Abstract
Urocortin-2 (UCn2) peptide infusion increases cardiac function in patients with heart failure, but chronic peptide infusion is cumbersome, costly, and provides only short-term benefits. Gene transfer would circumvent these shortcomings. Here we ask whether a single intravenous injection of adeno-associated virus type 8 encoding murine urocortin-2 (AAV8.UCn2) could provide long-term elevation in plasma UCn2 levels and increased left ventricular (LV) function. Normal mice received AAV8.UCn2 (5×1011 genome copies, intravenous). Plasma UCn2 increased 15-fold 6 weeks and >11-fold 7 months after delivery. AAV8 DNA and UCn2 mRNA expression was persistent in LV and liver up to 7 months after a single intravenous injection of AAV8.UCn2. Physiological studies conducted both in situ and ex vivo showed increases in LV +dP/dt and in LV −dP/dt, findings that endured unchanged for 7 months. SERCA2a mRNA and protein expression was increased in LV samples and Ca2+ transient studies showed an increased rate of Ca2+ decline in cardiac myocytes from mice that had received UCn2 gene transfer. We conclude that a single intravenous injection of AAV8.UCn2 increases plasma UCn2 and increases LV systolic and diastolic function for at least 7 months. The simplicity of intravenous injection of a long-term expression vector encoding a gene with paracrine activity to increase cardiac function is a potentially attractive strategy in clinical settings. Future studies will determine the usefulness of this approach in the treatment of heart failure.
Gao and colleagues utilize systemic adeno-associated virus–mediated gene delivery to achieve stable serum concentrations of urocortin 2 (UCn2), a peptide with demonstrated capacity to support cardiac function in congestive heart failure but limited utility due to an extremely short half-life. Mice treated with the UCn2-encoding vector exhibit sustained supraphysiologic expression of UCn2 and enhanced left ventricular function for up to 7 months.
Introduction
Congestive heart failure (CHF) is associated with unacceptably high morbidity and mortality. Even with advanced medical and device management, 50% of patients with class III and class IV CHF die within 4 years. In the United States, the prevalence for CHF is 6 million patients; 670,000 new cases are diagnosed annually (Roger et al., 2012). Because the prevalence of CHF is increasing and the outlook remains dismal, gene transfer is rational and may fulfill an unmet medical need.
Current methods of gene transfer for heart diseases include intramuscular injection into heart muscle and intracoronary delivery, which are cumbersome to apply. Gene therapy trials for heart disease have thus far been disappointing because of inadequate levels of gene expression in the heart. To circumvent this impediment, we have proposed a different approach: intravenous delivery of a transgene with paracrine activity affecting the heart. In paracrine-based gene transfer, a peptide with beneficial cardiovascular effects is released to the circulation from distant sites after systemic delivery of a long-term expression vector. This approach enables gene transfer via a simple intravenous injection during an office visit, abrogates the need for more invasive procedures, and potentially may give cardiac gene therapy a needed boost.
Clinical trials of systemic delivery of genes are underway for hemophilia B (Nathwani et al., 2011; Buchlis et al., 2012) and α1-antitrypsin deficiency (Flotte et al., 2011). However, systemic delivery of paracrine-based transgenes has not been performed in clinical CHF trials, and is relatively uncommon in preclinical studies (Rivera et al., 1999; Lai et al. 2012). Intravenous infusion of potentially beneficial peptides such as urodilatin (Mitrovic et al., 2006), relaxin-2 (Teerlink et al., 2013), and urocortin-2 (Davis et al., 2007b) shows promise in clinical CHF trials.
Urocortins 1, 2, and 3 (38–40 amino acids) belong to the corticotropin-releasing factor (CRF) family. These peptides stimulate corticotropin-releasing factor receptors 1 and 2 (CRFR1, CRFR2). UCn1 binds to CRFR1 and CRFR2, but UCn2 and UCn3 exclusively bind CRFR2. CRFR2 are expressed in cardiac myocytes, vasculature, gut, brain, and skeletal muscle (Wiley and Davenport, 2004; Davidson and Yellon, 2009; Davidson et al., 2009). Although UCn1 has been implicated in tissue permeability associated with lipopolysaccharide (LPS)-induced inflammation (Singh et al., 1999), UCn2, through selective CRFR2 activation, mediates protean beneficial effects including reduced renin–aldosterone activation, and is a potent inotrope with minimal effects on cardiac cyclic AMP (cAMP) (Bale et al., 2004). Peptide infusions of UCn2 in preclinical and clinical CHF have shown favorable effects on left ventricular (LV) function (Davis et al., 2007b; Rademaker et al., 2011).
Because the plasma half-life of UCn2 is 15 min (similar to those of other peptides), chronic infusion is required (Davis et al., 2007b). The inconvenience, associated hazards, need for hospitalization, and expense of therapeutic peptide infusions for CHF are considerable, and impede the broad use of these otherwise attractive peptides. The present study was conducted to address and resolve these shortcomings, using gene transfer.
Adeno-associated viral (AAV) vectors enable prolonged transgene expression. For example, persistent transgene expression has been shown in large animals years after a single injection of AAV vector (Rivera et al., 1999, 2005; De et al., 2006; Nathwani et al., 2011). We have confirmed this in rats (Lai et al., 2012). Although clinical trials have found that some AAV serotypes incite immune responses after intramuscular injection (Manno et al., 2006; Mingozzi et al., 2009), newer generation AAV vectors (AAV5, 6, 8, and 9) do not have similar problems in primates (Hildinger et al., 2001). Intravenous AAV delivery is superior to intramuscular delivery vis-à-vis serum transgene levels, and AAV9 and AAV8 are superior to AAV5 (Fang et al., 2012). Moreover, preexisting anti-AAV8 antibodies are not as prevalent in humans (19%) as are other AAV serotypes including AAV1 and AAV2 (50–59%) (Boutin et al., 2010). These data indicate that intravenous AAV8 is an attractive delivery route and vector with which to attain sustained increased levels of plasma UCn2.
Intravenous delivery of an AAV vector encoding a paracrine gene, as compared with intravenous peptide infusion, has the potential to circumvent infection and reduce repeated and prolonged hospital stays, thereby reducing costs. Systemic vector delivery may be an advantage in paracrine gene transfer—it provides the highest level of expression for any given AAV dose—by exploiting widespread distribution of the vector. The purposes of the present studies were twofold: First, to select and test a suitable vector to attain enduring increases in plasma UCn2 after a single intravenous injection; and second, to assess the effects of chronic elevation in UCn2 on cardiovascular function in normal mice.
Materials and Methods
AAV8.UCn2 vector production
A helper virus-free AAV8 vector encoding murine urocortin-2 (UCn2) driven by a chicken β-actin (CBA) promoter (AAV8.CBA.UCn2; Fig. 1) was produced by transient transfection of HEK293T cells with the vector plasmid pRep2/Cap8 and pAd-Helper plasmid (Xiao et al., 1998). Plasmid pRep2/Cap8 was obtained from the University of Pennsylvania Vector Core (Philadelphia, PA). Cell lysates prepared after 72 hr of transfection were treated with Benzonase and viruses were consolidated by 25% sucrose cushion ultracentrifugation. The pellets were resuspended for further purification of the virus by anion-exchange column chromatography (Q-Sepharose; GE Healthcare Life Sciences, Piscataway, NJ) and concentrated by 25% sucrose cushion ultracentrifugation (Gao et al., 2000; Zolotukhin et al., 2002). Subsequently the pellets were resuspended in 10 mM Tris-HCl (pH 7.9, 1 mM MgCl2, 3% sucrose). Viral titers were determined by real-time qPCR with viral genome DNA prepared from purified virus. To compare the efficacy of AAV8 versus AAV9 and CBA versus the cytomegalovirus (CMV) promoter, we also constructed AAV9.CBA.UCn2 and AAV9.CMV.UCn2 (maps not shown) for experiments in mice (Fig. 1).
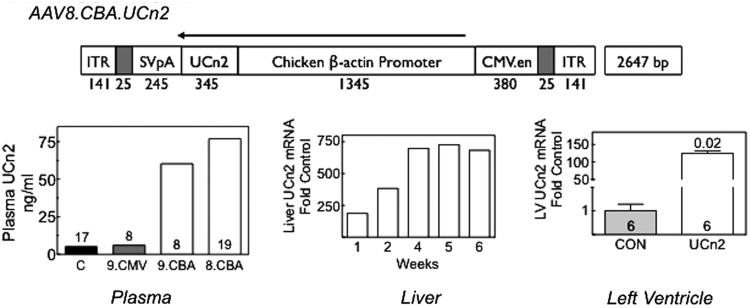
FIG. 1.
AAV8.CBA.UCn2 map and urocortin-2 expression after intravenous delivery (5×1011 genome copies [GC]). AAV8.CBA.UCn2 vector map: ITR, inverted terminal repeat; SVpA, poly(A) from the simian virus 40 (SV40) viral genome; UCn2, urocortin-2; CBA, chicken β-actin promoter; CMV.en, human cytomegalovirus enhancer. Plasma: Three AAV vectors were compared in their ability to increase plasma UCn2 levels 6 weeks after intravenous delivery (5×1011 GC): AAV9.CMV.UCn2 (9.CMV, n=8), AAV9.CBA.UCn2 (9.CBA, n=8), and AAV8.CBA.UCn2 (8.CBA, n=19). To obtain an estimate of variability among animals, among 4 subgroups of pooled samples in the 17 control (C) mice, the SE was 17% of the mean. Plasma UCn2 was increased 15-fold over control (n=17) after AAV8.CBA.UCn2 delivery. Columns denote mean values of pooled samples; numbers above and within columns denote group size. Liver: Time course of UCn2 mRNA expression in liver. Livers were obtained sequentially 1–6 weeks after AAV8.UCn2 gene transfer (n=2 per time point). Copies of UCn2 mRNA are presented as fold increase versus endogenous UCn2 mRNA from saline-injected control mice. Left ventricle: LV samples were obtained 6 weeks after UCn2 gene transfer. Copies of UCn2 mRNA are presented as fold increase versus endogenous UCn2 mRNA from saline-injected control mice. UCn2 transgene mRNA was increased 120-fold versus control. Values represent means±SE. Values of p were determined by Student t test (unpaired, two-tailed). Numbers in columns denote group size.
Intravenous vector delivery
One hundred and seventy-one male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) aged 10–12 weeks were used in these experiments, which were approved by our Animal Use Committee in accordance with National Institutes of Health (NIH) guidelines. Sixty-six mice were used to assess LV function, 9 for dose–response, 52 for plasma UCn2 measurements, 34 for isolation of cardiac myocytes, and 10 for liver expression time course. Under anesthesia (1.5% isoflurane), a small incision was made to expose the jugular vein for intravenous vector delivery. In studies conducted to determine the better AAV serotype (8 vs. 9) and promoter (CBA vs. CMV) we injected 5×1011 genome copies (GC) of the following vectors (Fig. 1): AAV8.CBA.UCn2 (n=19), AAV9.CMV.UCn2 (n=8), and AAV9.CBA.UCn2 (n=8). After completion of these vector-selecting studies, AAV8.CBA.UCn2 was used exclusively. With the exception of the dose–response studies, mice each received 5×1011 GC (in 50 μl) of a single-strand AAV8.CBA.UCn2 or a similar volume of saline as control. Four weeks to 7 months later blood samples were obtained, physiological studies were conducted, and necropsies were performed.
LV function in situ
Mice were anesthetized with sodium pentobarbital (80 mg/kg, intraperitoneal) and a 1.4F conductance-micromanometer catheter (SPR 839; Millar Instruments, Houston, TX) was advanced via the right carotid artery across the aortic valve and into the LV cavity. Left ventricular pressure was recorded and stored digitally for processing (IOX1.8; Emka Technologies, Christchurch, VA) as previously reported (Lai et al., 2012). Subsequently, blood and tissue samples were then obtained. After acquisition, the first derivatives of LV pressure development (LV +dP/dt) and decline (LV −dP/dt) were used to assess LV systolic and diastolic function. Data were acquired and analyzed without knowledge of group identity.
LV function ex vivo
Cardiac function was investigated in isolated perfused hearts to assess LV contractile function in a manner unaffected by reflex activation or anesthesia, as previously reported (Reichelt et al., 2009; Roth et al., 2002). An intraventricular balloon catheter was deployed to measure isovolumetric LV pressure (LV end-diastolic pressure, 10 mmHg; 2.5 mM CaCl2). After a 15-min stabilization period, LV pressure was recorded and subsequently the first derivatives of LV pressure development (LV +dP/dt) and decline (LV −dP/dt) were used to assess LV systolic and diastolic function. Data were acquired continuously for 2 min. Data acquisition and analysis were performed without knowledge of group identity.
Plasma urocortin-2
In a terminal study under anesthesia, approximately 1.0 ml of blood was collected (with EDTA) and centrifuged (1600×g, 15 min). Plasma was collected and stored at −80°C. Peptides were extracted from plasma samples (pooled by group) using buffer A, buffer B, and a SEP-Column according to the manufacturer's instructions (Phoenix Pharmaceuticals, Burlingame, CA). Free UCn2 was measured with a mouse urocortin-2 enzyme immunoassay (EIA) kit (Kamiya Biomedical, Seattle, WA). To obtain an estimate of variability among animals, among 4 subgroups of pooled samples in the 17 control mice, the SE was 17% of the mean.
PCR, RT-PCR, immunoblotting
LV and liver samples were collected and stored at −80°C for quantitative RT-PCR and Western blotting. Tissues were processed with a DNeasy blood & tissue kit (Qiagen, Valencia, CA) and vector DNA was detected by real-time PCR with the following primers: forward, GGCATTATGCCCAGTACATGAC; reverse, CATCACCATGGTAATAGCG; detection probe, tetrachloro-6-carboxyfluorescein TGGGACTTTCCTACTTGGCAGTACATCTACGTATT. Transgene UCn2 mRNA was measured by quantitative RT-PCR with the following transgene-specific primers: forward, ACTCCTATCCCCACCTTCCA; reverse, AAGATCCGTAGGAGGCCAAT. Immunoblotting was performed as described previously (Gao et al., 2008). The following antibodies were used: phospho-PKA (protein kinase A) catalytic subunit, PKA catalytic subunit, troponin I, 22/23-phospho-troponin I (Cell Signaling Technology, Danvers, MA); phospholamban (PLB) (Thermo Fisher Scientific, Waltham, MA); Ser-16 and Thr-17-phospho-PLB (Badrilla, Leeds, UK); and SERCA2a (sarcoplasmic/endoplasmic reticulum calcium ATPase 2) (Enzo Life Sciences, Farmingdale, NY).
Cardiac myocyte isolation
Cardiac myocytes were isolated as previously described (Gao et al., 1999).
Cardiac myocyte cyclic AMP and protein kinase A activity
Cardiac myocytes underwent cAMP measurement and PKA activity assays before and after stimulation with isoproterenol (10 μM, 10 min) and NKH477 (10 μM, 10 min). Cyclic AMP was measured with the Biotrak EIA system (GE Healthcare Life Sciences) as previously described (Gao et al., 2011). PKA activity was determined in pooled cardiac myocytes from each group, using a DetectX PKA activity kit (Arbor Assays, Ann Arbor, MI).
Ca2+ transient
Cytosolic Ca2+ transients were measured with Indo-1, as described previously (Suarez et al., 2008) with modifications. Cardiac myocytes were plated onto laminin-coated glass coverslips and loaded with Indo-1/AM (3 μM; Calbiochem, La Jolla, CA) and the dispersing agent Pluronic F-127 (0.02 mg/ml; Calbiochem, La Jolla, CA) for 30 min. After dye loading, coverslips were mounted in a superfusion chamber, rinsed to remove excess Indo-1/AM, and mounted on a Nikon Diaphot epifluorescence microscope equipped with a ×40 objective interfaced to a photometry system (Photon Technologies, Birmingham, NJ) with the excitation wavelength set to 365 nm via a monochromator. Fluorescence emission was split and directed to two photomultiplier tubes through 20-nm band-pass filters centered at 405 and 485 nm, respectively. The ratio F405/F485 represents a measure for [Ca2+]i. During these measurements, cardiac myocytes were superfused with 25 mM HEPES (pH 7.3) containing 2 mM CaCl2. Myocytes were field-stimulated at 0.3 Hz. Ca2+ transients were recorded from 112 cardiac myocytes obtained from 6 hearts (3 per group). Diastolic and systolic intracellular Ca2+ levels were inferred from the basal and maximal Indo-1 ratio per cycle, respectively. Diastolic decay time was calculated from the normalized Ca2+ transient.
Histology
Samples of liver and transmural sections of the LV free wall were formalin-fixed and paraffin-imbedded. Five-micron-thick sections were mounted and counterstained with hematoxylin and eosin and with Masson's trichrome and examined for fibrosis and inflammation.
Statistical analysis
Data represent means±SE; group differences were tested for statistical significance by one-way analysis of variance (ANOVA) followed by Bonferroni t testing. Between-group comparisons were made by Student t test (unpaired, two-tailed). The null hypothesis was rejected when p<0.05. Pooled data (plasma UCn2; and PKA activity in isolated cardiac myocytes) did not undergo statistical analysis.
Results
Plasma urocortin-2 levels
The vector comparison studies (AAV8.CBA.UCn2 vs. AAV9.CBA.UCn2 vs. AAV9.CMV.UCn2) showed AAV8.CBA.UCn2 to be the most effective of the three in increasing plasma UCn2 levels. Six weeks after intravenous delivery of AAV8.UCn2 (5×1011 GC) we saw a 15-fold increase in plasma UCn2 (vs. control; Fig. 1), confirming that a single intravenous injection of AAV8.UCn2 results in marked increases in plasma UCn2 levels 6 weeks later. In separate studies, we found that a >11-fold increase in plasma UCn2 levels persisted 7 months after a single intravenous delivery of this same dose of AAV8.UCn2 (pooled plasma from n=6 both groups). It is noteworthy that although AAV8.CBA.UCn2 provided the highest plasma levels of UCn2, AAV9.CBA.UCn2 was far superior to AAV9.CMV.UCn2 (Fig. 1).
Vector quantification in LV and Liver
Six weeks after gene transfer, AAV8.UCn2 was detected (liver, 4.4×106±8.8×105 copies/μg DNA, n=8; LV, 2×104±3.5×103 copies/μg DNA, n=8). Seven months after gene transfer AAV8.UCn2 DNA was still detectable (liver, 106±2.1×105 copies/μg DNA, n=6; LV, 1.8×103±1.6×102 copies/μg DNA, n=6).
Urocortin-2 expression
A time course of liver expression of UCn2 was performed. UCn2 mRNA expression was evident 1 week after AAV8.UCn2 delivery and reached a plateau 4–6 weeks after injection (Fig. 1). Liver expression of UCn2 was sustained 7 months after AAV8.UCn2 delivery (data not shown), indicating that sustained expression of UCn2 can be achieved under the direction of the CBA promoter. Increased UCn2 mRNA was also detected in LV (Fig. 1).
Echocardiography
Echocardiography performed 4 months after AAV8.UCn2 delivery showed increased ejection fraction (p<0.05) and velocity of circumferential fiber shortening (p=0.035). Mice that had received AAV8.UCn2 exhibited small but significant reductions in LV end-diastolic diameter (EDD; p<0.02) and LV end-systolic diameter (ESD; p=0.02). These reductions in chamber dimension were not the result of altered heart rates during the measurements, which showed no group differences. Posterior and septal wall thickness showed no group differences (Table 1).
Table 1.
Echocardiography 4 Months After AAV8.UCn2 Gene Transfer
| Con (n=8) | AAV8.UCn2 (n=8) | p Value | |
|---|---|---|---|
| EDD, mm | 4.2±0.1 | 3.8±0.1 | <0.02 |
| ESD, mm | 2.5±0.2 | 1.9±0.1 | 0.02 |
| PWD, mm | 0.6±0.1 | 0.7±0.1 | 0.49 |
| IVSD, mm | 0.6±0.1 | 0.7±0.1 | 0.49 |
| VCF, circ/sec | 8.7±0.6 | 10.6±0.5 | 0.035 |
| EF, % | 79±3 | 87±1 | <0.05 |
| HR, beats/min | 563±8 | 562±8 | 0.97 |
Con, Control; EDD, LV end-diastolic dimension; EF, LV ejection fraction; ESD, LV end-systolic dimension; HR, heart rate; IVSD, interventricular wall thickness, end diastole; PWD, posterior wall thickness, end diastole; VCF, velocity of circumferential fiber shortening.
Note: Values represent means±SE; p values were determined by Student t-test (unpaired, two-tailed).
LV function in situ
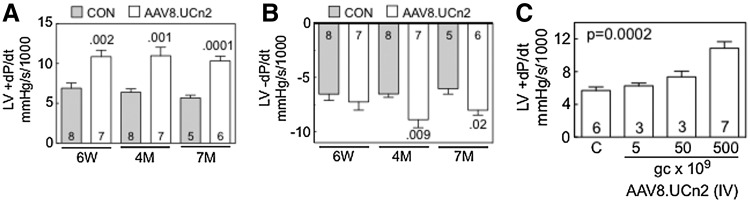
In vivo assessments of LV volume and LV pressure development, using LV pressure-conductance catheters, showed substantial increases in rates of LV pressure development (LV +dP/dt; p values ranging from 0.002 to 0.0001), which were seen 6 weeks, and 4 and 7 months after UCn2 gene transfer (Table 2 and Fig. 2). LV −dP/dt, a measure of LV relaxation, also was increased 4 months (p=0.009) and 7 months (p<0.02) after UCn2 gene transfer (Table 2 and Fig. 2). Heart rate and cardiac output at 7 months were higher in mice that had received AAV8.UCn2 gene transfer.
Table 2.
Left Ventricular Physiological Studies
| |
6 Weeks |
4 Months |
7 Months |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n=8) | AAV8.UCn2 (n=7) | p Value | Control (n=8) | AAV8.UCn2 (n=7) | p Value | Control (n=5) | AAV8.UCn2 (n=6) | p Value | |
| LVP (mmHg) | 87±4 | 95±5 | 0.36 | 94±4 | 96±5 | 0.76 | 88±3 | 91±4 | 0.58 |
| LV +dP/dt (mmHg/sec) | 6899±658 | 10860±810 | 0.002 | 6380±430 | 10980±1067 | 0.001 | 5676±347 | 10330±578 | 0.0001 |
| LV −dP/dt (mmHg/sec) | −6532±580 | −7245±756 | 0.46 | −6516±312 | −8883±741 | 0.009 | −6030±502 | −8010±451 | <0.02 |
| CO (ml/min) | 13±4 | 18±3 | 0.35 | ND | ND | ND | 7±1 | 13±1 | 0.015 |
| MAP (mmHg) | 70±3 | 73±6 | 0.43 | ND | ND | ND | 67±3 | 65±1 | 0.69 |
| MAP/CO mmHg/ml | 13±4 | 6±3 | 0.35 | ND | ND | ND | 12±2 | 10±5 | 0.74 |
| HR (beats/min) | 453±18 | 511±21 | 0.05 | 461±21 | 520±27 | 0.10 | 432±23 | 539±13 | 0.003 |
CO, cardiac output; Con, control; LVP, left ventricular developed pressure; HR, heart rate; MAP, mean arterial pressure; ND, no data.
Note: Values represent means±SE. Values of p were determined by Student t test between groups (unpaired, two-tailed).
FIG. 2.
LV function in situ and AAV8.UCn2 dose–response effect. (A and B) Six weeks after AAV8.UCn2 (5×1011 GC, intravenous) or saline (CON) administration in vivo studies were performed to measure the rates of LV pressure development (LV +dP/dt) and decay (LV −dP/dt), which were used to assess LV systolic and diastolic function. AAV8.UCn2 increased LV +dP/dt at 6 weeks (p=0.002), 4 months (p=0.001), and 7 months (p=0.0001) after gene transfer, indicating that UCn2 gene transfer increases LV contractile function. Similarly, LV −dP/dt, a measure of LV diastolic function, was increased 4 months (p=0.009) and 7 months (p<0.02) after AAV8.UCn2 delivery. Studies were performed without knowledge of group identity. Values of p in (A) and (B) were determined by Student t test (unpaired, two-tailed). (C) To determine whether there was a relationship between the amount of AAV8.UCn2 delivered and its effect on LV function, LV +dP/dt was measured 6 weeks after delivery of AAV8.UCn2 in various doses: 5×109 GC (n=3); 5×1010 GC (n=3); and 5×1011 GC (n=7) versus saline-injected control mice (C) (n=6). Increased LV +dP/dt showed a dose-related effect; values of p were determined by ANOVA. 6W, 6 weeks; 4M, 4 months; 7M, 7 months; GC, genome copies; IV, intravenous; LV, left ventricle; UCn2, urocortin-2. In all graphs, data represent means±SE, and numbers in columns denote group size.
LV function ex vivo
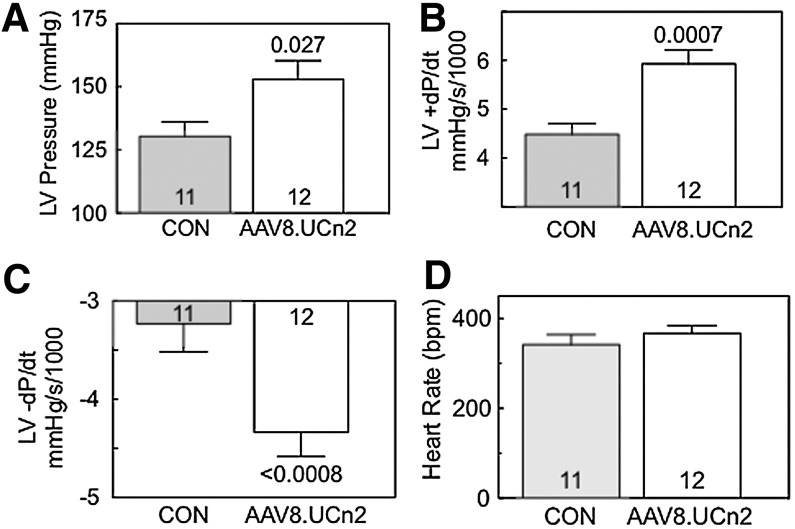
Studies of isolated perfused hearts confirmed the presence of group differences in both LV +dP/dt (p=0.0007) and LV −dP/dt (p<0.0008), as was seen in vivo (Fig. 3). These findings confirm that the AAV8.UCn2 effects were not due to vasodilation from increased plasma levels of UCn2, but, rather, reflect intrinsic increases in LV systolic and diastolic function conferred by UCn2 gene transfer. In these studies there were no group differences in heart rate, suggesting that intrinsic heart rate is not altered by UCn2 gene transfer (Fig. 3).
FIG. 3.
LV function ex vivo. To ensure that differences in LV function measured in vivo were not influenced by the effects of anesthesia, vasodilation, or autonomic reflexes, isolated perfused hearts were studied. Six weeks after AAV8.UCn2 (5×1011 GC, intravenous) or saline (CON) administration, hearts were isolated and perfused and LV pressure was measured, blinded to group identity. (A) AAV8.UCn2 increased LV developed pressure. (B and C) LV +dP/dt (B), and LV −dP/dt (C) were increased in hearts from mice that had received AAV8.UCn2, confirming data obtained in vivo (Fig. 2 and Table 2). (D) Heart rates were not different between groups. These data (A–D) confirm that alterations in systolic and diastolic function are intrinsic to the heart, and do not reflect vasodilation due to increased levels of urocortin-2 alone. In all graphs, data represent means±SE, and numbers in columns denote group size. Values of p were determined by Student t test (unpaired, two-tailed).
Ca2+ handling assessment
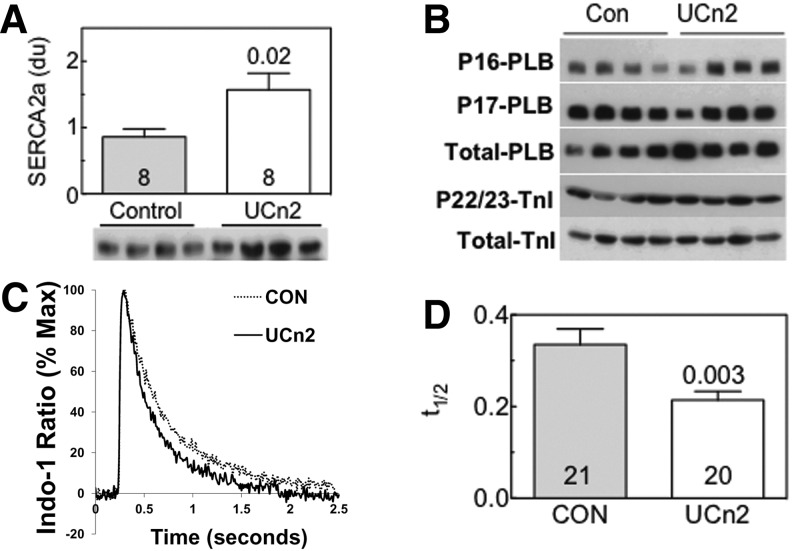
UCn2 gene transfer increased LV SERCA2a protein (Fig. 4A) and mRNA expression (data not shown). LV expression levels of troponin I (TnI) and phospholamban (PLB) were unchanged. Phosphorylation of LV PLB at Ser-16 or Thr-17 and of troponin I at S22/23 showed no group differences (Fig. 4B).
FIG. 4.
Left ventricular Ca2+ handling. (A) SERCA2a protein expression was increased in LV samples 4 weeks after AAV8.CBA.UCn2 gene transfer (5×1011 GC, intravenous) (p=0.02). A significant increase in SERCA2a mRNA was also seen (data not shown). Columns and error bars denote means and SE; numbers in columns denote group size; the number above the column on the right indicates the p value determined by Student t test (unpaired, two-tailed). (B) Phospholamban and troponin I. Phosphorylation and expression of phospholamban and troponin I were unaltered in LV samples 6 weeks after AAV8.UCn2 gene transfer. (C) Cytosolic Ca2+ transients in cardiac myocytes isolated from mice 6 weeks after UCn2 administration versus saline-treated control mice. The rate of Ca2+ decline was increased in cardiac myocytes from mice that had received AAV8.UCn2 gene transfer. (D) Summary of Ca2+ transient experiments. Time to Ca2+ decline was shortened in cardiac myocytes obtained from mice 6 weeks after gene transfer. Experiments were repeated three times and are derived from studies of 112 cardiac myocytes. Columns and error bars denote means and SE; numbers in columns denote number of cardiac myocytes obtained from a subset of n=3 mice per group; the number above the column on the right indicates the p value determined by Student t test (unpaired, two-tailed), confirming a group difference in these cardiac myocytes.
Ca2+ transients
Cardiac myocytes isolated from control mice and mice that had received AAV8.UCn2 gene transfer 4 weeks previously were field-stimulated at 0.3 Hz and analyzed by real-time [Ca2+]i imaging in the presence of the ratiometric dye Indo-1. Cardiac myocytes isolated from mice 4 weeks after UCn2 gene transfer showed an increased rate of Ca2+ decline (Fig. 4C and D; p=0.003).
Cardiac myocyte cAMP and PKA activity
There were no apparent group differences in basal or adenylyl cyclase (AC)-stimulated cAMP production in cardiac myocytes 6 weeks after gene transfer versus control (Fig. 5). However, β-adrenergic receptor-stimulated cAMP production was increased in cardiac myocytes isolated from mice that had received UCn2 gene transfer (Fig. 5; p=0.04). No group differences in the expression or phosphorylation of the PKA catalytic subunit were seen (Fig. 5). PKA activity appeared to be somewhat increased (22–34%) in pooled cardiac myocytes isolated from mice 6 weeks after AAV8.UCn2 gene transfer (Fig. 5).
FIG. 5.
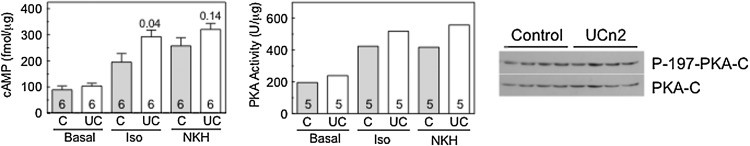
Cardiac myocyte cAMP–PKA signaling. Cardiac myocytes were isolated from control (C) mice and from mice that had received AAV8.UCn2 (UC) gene transfer 6 weeks previously. Cyclic AMP and protein kinase A (PKA) activity were assesses in the unstimulated (basal) state and after stimulation with isoproterenol (Iso, 10 μM, 10 min) and, in separate experiments, NKH477 (NKH, 10 μM, 10 min). NKH477 is a water-soluble forskolin analog that stimulates adenylate cyclase independently of β-adrenergic receptors. Numbers in columns denote group size. Left: cAMP production. No group differences were seen in basal or NKH477-stimulated cAMP production. However, β-adrenergic receptor-dependent cAMP production was increased after UCn2 gene transfer (Iso; p=0.04). Columns and error bars denote means and SE; numbers in columns denote group size; numbers above the columns indicate p values determined by Student t test (unpaired, two-tailed). Middle: PKA activity. PKA activity was assessed in cardiac myocytes pooled from each group; the means of five replicates are shown. PKA activity appeared to show small increases (22–34%) in PKA activity after UCn2 gene transfer. Right: PKA expression. No group differences in the expression or phosphorylation of the PKA catalytic subunit (PKA-C) were seen.
Necropsy
Liver and lung weights showed no group differences. Compared with control mice at 6 weeks, LV weights of control mice at 4 and 7 months were 12 and 11% increased, presumably reflecting LV hypertrophy associated with long-term captivity. Among AAV8.UCn2-treated mice, by contrast, LV weight increased 2% at 4 months and 6% at 7 months. Consequently, group differences in LV weight were seen in the 4- and 7-month group comparisons, and primarily reflect increased LV hypertrophy in control mice (Table 3).
Table 3.
Necropsy
| |
6 Weeks |
4 Months |
7 Months |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n=4) | AAV8.UCn2 (n=6) | p Value | Control (n=8) | AAV8.UCn2 (n=8) | p Value | Control (n=6) | AAV8.UCn2 (n=6) | p Value | |
| BW, g | 28±1 | 29±1 | 0.16 | 30±1 | 32±1 | 0.01 | 31±1 | 32±1 | 0.30 |
| LV, mg | 93±5 | 87±3 | 0.31 | 104±5 | 89±2 | 0.03 | 103±3 | 92±3 | 0.01 |
| LV/BW, mg/g | 3.3±0.2 | 3.0±0.1 | 0.09 | 3.5±0.2 | 2.8±0.1 | 0.002 | 3.3±0.1 | 2.9±0.1 | 0.006 |
| Liver, g | 1.5±0.4 | 1.4±0.6 | 0.16 | 1.4±0.6 | 1.4±0.3 | 0.27 | 1.5±0.7 | 1.4±0.2 | 0.18 |
| Lung, mg | 150±7 | 170±6 | 0.07 | 161±4 | 159±7 | 0.74 | 170±6 | 173±5 | 0.64 |
BW, body weight; LV, left ventricle.
Note: Values of p were determined by Student t test (unpaired, two tailed).
LV histology
Hematoxylin and eosin staining of samples of liver and LV showed no evidence of group differences in histological features. Masson's trichrome staining revealed no group differences in fibrosis in liver or LV (Fig. 6). Markers of inflammation and tissue injury (interleukin [IL]-1, IL-6, monocyte chemotactic protein [MCP]-1, transforming growth factor [TGF]-β, tumor necrosis factor [TNF]-α, α-smooth muscle actin [α-SMA], tissue inhibitor of metalloproteinase-1 [TIMP1]) were examined by RT-PCR and no group differences were found.
FIG. 6.
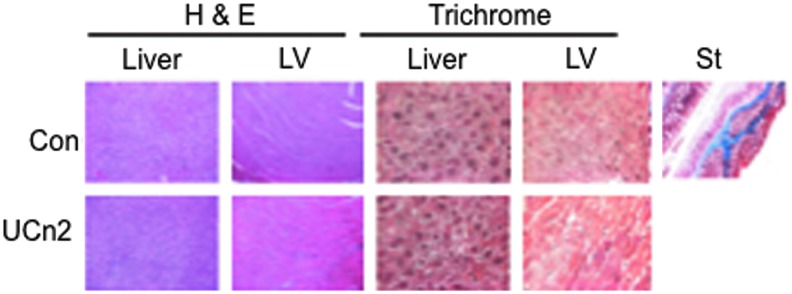
Histology: Liver and left ventricle. Hematoxylin and eosin (H&E) and Masson's trichrome staining was performed on sections of liver (original magnification,×10) and transmural sections of left ventricle (LV; original magnification,×40), 7 months after intravenous delivery of AAV8.UCn2 (5×1011 GC). AAV8.UCn2 delivery and sustained urocortin-2 expression had no adverse effects even after 7 months. St, standard control stain for Masson's trichrome. Color images available online at www.liebertpub.com/hum
Discussion
The most important finding in this study is that a single intravenous injection of AAV8.UCn2 provided elevation in plasma UCn2 levels and increased heart function for up to 7 months, demonstrating the feasibility and effectiveness of paracrine-based gene transfer via intravenous delivery.
Three measures of cardiac function all confirmed increased LV function 6 weeks to 7 months after intravenous AAV8.UCn2 delivery. Echocardiography showed significant increases in LV systolic function, and small changes in LV volume (Table 1). The in vivo studies (anesthetized) and ex vivo studies (anesthesia no longer present) showed that UCn2 gene transfer increased peak LV +dP/dt, indicating enhanced LV contractile function, and reduced LV −dP/dt, indicating enhanced LV diastolic function (Table 2, Fig. 2, and Fig. 3). Studies of isolated perfused hearts (Fig. 3) provided important data, effectively excluding the possibility that the changes in LV function detected in vivo were secondary to vasodilation conferred by increased plasma UCn2, or differences in the effects of anesthesia or autonomic reflexes. We suspect an autocrine effect of UCn2 on the isolated perfused heart, because intravenous delivery of AAV8.UCn2 was associated not only with increased UCn2 expression in liver, a large source of plasma UCn2, but also in left ventricle (Fig. 1), enabling the synthesis and release of UCn2 directly from cardiac myocytes. Transgene UCn2 peptide could then have direct intracellular effects via protein:protein interactions, or could be secreted from cardiac myocytes, and subsequently activate cardiac myocyte CRF type 2 receptors in an autocrine manner. Our study was not designed to determine which of these pathways is primarily responsible for the effects of UCn2 on LV contractile function.
It is important to consider whether UCn2 gene transfer will also increase function of the failing heart as it did in the present study in normal mice—such studies are underway in our laboratory. In clinical settings, UCn2 gene transfer will circumvent many adverse effects associated with intravenous peptide infusions: discomfort, susceptibility to infection and phlebitis due to intravenous catheters, and the cost and inconvenience of hospitalization.
The present study focused on the feasibility and cardiovascular physiological consequences of long-term UCn2 expression. The determination of precise molecular underpinnings for the physiological changes is better addressed in focused studies in isolated cells alone, which are underway. Nevertheless, LV samples from mice that received UCn2 gene transfer showed increased expression of SERCA2a—both mRNA and protein were increased (Fig. 4A). Furthermore, Ca2+ transient studies provided a physiological correlate of increased SERCA2a expression. SERCA2a returns cytosolic Ca2+ to the sarcoplasmic reticulum. An increased amount of SERCA2a would be anticipated to yield a more rapid cytosolic Ca2+ decline, which is what we found (Fig. 4C and D), and consequently to increase the rate of LV pressure decline (LV −dP/dt), as we also found (Fig. 3C). SERCA2a activity controls both the rate of cytosolic Ca2+ removal and the extent of SR Ca2+ load (Maier et al., 2005), thereby affecting both LV relaxation and contraction (He et al., 1997). Although we did not directly measure SR Ca2+ loading, this effect of increased SERCA2a is anticipated, and is reflected in the substantial increases in LV +dP/dt (Figs. 2A and 3A). The mechanism by which UCn2 influences SERCA2a mRNA expression was not addressed, and is not known. These data, acquired from extensive studies in normal mice, warrant additional studies to determine the effects of UCn2 gene transfer in heart failure.
Although the present study is the first report of the effects of UCn2 gene transfer on cardiac function, there have been several studies indicating favorable effects of UCn2 peptide infusion on the normal and failing heart in preclinical studies (Rademaker et al., 2002, 2005, 2011; Davis et al., 2007a). UCn2 peptide infusion has also been used in clinical trials in heart failure (Davis et al., 2007b). UCn2 peptide infusion increases contractile function independent of loading conditions in normal mice, indicating a direct cardiac effect (Bale et al. 2004). The mechanisms for inotropic effects have not been defined, but studies suggest beneficial effects on Ca2+ handling (Yang et al., 2011). The assumption is that the beneficial direct cardiac effects of UCn2 occur via activation of CRFR2 on cardiac myocytes. Although intracellular cAMP may play a role in postreceptor events, its increase on UCn2 stimulation is small. In the present study we saw no difference in basal cAMP production in cardiac myocytes from the two groups of mice (Fig. 5), despite pronounced changes in LV dP/dt, although β-adrenergic receptor-stimulated cAMP was increased (Fig. 5), suggesting an unanticipated synergistic interaction between β-adrenergic receptor stimulation and the autocrine effects of UCn2 on CRFR2 activation.
The relative LV hypertrophy seen in control mice versus mice that received AAV8.UCn2 merits comment. Previous studies have documented a linear increase in LV weight with age in cage-confined mice, which appears to begin at 5 months of age, and has been attributable, at least in part, to activation of the renin–angiotensin–aldosterone system (RAAS) (Dai and Rabinovitch, 2009). The relative reduction in LV mass associated with AAV8.UCn2 gene transfer may reflect the inhibition by UCn2 of RAAS activation (Rademaker et al., 2011)
The AAV8.UCn2 dose–response data (Fig. 2C) indicate a potential effect on LV +dP/dt with a dose of 5×1010 GC. A weight-proportional dose in a 70-kg human subject would be 1014 GC, a dose similar to the range used in clinical trial in subjects with hemophilia B (Nathwani et al., 2011). Limiting expression using a liver-specific promoter would potentially simplify surveillance of toxicity in a subsequent clinical trial, but would come at a cost. First, the data from studies of isolated hearts (Fig. 2) indicate an autocrine action of transgene UCn2, which would be abrogated if one limited expression to the liver. Second, tissue-specific promoters are less robust than the CBA promoter, and therefore an increased AAV8 dose would be required for an equivalent effect on plasma UCn2. CMV performed less well than CBA, presumably due to methylation in liver (Fig. 1; Everett et al., 2004). We selected AAV8 after a comparison with AAV9 (Fig. 1). Our data indicate that UCn2 expression is important in both liver and heart. Both AAV8 and AAV9 provide reasonable transgene expression in liver and heart after intravenous delivery; AAV9 somewhat better in heart, and AAV8 somewhat better in liver (Inagaki et al., 2006). Because there is less preexisting immunity in human subjects to AAV8 than to AAV9 (Boutin et al., 2010) we favor AAV8, because it will enable us to recruit from a substantially larger subject pool—those without preexisting immunity to AAV8. The addition of a regulated expression system to the vector would enable turning transgene expression on or off at will, and may be a required feature when translated to clinical trials. We have designed such AAV8 vectors using tetracycline and rapamycin regulation systems and are initiating preclinical studies.
In summary, a single intravenous injection of AAV8.UCn2 provides sustained elevation of plasma UCn2. Paracrine and autocrine benefits of transgene UCn2 include enhanced systolic and diastolic function of the heart for at least 7 months. Systemic delivery of the vector ensures that the transgene is released into the circulation from multiple tissue sources, an advantage of systemic delivery. Other theoretical advantages of gene transfer as compared with intravenous infusion of paracrine-acting peptides include reduction in catheter-based infections, the absence of need for hospitalization, and reduced costs. It remains to be shown whether this strategy will be effective in increasing function of the failing heart.
Acknowledgments
This work was supported by National Institutes of Health grants (P01 HL66941, HL088426, HL081741, and HL107200), merit grants from the Department of Veterans Affairs (1l01BX001515 and 1l01BX000783), and a grant-in-aid from the American Heart Association Western States Affiliate.
Author Disclosure Statement
Dr. Hammond is founder, consultant, and equity holder for Renova Therapeutics. Renova did not fund the work and was not involved in its planning, interpretation or writing.
References
- Bale T.L. Hoshijima M. Gu Y., et al. The cardiovascular physiologic actions of urocortin II: Acute effects in murine heart failure. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3697–3702. doi: 10.1073/pnas.0307324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin S. Monteilhet V. Veron P., et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- Buchlis G. Podsakoff G.M. Radu A., et al. Factor IX expression in skeletal muscle of a severe hemophilia B patient 10 years after AAV-mediated gene transfer. Blood. 2012;119:3038–3041. doi: 10.1182/blood-2011-09-382317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D.-F. Rabinovitch P.S. Cardiac aging in mice and humans: The role of mitochondrial oxidative stress. Trends Cardiovasc. Med. 2009;19:213–220. doi: 10.1016/j.tcm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S.M. Yellon D.M. Urocortin: A protective peptide that targets both the myocardium and vasculature. Pharmacol. Rep. 2009;61:172–182. doi: 10.1016/s1734-1140(09)70019-2. [DOI] [PubMed] [Google Scholar]
- Davidson S.M. Rybka A.E. Townsend P.A. The powerful cardioprotective effects of urocortin and the corticotropin releasing hormone (CRH) family. Biochem. Pharmacol. 2009;77:141–150. doi: 10.1016/j.bcp.2008.08.033. [DOI] [PubMed] [Google Scholar]
- Davis M.E. Pemberton C.J. Yandle T.G., et al. Urocortin 2 infusion in healthy humans: Hemodynamic, neurohormonal, and renal responses. J. Am. Coll. Cardiol. 2007a;49:461–471. doi: 10.1016/j.jacc.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Davis M.E. Pemberton C.J. Yandle T.G. Urocortin 2 infusion in human heart failure. Eur. Heart J. 2007b;28:2589–2597. doi: 10.1093/eurheartj/ehm340. [DOI] [PubMed] [Google Scholar]
- De B.P. Heguy A. Hackett N.R., et al. High levels of persistent expression of α1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol. Ther. 2006;13:67–76. doi: 10.1016/j.ymthe.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Everett R.S. Evans H.K. Hodges B.L., et al. Strain-specific rate of shutdown of CMV enhancer activity in murine liver confirmed by use of persistent [E1−, E2b−] adenoviral vectors. Virology. 2004;325:96–105. doi: 10.1016/j.virol.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Fang H. Lai N.C. Gao M.H., et al. Comparison of adeno-associated virus serotypes and delivery methods for cardiac gene transfer. Hum. Gene Ther. Methods. 2012;23:234–241. doi: 10.1089/hgtb.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte T.R. Trapnell B.C. Humphries M., et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: Interim results. Hum. Gene Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Ou G. Burnham M.S., et al. Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum. Gene Ther. 2000;11:2079–2091. doi: 10.1089/104303400750001390. [DOI] [PubMed] [Google Scholar]
- Gao M.H. Lai N.C. Roth D.M., et al. Adenylylcyclase increases responsiveness to catecholamine stimulation in transgenic mice. Circulation. 1999;99:1618–1622. doi: 10.1161/01.cir.99.12.1618. [DOI] [PubMed] [Google Scholar]
- Gao M.H. Tang T. Guo T., et al. Adenylyl cyclase type VI increases Akt activity and phospholamban phosphorylation in cardiac myocytes. J. Biol. Chem. 2008;283:33527–33535. doi: 10.1074/jbc.M805825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M.H. Tang T. Lai N.C., et al. Beneficial effects of adenylyl cyclase type 6 (AC6) expression persist using a catalytically inactive AC6 mutant. Mol. Pharmacol. 2011;79:381–388. doi: 10.1124/mol.110.067298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H. Giordano F.J. Hilal-Dandan R., et al. Overexpression of the rat sarcoplasmic reticulum Ca2+ ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. J. Clin. Invest. 1997;100:380–389. doi: 10.1172/JCI119544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildinger M. Auricchio A. Gao G., et al. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J. Virol. 2001;75:6199–6203. doi: 10.1128/JVI.75.13.6199-6203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K. Fuess S. Storm T.A., et al. Robust systemic transduction with AAV9 vectors in mice: Efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai N.C. Tang T. Gao M.H., et al. Improved function of the failing rat heart by regulated expression of insulin-like growth factor I via intramuscular gene transfer. Hum. Gene Ther. 2012;23:255–261. doi: 10.1089/hum.2011.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier L.S. Wahl-Schott C. Horn W., et al. Increased SR Ca2+ cycling contributes to improved contractile performance in SERCA2a-overexpressing transgenic rats. Cardiovasc. Res. 2005;67:636–646. doi: 10.1016/j.cardiores.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Manno C.S. Pierce G.F. Arruda V.R., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mingozzi F. Meulenberg J.J. Hui D.J., et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic V. Seferovic P.M. Simeunovic D., et al. Haemodynamic and clinical effects of ularitide in decompensated heart failure. Eur. Heart J. 2006;27:2823–2832. doi: 10.1093/eurheartj/ehl337. [DOI] [PubMed] [Google Scholar]
- Nathwani A.C. Tuddenham E.G.D. Rangarajan S., et al. Adenovirus-associated virus vector–mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademaker M.T. Charles C.J. Espiner E.A., et al. Beneficial hemodynamic, endocrine, and renal effects of urocortin in experimental heart failure: Comparison with normal sheep. J. Am. Coll. Cardiol. 2002;40:1495–1505. doi: 10.1016/s0735-1097(02)02170-8. [DOI] [PubMed] [Google Scholar]
- Rademaker M.T. Charles C.J. Espiner E.A., et al. Four-day urocortin-I administration has sustained beneficial haemodynamic, hormonal, and renal effects in experimental heart failure. Eur. Heart J. 2005;26:2055–2062. doi: 10.1093/eurheartj/ehi351. [DOI] [PubMed] [Google Scholar]
- Rademaker M.T. Charles C.J. Ellmers L.J., et al. Prolonged urocortin 2 administration in experimental heart failure: Sustained hemodynamic, endocrine, and renal effects. Hypertension. 2011;57:1136–1144. doi: 10.1161/HYPERTENSIONAHA.111.173203. [DOI] [PubMed] [Google Scholar]
- Reichelt M.E. Willems L. Hack B.A., et al. Cardiac and coronary function in the Langendorff-perfused mouse heart model. Exp. Physiol. 2009;94:54–70. doi: 10.1113/expphysiol.2008.043554. [DOI] [PubMed] [Google Scholar]
- Rivera V.M. Ye X. Courage N.L., et al. Long-term regulated expression of growth hormone in mice after intramuscular gene transfer. Proc. Natl. Acad. Sci. 1999;96:8657–8662. doi: 10.1073/pnas.96.15.8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera V.M. Gao G.P. Grant R.L., et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- Roger V.L. Go A.S. Lloyd-Jones D.M., et al. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D.M. Bayat H. Drumm J.D., et al. Adenylyl cyclase increases survival in cardiomyopathy. Circulation. 2002;105:1989–1994. doi: 10.1161/01.cir.0000014968.54967.d3. [DOI] [PubMed] [Google Scholar]
- Singh L.K. Boucher W. Pang X., et al. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of corticotropin-releasing hormone receptors. J. Pharmacol. Exp. Ther. 1999;288:1349–1356. [PubMed] [Google Scholar]
- Suarez J. Scott B. Dillmann W.H. Conditional increase in SERCA2a protein is able to reverse contractile dysfunction and abnormal calcium flux in established diabetic cardiomyopathy. Am. J. Physiol. 2008;295:R1439–R1445. doi: 10.1152/ajpregu.00736.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teerlink J.R. Cotter G. Davison B.A., et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): A randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- Wiley K.E. Davenport A.P. CRF2 receptors are highly expressed in the human cardiovascular system and their cognate ligands urocortins 2 and 3 are potent vasodilators. Br. J. Pharmacol. 2004;143:508–514. doi: 10.1038/sj.bjp.0705985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X. Li J. Samulski R.J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.Z. Kockskämper J. Khan S., et al. Cyclic AMP- and Ca2+/calmodulin-dependent protein kinases mediate inotropic, lusitropic and arrhythmogenic effects of urocortin 2 in mouse ventricular myocytes. Br. J. Pharmacol. 2011;162:544–556. doi: 10.1111/j.1476-5381.2010.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S. Potter M. Zolotukhin I., et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]