader et al., 2009).
The finding of higher cigarette craving among active smokers, including those treated with clonidine, compared with patients who were not allowed to smoke and did not receive clonidine may seem counterintuitive. Smokers frequently report intense craving even without nicotine deprivation (Hughes, 1992), and many endorse more severe urges than during abstinence (Cox et al., 2001; Piper et al., 2011). In this regard, clonidine has shown to reduce craving following smoking cessation and it seems less effective in attenuating smoking-induced craving (Knott et al., 2005). The observation that only “positive” tobacco urges were significantly elevated among intreatment smokers is consistent with laboratory findings that smoking does not extinguish craving but may generate expectations of more reward through more smoking (Dols, van den Hout, Kindt, & Willems, 2002), as confirmed by higher number of cigarettes smoked at early follow-up in our sample. Such findings are of clinical interest, given the frequent focus of smoking cessation treatment on craving during abstinence.
The efficacy of VLNTX treatment in reducing opioid withdrawal symptoms among active smokers confirms results observed in the whole sample (Mannelli et al., 2009), though it was significantly less effective than the combination VLNTX–clonidine in modifying smoking behaviors. This is in line with mechanistic evidence. Early studies have indicated that naltrexone reduces smoking reward (Gorelick, Rose, & Jarvik, 1988). However, naltrexone does not affect negative reinforcing properties of tobacco and its use at higher doses is associated with side effects such as sedation (Epstein & King, 2004). In addition, even when naltrexone is administered following detoxification to avoid severe withdrawal, its antirewarding effects may contribute to worsen persisting anhedonic symptoms in opioid-dependent patients (Martinotti, Cloninger, & Janiri, 2008). VLNTX downregulates activity at the mu-opioid receptor (Mannelli et al., 2004) that is selectively involved with dopamine response to hedonic stimuli (Wise, 2008), as well as to smoking and tobacco reinforcement (Domino et al., 2012; Liu & Jernigan, 2011). Clonidine moderates positive and negative smoking reinforcement through alpha-2-adrenergic agonism and GABA(B) receptor modulation (Bruijnzeel et al., 2011; Vlachou et al., 2011), though its clinical use is limited by orthostatic hypotension (Herman & Sofuoglu, 2010). A synergistic action on cigarette craving and smoking would explain the stronger efficacy of VLNTX + clonidine than each medication separately and allow use of lower doses of each drug, with reduced adverse events.
This study has several limitations. Tobacco use and smoking cessation were not taken into account in the design and tobacco withdrawal was not assessed. The intreatment smoking condition did not result from prospective randomization of subjects and may be a confounding factor. Patients were randomized to receive VLNTX or placebo, not clonidine, and a comparison of VLNTX–clonidine treatment response between active and smokers who were not allowed to smoke could not be performed. Although confounding by individual or treatment variables is unlikely because patients did not significantly differ in such characteristics, it is possible that the association between nicotine dependence, smoking, and response to treatment is accounted for by unmeasured confounds.
The investigation was conducted in community treatment programs, a “real world” setting. Although this can be considered one strong point of the study, the exclusion of patients with dependence from substances other than opioids and tobacco, or major psychiatric comorbidity may limit the generalizability of the results. However, a high proportion of patients who were screened were included in the study (86%, Figure 1), and rates of other drug use or ASI composite scores of comorbid psychiatric burden (Table 1) were comparable with those of large opioid-dependent populations (Back et al., 2011).
This investigation shows that tobacco use negatively affects pharmacologically managed opioid agonist detoxification. Patients who smoked during detoxification displayed worse OA and smoking outcomes than those who were not allowed to smoke and experienced unassisted smoking cessation. Under these circumstances, failure to address tobacco use during substance abuse treatment may be a clinical liability more than an erroneous application of “harm-reduction” principles (Prochaska, 2010).
The advantage of including smoking cessation in detoxification programs may be twofold: first a reciprocal pharmacological gain from combining treatments and second, the possibility of extending the intervention to otherwise undertreated populations, similar to what done with drug abusers attending primary care visits (Ong, Zhou, & Sung, 2011).
Despite its limitations, this study suggests that VLNTX + low dose clonidine is well tolerated and associated with improved smoking behaviors in OA. Further investigations should test the effectiveness of this pharmacological combination for smoking cessation in patients with or without comorbid substance abuse disorders.
FUNDING
This work was supported by the National Institute on Drug Abuse (DA 15469 to PM). DAG was supported by the Intramural Research Program, NIDA, National Institutes of Health (NIH).
DECLARATION OF INTERESTS
Dr. Mannelli is receiving support from Alkermes, Inc., Forest Research Institute, Pfizer Inc., and Sunovion Pharmaceuticals. Drs. Wu, Peindl, and Gorelick have no conflicts of interest to disclose.
ACKNOWLEDGMENTS
The research was carried out at Duke University, Durham, NC, and Thomas Jefferson University, Philadelphia, PA. The ID number of this study in www.ClinicalTrials.gov is NCT00135759. Portions of this research were presented at the American Society for Addiction Medicine (ASAM) annual conference in Atlanta, GA, April 19–21, 2012, and at the American Psychiatric Association annual conference in Philadelphia, PA, May 6–10, 2012.
REFERENCES
- Back S. E., Payne R. L., Wahlquist A. H., Carter R. E., Stroud Z., Haynes L., et al. (2011). Comparative profiles of men and women with opioid dependence: Results from a national multisite effectiveness trial. American Journal of Drug and Alcohol Abuse, 37, 313–323.10.3109/00952990.2011.596982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F., Robledo P., Trigo J. M., Martin-Garcia E., Maldonado R. (2010). Neurobiological mechanisms involved in nicotine dependence and reward: Participation of the endogenous opioid system. Neuroscience and Biobehavioral Review, 35, 220–231.10.1016/j.neubiorev.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow G. E., Stitzer M. I., Griffiths R. R., Liebson I. A. (1981). Human methadone detoxification: Opioid self-administration behavior, cigarette smoking, and withdrawal signs and symptoms as a function of progressive dose reductions. Federation Proceedings, 40(3),296 [Google Scholar]
- Blazer D. G., Wu L. T. (2012). Patterns of tobacco use and tobacco-related psychiatric morbidity and substance use among middle-aged and older adults in the United States. Aging and Mental Health, 16, 296–304.10.1080/13607863.2011.615739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel A. W., Bauzo R. M., Munikoti V., Rodrick G. B., Yamada H., Fornal C. A., et al. (2011). Tobacco smoke diminishes neurogenesis and promotes gliogenesis in the dentate gyrus of adolescent rats. Brain Research, 1413, 32–42.10.1016/j.brainres.2011.07.041 [DOI] [PubMed] [Google Scholar]
- Campbell B. K., Wander N., Stark M. J., Holbert T. (1995). Treating cigarette smoking in drug-abusing clients. Journal of Substance Abuse Treatment, 12, 89–94 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2011). Vital signs: Current cigarette smoking among adults aged ≥18 years—United States, 2005–2010. Morbidity and Mortality Weekly Report, 60, 1207–1212 [PubMed] [Google Scholar]
- Clark R. E., Samnaliev M., Baxter J. D., Leung G. Y. (2011). The evidence doesn’t justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Affairs (Millwood), 30, 1425–1433.10.1377/hlthaff.2010.0532 [DOI] [PubMed] [Google Scholar]
- Clarke J. G., Stein M. D., McGarry K. A., Gogineni A. (2001). Interest in smoking cessation among injection drug users. American Journal of Addictions, 10, 159–166 [DOI] [PubMed] [Google Scholar]
- Clemmey P., Brooner R., Chutuape M. A., Kidorf M., Stitzer M. (1997). Smoking habits and attitudes in a methadone maintenance treatment population. Drug and Alcohol Dependence, 44, 123–132 [DOI] [PubMed] [Google Scholar]
- Conner B. T., Stein J. A., Longshore D., Stacy A. W. (1999). Associations between drug abuse treatment and cigarette use: Evidence of substance replacement. Experimental and Clinical Psychopharmacology, 7, 64–71 [DOI] [PubMed] [Google Scholar]
- Cornish R., Macleod J., Strang J., Vickerman P., Hickman M. (2010). Risk of death during and after opiate substitution treatment in primary care: Prospective observational study in UK General Practice Research Database. British Medical Journal, 341, c5475.10.1136/bmj.c5475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L. S., Tiffany S. T., Christen A. G. (2001). Evaluation of the brief questionnaire of smoking urges (QSU-Brief) in laboratory and clinical settings. Nicotine & Tobacco Research, 3, 7–16.10.1080/14622200020032051 [DOI] [PubMed] [Google Scholar]
- David S., Lancaster T., Stead L. F., Evins A. E. (2006). Opioid antagonists for smoking cessation. Cochrane Database of Systematic Reviews, (4)CD003086.10.1002/14651858.CD003086.pub2 [DOI] [PubMed] [Google Scholar]
- Dols M., van den Hout M., Kindt M., Willems B. (2002). The urge to smoke depends on the expectation of smoking. Addiction, 97, 87–93 [DOI] [PubMed] [Google Scholar]
- Domino E. F., Evans C. L., Ni L., Guthrie S. K., Koeppe R. A., Zubieta J. K. (2012). Tobacco smoking produces greater striatal dopamine release in G-allele carriers with mu opioid receptor A118G polymorphism. Progress in Neuropsychopharmacology and Biological Psychiatry, 38, 236–240.10.1016/j.pnpbp.2012.04.003 [DOI] [PubMed] [Google Scholar]
- Elkader A. K., Brands B., Selby P., Sproule B. A. (2009). Methadone-nicotine interactions in methadone maintenance treatment patients. Journal of Clinical Psychopharmacology, 29, 231–238.10.1097/JCP.0b013e3181a39113 [DOI] [PubMed] [Google Scholar]
- Engström A., Adamsson C., Allebeck P., Rydberg U. (1991). Mortality in patients with substance abuse: A follow-up in Stockholm County, 1973-1984. International Journal of the Addictions, 26, 91–106 [DOI] [PubMed] [Google Scholar]
- Epstein A. M., King A. C. (2004). Naltrexone attenuates acute cigarette smoking behavior. Pharmacology, Biochemistry, and Behavior, 77, 29–37 [DOI] [PubMed] [Google Scholar]
- Faber M. S., Fuhr U. (2004). Time response of cytochrome P450 1A2 activity on cessation of heavy smoking. Clinical and Pharmacological Therapeutics, 76, 178–184.10.1016/j.clpt.2004.04.003 [DOI] [PubMed] [Google Scholar]
- First M., Spitzer R., Gibbon M., Williams J. (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders (Research Version, Patient Edition (SCID-I/P)). New York, NY: Biometrics Research; [Google Scholar]
- Frosch D. L., Nahom D., Shoptaw S. (2002). Optimizing smoking cessation outcomes among the methadone maintained. Journal of Substance Abuse Treatment, 23, 425–430 [DOI] [PubMed] [Google Scholar]
- Frosch D. L., Shoptaw S., Jarvik M. E., Rawson R. A., Ling W. (1998). Interest in smoking cessation among methadone maintained outpatients. Journal of Addictive Disorders, 17, 9–19.10.1300/J069v17n02_02 [DOI] [PubMed] [Google Scholar]
- Frosch D. L., Shoptaw S., Nahom D., Jarvik M. E. (2000). Associations between tobacco smoking and illicit drug use among methadone-maintained opiate-dependent individuals. Experimental and Clinical Psychopharmacology, 8, 97–103 [DOI] [PubMed] [Google Scholar]
- Gorelick D. A., Rose J., Jarvik M. E. (1988). Effect of naloxone on cigarette smoking. Journal of Substance Abuse, 1, 153–159 [DOI] [PubMed] [Google Scholar]
- Gourlay S., Stead L., Benowitz N. (2004). Clonidine for smoking cessation. Cochrane Database of Systematic Reviews, (3)CD000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guydish J., Passalacqua E., Tajima B., Chan M., Chun J., Bostrom A. (2011). Smoking prevalence in addiction treatment: A review. Nicotine & Tobacco Research, 13, 401–411.10.1093/ntr/ntr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A. L., Sorensen J. L., Hall S. M., Lin C., Delucchi K., Sporer K., et al. (2008). Cigarette smoking in opioid- using patients presenting for hospital-based medical services. American Journal of Addictions, 17, 65–69.10.1080/10550490701756112 [DOI] [PubMed] [Google Scholar]
- Handelsman L., Cochrane K. J., Aronson M. J., Ness R., Rubinstein K. J., Kanof P. D. (1987). Two new rating scales for opiate withdrawal. American Journal of Drug and Alcohol Abuse, 13, 293–308.10.3109/00952998709001515 [DOI] [PubMed] [Google Scholar]
- Harrell P. T., Montoya I. D., Preston K. L., Juliano L. M., Gorelick D. A. (2011). Cigarette smoking and short-term addiction treatment outcome. Drug and Alcohol Dependence, 115, 161–166.10.1016/j.drugalcdep.2010.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127 [DOI] [PubMed] [Google Scholar]
- Herman A. I., Sofuoglu M. (2010). Comparison of available treatments for tobacco addiction. Current Psychiatry Reports, 12, 433–440.10.1007/s11920-010-0134-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten W. M., Townsend C. O., Bruce B. K., Warner D. O. (2009). The effects of smoking status on opioid tapering among patients with chronic pain. Anesthesia and Analgesia, 108, 308–315.10.1213/ane.0b013e31818c7b99 [DOI] [PubMed] [Google Scholar]
- Hser Y. I., McCarthy W. J., Anglin M. D. (1994). Tobacco use as a distal predictor of mortality among long-term narcotics addicts. Prevention Medicine, 23, 61–69.10.1006/pmed.1994.1009 [DOI] [PubMed] [Google Scholar]
- Hughes J. R. (1992). Tobacco withdrawal in self-quitters. Journal of Consulting and Clinical Psychology, 60, 689–697 [DOI] [PubMed] [Google Scholar]
- Hughes J. R., Gulliver S. B., Fenwick J. W., Valliere W. A., Cruser K., Pepper S., et al. (1992). Smoking cessation among self-quitters. Health Psychology, 11, 331–334 [DOI] [PubMed] [Google Scholar]
- Kalman D., Morissette S. B., George T. P. (2005). Co-morbidity of smoking in patients with psychiatric and substance use disorders. American Journal of Addictions, 14, 106–123.10.1080/10550490590924728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanof P. D., Handelsman L., Aronson M. J., Ness R., Cochrane K. J., Rubinstein K. J. (1992). Clinical characteristics of naloxone-precipitated withdrawal in human opioid-dependent subjects. Journal of Pharmacology and Experimental Therapeutics, 260, 355–363 [PubMed] [Google Scholar]
- Kapur B. M., Hutson J. R., Chibber T., Luk A., Selby P. (2011). Methadone: A review of drug-drug and pathophysiological interactions. Critical Review of Clinical and Laboratory Science, 48, 171–195.10.3109/10408363.2011.620601 [DOI] [PubMed] [Google Scholar]
- Knott V. J., Raegele M., Fisher D., Robertson N., Millar A., McIntosh J., et al. (2005). Clonidine pre-treatment fails to block acute smoking-induced EEG arousal/mood in cigarette smokers. Pharmacology Biochemistry and Behavior, 80, 161–171.10.1016/j.pbb.2004.10.025 [DOI] [PubMed] [Google Scholar]
- Liu X., Jernigan C. (2011). Activation of the opioid mu1, but not delta or kappa, receptors is required for nicotine reinforcement in a rat model of drug self-administration. Progress in Neuropsychopharmacology and Biological Psychiatry, 35, 146–153.10.1016/ j.pnpbp.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli P., Gottheil E., Peoples J. F., Oropeza V. C., Van Bockstaele E. J. (2004). Chronic very low dose naltrexone administration attenuates opioid withdrawal expression. Biological Psychiatry, 56, 261–268.10.1016/j.biopsych.2004.05.013 [DOI] [PubMed] [Google Scholar]
- Mannelli P., Patkar A. A., Peindl K., Gorelick D. A., Wu L. T., Gottheil E. (2009). Very low dose naltrexone addition in opioid detoxification: A randomized, controlled trial. Addiction Biology, 14, 204–213.10.1111/j.1369-1600.2008.00119.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti G., Cloninger C., Janiri L. (2008). Temperament and character inventory dimensions and anhedonia in detoxified substance-dependent subjects. American Journal of Drug and Alcohol Abuse, 34, 177–183.10.1080/ 00952990701877078 [DOI] [PubMed] [Google Scholar]
- McLellan A. T., Kushner H., Metzger D., Peters R., Smith I., Grissom G., et al. (1992). The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment, 9, 199–213 [DOI] [PubMed] [Google Scholar]
- Mello N. K., Lukas S. E., Mendelson J. H. (1985). Buprenorphine effects on cigarette smoking. Psychopharmacology (Berlin), 86, 417–425 [DOI] [PubMed] [Google Scholar]
- Mello N. K., Mendelson J. H., Sellers M. L., Kuehnle J. C. (1980). Effects of heroin self-administration on cigarette smoking. Psychopharmacology (Berlin), 67, 45–52 [DOI] [PubMed] [Google Scholar]
- Mutschler N. H., Stephen B. J., Teoh S. K., Mendelson J. H., Mello N. K. (2002). An inpatient study of the effects of buprenorphine on cigarette smoking in men concurrently dependent on cocaine and opioids. Nicotine & Tobacco Research, 4, 223–228.10.1080/14622200 210124012 [DOI] [PubMed] [Google Scholar]
- Okoli C. T., Khara M., Procyshyn R. M., Johnson J. L., Barr A. M., Greaves L. (2010). Smoking cessation interventions among individuals in methadone maintenance: A brief review. Journal of Substance Abuse Treatment, 38, 191–199.10.1016/j.jsat.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Ong M. K., Zhou Q., Sung H. Y. (2011). Primary care providers advising smokers to quit: Comparing effectiveness between those with and without alcohol, drug, or mental disorders. Nicotine & Tobacco Research, 13, 1193–1201.10.1093/ntr/ntr167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M. E., Schlam T. R., Cook J. W., Sheffer M. A., Smith S. S., Loh W. Y., et al. (2011). Tobacco withdrawal components and their relations with cessation success. Psychopharmacology (Berlin), 216, 569–578.10.1007/s00213-011-2250-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska J. J. (2010). Failure to treat tobacco use in mental health and addiction treatment settings: A form of harm reduction? Drug and Alcohol Dependence, 110, 177–182.10.1016/j.drugalcdep.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M. S., Jiang H., Fallon B., Sonne S., Rinaldi P., Turrigiano E., et al. (2011). Smoking cessation treatment among patients in community-based substance abuse rehabilitation programs: Exploring predictors of outcome as clues toward treatment improvement. American Journal of Drug and Alcohol Abuse, 37, 472–478.10.3109/00952990.2011.596981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K. P., Choi W. S., McCool R. M., Harris K. J., Ahluwalia J. S. (2004). Smoking cessation services in U.S. methadone maintenance facilities. Psychiatric Services, 55, 1258–1264.10.1176/appi.ps.55.11.1258 [DOI] [PubMed] [Google Scholar]
- Richter K. P., Gibson C. A., Ahluwalia J. S., Schmelzle K. H. (2001). Tobacco use and quit attempts among methadone maintenance clients. American Journal of Public Health, 91, 296–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K. P., Hamilton A. K., Hall S., Catley D., Cox L. S., Grobe J. (2007). Patterns of smoking and methadone dose in drug treatment patients. Experimental Clinical Psychopharmacology, 15, 144–153.10.1037/ 1064-1297.15.2.144 [DOI] [PubMed] [Google Scholar]
- Sheskin D. (2004). Handbook of parametric and nonparametric statistical procedures, (3rd ed.). Boca Raton, FL: Chapman & Hall/CRC; [Google Scholar]
- Shoptaw S., Rotheram-Fuller E., Yang X., Frosch D., Nahom D., Jarvik M. E., et al. (2002). Smoking cessation in methadone maintenance. Addiction, 97, 1317–1328 [DOI] [PubMed] [Google Scholar]
- Sobell L. C., Brown J., Leo G. I., Sobell M. B. (1996). The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug and Alcohol Dependence, 42, 49–54 [DOI] [PubMed] [Google Scholar]
- Spiga R., Martinetti M. P., Meisch R. A., Cowan K., Hursh S. (2005). Methadone and nicotine self-administration in humans: A behavioral economic analysis. Psychopharmacology (Berlin), 178, 223–231.10.1007/s00213-004-2020-6 [DOI] [PubMed] [Google Scholar]
- Spiga R., Schmitz J., Day J., II (1998). Effects of nicotine on methadone self-administration in humans. Drug and Alcohol Dependence, 50, 157–165 [DOI] [PubMed] [Google Scholar]
- Stapleton J. A., Keaney F., Sutherland G. (2009). Illicit drug use as a predictor of smoking cessation treatment outcome. Nicotine & Tobacco Research, 11, 685–689.10.1093/ntr/ntp050 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2010). Heroin and other opiate admissions to substance abuse treatment. The TEDS report. Rockville, MD: Author; [Google Scholar]
- van den Brink W. (2012). Evidence-based pharmacological treatment of substance use disorders and pathological gambling. Current Drug Abuse Reviews, 5, 3–31 [DOI] [PubMed] [Google Scholar]
- Vlachou S., Paterson N. E., Guery S., Kaupmann K., Froestl W., Banerjee D., et al. (2011). Both GABAB receptor activation and blockade exacerbated anhedonic aspects of nicotine withdrawal in rats. European Journal of Pharmacology, 655, 52–58.10.1016/j.ejphar.2011.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahawisan J., Kolluru S., Nguyen T., Molina C., Speake J. (2011). Methadone toxicity due to smoking cessation: A case report on the drug–drug interaction involving cytochrome P450 isoenzyme 1A2. Annals of Pharmacotherapy, 45, e34.10.1345/aph.1P759 [DOI] [PubMed] [Google Scholar]
- Weinberger A. H., Reutenauer E. L., Vessicchio J. C., George T. P. (2008). Survey of clinician attitudes toward smoking cessation for psychiatric and substance abusing clients. Journal of Addictive Disorders, 27, 55–63.10.1300/J069v27n01_06 [DOI] [PubMed] [Google Scholar]
- Wise R. A. (2008). Dopamine and reward: The anhedonia hypothesis 30 years on. Neurotoxicology Research, 14, 169–183.10.1007/BF03033808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziedonis D. M., Amass L., Steinberg M., Woody G., Krejci J., Annon J. J., et al. (2009). Predictors of outcome for short-term medically supervised opioid withdrawal during a randomized, multicenter trial of buprenorphine-naloxone and clonidine in the NIDA clinical trials network drug and alcohol dependence. Drug and Alcohol Dependence, 99, 28–36.10.1016/j.drugalcdep.2008.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]