Abstract
Citrus Variegated Chlorosis (CVC) is an economically important, destructive disease in Brazil and is caused by Xylella fastidiosa and transmitted by sharpshooter insects. In this study, the efficacy of the fungus Metarhizium anisopliae in controlling the sharpshooter Oncometopia facialis was studied by bioassay conditions. In the bioassay, insects were sprayed with a suspension containing 5 X 107 conidia mL-1. Adults captured in the field were treated in groups of 10 in a total of 11 replications per treatment. Significant differences between the natural mortality and the mortality of insects treated with the fungus were observed 6 days after inoculations (P<0.05). These significant differences increased until 10 days after treatment. The fungus caused 87.1% mortality, with the LT50 varying from 5 to 6 days. The LC50 was 1.2 X 106 conidia mL-1, varying from 7.7 X 105 to 2 X 106 conidia mL-1. The results showed that the sharpshooter O. facialis was susceptible to the entomopathogenic action of M. anisopliae in controlled condition during bioassay.
Keywords: microbial control, entomopathogen, Citrus Variegated Chlorosis (CVC), bioassay
INTRODUCTION
The entomopathogenic fungus Metarhizium anisopliae (Metschnikoff) Sorokin has been isolated from many insect species including the orders Lepidoptera, Coleoptera, Orthoptera and Hemiptera (6,34). M. anisopliae is used commercially in Brazil to control the sugar cane spittlebug, Mahanarva posticata (Homoptera: Cercopidae), and it is also used in other countries, including Colombia, Australia and the U.S.A. to control a variety of pests. The wide insect host range of the genus Metarhizium makes it commercially attractive as a biocontrol agent (24). Excessive use of conventional chemical insecticides has resulted in pest resistance, elimination of economically beneficial insects, residue persistence in the environment, toxicity to humans and wildlife, and a higher cost of crop production (22).
Xylella fastidiosa is a fastidious Gram-negative, xylem-limited bacterium (5, 30). Specific strains cause diseases in many crops of economic importance, such as grape, almond, peach, coffee, plum and citrus (12,15-17,25,30). In Brazil, the most economically important disease caused by X. fastidiosa is Citrus Variegated Chlorosis (CVC) (15, 31). In Brazil, over 70 million sweet orange trees (38%) are affected annually. CVC is responsible for losses of US$ 100 million per year to the Brazilian citrus industry and affects all commercial sweet orange varieties (7,8). X. fastidiosa is disseminated rapidly from the use of infected nursery trees and transmission of X. fastidiosa by several xylem-feeding sharpshooter insect vectors (4).
The insect vectors that transmit X. fastidiosa to plants are leafhoppers of the subfamily Cicadellinae (Hemiptera: Cicadellidae), known as sharpshooters. In citrus plants, Oncometopia facialis (Hemiptera: Cicadellidae: Cicadellinae) is the principal vector of the bacterium X. fastidiosa (28). The principal means of controlling this vector has been the use of chemical insecticides (34,35) with consequent ecological problems.
In developing effective replacements for toxic chemicals, entomopathogenic fungi have been considered as an alternative (29). Adoption of a biological control agent, such as Metarhizium, in integrated pest management (IPM) often results in overall reduction in the total amount of pesticide applied (26).
The aim of this study was to investigate the efficacy of the entomopathogenic fungus M. anisopliae to control the sharpshooter O. facialis under conditions of bioassay in citrus plants.
MATERIALS AND METHODS
Strain of M. anisopliae
We used strain E9 of M. anisopliae var. anisopliae, isolated from Deois flavopicta (Hemiptera:Cercopidae), obtained from a stock collection of the Department of Genetics and Evolution, Universidade Estadual de Campinas, Campinas, São Paulo, Brazil. This strain is largely used in bioassays and genetic studies (9,11,20,23,32). The conidia used in these studies were producted on complete culture medium (CM) consisting of yeast extract, glucose, minerals, agar, and water, according to Alves et, 1998 (3). The E9 strain was plated on Petri dishes containing CM and incubated in a B.O.D. chamber at 27°C ± 1°C for 6 to 7 days.
Insects
Adults of O. facialis were collected from orchards of sweet-orange (Citrus sinensis [L.] Osbeck cv. pera) containing CVC-symptomatic orange trees in Araraquara, São Paulo, Brazil. The insects were carefully transported from the field to cages. Each cage (20cm × 50cm) contained one potted sweet-orange seedling. The potted plants were maintained in the greenhouse at a temperature of 27°C ± 1°C, 63% ± 5% relative humidity.
Lethal Concentration Estimation (LC50)
Six suspensions (103, 104, 105, 106, 107, 108 conidia mL-1) of the M. anisopliae strain E9 were tested against field-collected O. facialis to determine the LC50. The LC50 values were analysed using POLO-PC, a computer programme for probit analysis (14) based on the method of Finney (10).
Conidial Viability
Conidial concentrations were determined with an improved hemocytometer adjusted to 107 conidia per milliliter by dilution with 0.1% Tween 20. One milliliter of each suspension was removed and diluted to 106 conidia mL-1 using 0.1% Tween 20. Spore viability was determined by plating 100 μl of the conidial suspensions on CM and counting colonies after 48h. Spore germination of M. anisopliae strain E9 exceeded 94% in all cases. All samples were adjusted to a final concentration of 107 conidia mL-1.
Bioassays
Experiments were conducted with field-captured adult sharpshooters. Plots consisted of 10 sharpshooters, totaling 110 sharpshooters per treatment in a completely randomized design with 11 replicates. Insects were sprayed with a suspension of conidia at a 5 X 107 concentration conidia mL-1 with a viability of 94.9%. Evaluations of the entomopathogenic action were done daily and after a period of 10 days. This experiment was conducted in a greenhouse with the same temperature and relative humidity cited above.
Dead sharpshooters were collected daily from the fungal treatment groups and the control groups and were tested to determine if mortality was due to infection. To confirm the identification of the primary pathogen, the sharpshooters were surface-sterilized by dipping them successively in 70% ethanol (10 min), 2% sodium hypochlorite solution (2 min), and sterile water (40 seconds) and transferred with a camel-hair brush to Petri dishes containing CM media (19). These plates were sealed with parafilm and incubated at 27°C ± 1°C for 7-14 days. The dead sharpshooters were observed daily for the presence of external fungal structure such as hyphae and conidia. Dead sharpshooters with external hyphae and conidia were counted and only sharpshooters that showed fungal growth were considered to have died of infection, and only these counts were used to compute the pathogenicity of the fungal pathogens.
Statistical Analysis
The daily mortality values were accumulated during the experiments to allow LT50 (lethal time) calculation by Probit analysis using the Mobae computer program (13). The mite mortality data were submitted to analysis of variance using the F test and the means were compared by Tukey test (P < 0.05) using the Sanest (version 3.0) software package.
RESULTS AND DISCUSSION
The M. anisopliae strain E9 was tested against O. facialis sharpshooters at six suspensions of 103, 104, 105, 106, 107, 108 conidia mL-1 and compared with controls. Each treatment and control group had 10 insects. The M. anisopliae strain E9 showed an LC50 of 1.2 X 106 conidia mL-1, varying from 7.7 X 105 to 2 X 106 conidia mL-1.
After 10 days, 96% mortality was observed for M. anisopliaeinfected O. facialis and 69% mortality was observed for noninfected O. facialis (negative control) (Fig. 1). Natural mortality was excluded because it happened in both treated and negative control groups. Mortality percentage was corrected using Abbott’s formula (1). Corrected mortality was 87.1%, which represented the percentage of dead sharpshooters due to action of M. anisopliae (Table 1).
Figure 1.
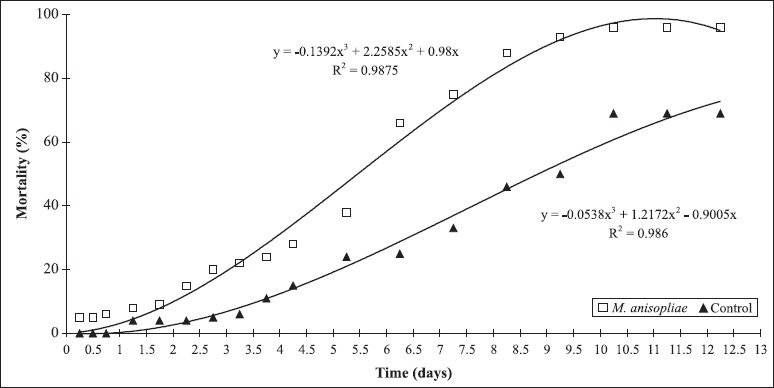
Mean cumulative mortality (%) of Oncometopia facialis during an observation period of 12 days after application of the Metarhizium anisopliae suspension of 5 X 107 conidia mL-1 and the untreated control.
Table 1.
Total mortality and confirmed mortality percentages of Oncometopia facialis by Metarhizium anisopliae strain E9 at suspension of 5 X 107 conidia mL-1
| Observations | Time (days) | Mortality (% ± SE) | CV (%) | Calculated χ2 |
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 |
| 2 | 0.25 | 5.0 ± 0.02 | l.7 | 8.3* |
| 3 | 0.5 | 5.0 ± 0.02 | l.7 | 8.3* |
| 4 | 0.75 | 6.0 ± 0.02 | l.7 | l7.5* |
| 5 | 1.25 | 4.2 ± 0.03 | 3.1 | 2.8ns |
| 6 | l.75 | 5.2 ± 0.03 | 3.4 | 3.5ns |
| 7 | 2.25 | 11.5 ± 0.04 | 4.1 | 9.8* |
| 8 | 2.75 | 15.8 ± 0.05 | 5.4 | l2.7* |
| 9 | 3.25 | l7.O ± 0.06 | 6.2 | 9.9* |
| 10 | 3.75 | 14.6 ± 0.07 | 7.6 | 4.7* |
| 11 | 4.25 | 15.3 ± 0.08 | 8.7 | 3.9ns |
| 12 | 5.25 | 18.4 ± 0.11 | 12.9 | 3.0ns |
| 13 | 6.25 | 54.7 ± 0.13 | l7.5 | 22.8* |
| 14 | 7.25 | 62.7 ± 0.13 | 19.6 | 25.8* |
| 15 | 8.25 | 77.8 ± 0.12 | 20.6 | 40.2* |
| l6 | 9.25 | 86.0 ± 0.10 | 18.6 | 59.7* |
| l7 | 10.25 | 87.l ± 0.12 | 25.0 | 25.0* |
| 18 | 11.25 | 87.l ± 0.12 | 25.0 | 25.0* |
| 19 | 12.25 | 87.l ± 0.12 | 25.0 | 25.0* |
Coefficient of variation (CV);
not significant (p>0.05);
significant (p<0.05).
Colonization of O. facialis by M. anisopliae occurred between 24h and 72h. As typical in M. anisopliae-infected insects, the external fungal growth initiated on the intersegmental regions before spreading over the entire host. Fungal growths were especially concentrated on the head, thorax, and legs (Fig. 2).
Figure 2.

Metarhizium anisopliae, sprayed at suspension of 5 X 107 conidia mL-1, emerging from dead glassy sharpshooters colleted from the treated samples after 6 days incubation.
The M. anisopliae E9 strain caused the highest total mortality (87.1%) and the shortest LT50 (5 to 6 days) (Table 1). These values are similar to those obtained by Kaya and Dara (21) for Homalodisca coagulata, a sharpshooter insect vector of X. fastidiosa in Pierce’s Disease (2), using M. anisopliae isolates at a concentration of 5 X 108 conidia mL-1. In addition, Kanga et al. (19), working with M. anisopliae at 108 conidia mL-1 and H. coagulata, verified a total mortality of 75% after 21 days and an LT50 value of 14 days. These authors also suggested the use of M. anisopliae in IPM to control H. coagulata. The results of the present study are promising because 87.1% mortality was obtained with a concentration of 107 conidia mL-1 and LT50 of 5 to 6 days.
Chemical control with pyrethroid and neonicotinoid insecticides looks promising against immature and adult of sharpshooters (34,35), but it is associated with residual contamination and interferes with biological control strategies (33). Jaramillo et al. (18) tested the use of combined applications of M. anisopliae with the neonicotinoid insecticide imidacloprid and did not observe colony growth inhibition for M. anisopliae in media containing this insecticide. These data reinforce that the use of insecticides to control O. facialis can be reduced when a fungus agent such as M. anisopliae is used as part of a biocontrol strategy.
Little is known about predators, parasitoids, and pathogens that attack sharpshooter nymphs and adults (18). The results obtained in this study suggest that M. anisopliae can be successfully used for the control of O. facialis, indicating the importance of entomopathogenic fungi in an IPM strategy.
This is the first report that describes the use of M. anisopliae against O. facialis and demonstrates the potential to develop IPM strategies for this insect vector. However, additional studies are needed to provide a better understanding of host-pathogen interactions, and identify the factors that enhance or limit pathogenicity in sharpshooter populations under natural conditions.
ACKNOWLEDGEMENTS
The authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES for the fellowship awarded to W.D. Pria Júnior and Fundo de Defesa da Citricultura FUNDECITRUS for financial support and Dr Thomas A. Miller (Entomology Department, University of California Riverside) for reviewing the manuscript.
RESUMO
Avaliação do bioensaio de Metarhizium anisopliae (metchnikoff) sorokin (deuteromycota: hyphomycetes) contra Oncometopia facialis (signoret) (hemiptera: cicadellidae)
A Clorose Variegada dos Citros (CVC) é uma doença economicamente importante e destrutiva no Brasil e é causada pela bactéria Xylella fastidiosa e transmitida por insetos vetores tal como Oncometopia facialis. Nesse estudo, a eficácia do fungo Metarhizium anisopliae em controlar o inseto vetor O. facialis foi estudada em condições de bioensaio. Nesse bioensaio, insetos foram pulverizados com uma suspensão de 5 X 107 conídio mL-1. Insetos-adultos capturados no campo foram tratados em grupos de 10, em um total de 11 replicatas por tratamento. Diferenças significativas entre a mortalidade natural e a mortalidade dos insetos tratados com o fungo foram observadas em 6 dias após a inoculação (P<0.05). Estas diferenças significativas aumentaram antes do décimo dia após o tratamento. O fungo causou uma mortalidade de 87,1%, com uma LT50 variando entre 5 e 6 dias. A LC50 foi de 1,2 X 106 conídio mL-1, variando de 7,7 X 105 a 2 X 106 conídio mL-1. Estes resultados mostraram que o vetor O. facialis foi susceptível a ação entomopatogênica de M. anisopliae em condições controladas durante o bioensaio.
Palavras-chave: controle microbiano, entomopatógeno, Clorose Variegada dos Citros (CVC), bioensaio
REFERENCES
- 1.Abbott W.S. A method of computing the effectiviness of an insecticide. J. Economics Entomol. 1925;18:265–267. [Google Scholar]
- 2.Almeida R.P.P., Purcel A.H. Transmission of Xylella fastidiosa to grapevines by Homalodisca coagulata (Hemiptera: Cicadellidae) J. Econ. Entomology. 2003;96:264–271. doi: 10.1093/jee/96.2.264. [DOI] [PubMed] [Google Scholar]
- 3.Alves S.B., Almeida J.E.M., Moino JR. A., Alves L.F.A. Técnicas de laboratório. In: Alves S.B., editor. Controle Microbiano de Insetos. Brasil: FEALQ, Piracicaba; 1998. pp. 637–771. [Google Scholar]
- 4.Brlansky R.H., Damsteegt V.D., Hartung J.S. Transmission of the citrus variegated chlorosis bacterium Xylella fastidiosa with the sharpshooter Oncometopia nigricans. Plant Dis. 2002;86:1237–1239. doi: 10.1094/PDIS.2002.86.11.1237. [DOI] [PubMed] [Google Scholar]
- 5.Chang C.J., Garnier M., Zreik L., Rossetti V., Bové J.M. Culture and serological etection of xylem-limited bacterium causing citrus variegated chlorosis and its identification as a strain of Xylella fastidiosa. Curr. Microbiol. 1993;27:137–142. doi: 10.1007/BF01576010. [DOI] [PubMed] [Google Scholar]
- 6.Charnley A.K. Mechanisms of fungal pathogenesis in insects. In: Whipps J.M., Lumsden R.D., editors. Biotechnology of Fungi for Improving Plant Growth. Cambridge, UK: Cambridge University Press; 1989. pp. 85–125. [Google Scholar]
- 7.Coletta-Filho H.D., Takita M.A., Souza A.A., Aguilar-Vildoso C.I., Machado M.A. Differentiation of strains of Xylella fastidiosa by a variable number of tandem repeat analysis. Appl. Environ. Microbiol. 2001;67:4091–4095. doi: 10.1128/AEM.67.9.4091-4095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Souza A.A., Takita M.A., Pereira E.O., Coletta-Filho H.D., Machado M.A. Expression of pathogenicity-related genes of Xylella fastidiosa in vitro and in planta. Curr. Microbiol. 2005;50:223–228. doi: 10.1007/s00284-004-4447-8. [DOI] [PubMed] [Google Scholar]
- 9.Destéfano R.H.R., Bechara I.J., Messias C.L., Piedrabuena A.E. Effectiveness of Metarhizium anisopliae against immature stages of Anastrepha fraterculus fruitfly (Diptera: Tephritidae) Braz. J. Microbiol. 2005;36:94–99. [Google Scholar]
- 10.Finney D. Probit Analyses. Cambridge: Cambridge University Press; 1971. p. 333. [Google Scholar]
- 11.Freire L.L.C., Costa A.B.L., Goés L.B., Oliveira N.T. DNA polymorphism and total protein in mutants of Metarhizium anisopliae var. anisopliae (Metsch.) Sorokin strain E9. Braz. J. Microbiol. 2001;32:93–97. [Google Scholar]
- 12.French W.J., Kitajima E.W. Occurrence of plum leaf scald in Brazil and Paraguay. Plant Dis. Reptr. 1978;62:1035–1038. [Google Scholar]
- 13.Haddad M.L., Moraes R.C.B., Parra J.R.P. Modelos bioestatísticos aplicados á entomologia (MOBAE) São Paulo: Editora ESALQ/USP, Piracicaba; [Google Scholar]
- 14.Haddad M.L. Utilização do Polo-PC para análise de Probit. In: Alves S.B., editor. Controle microbiano de insetos. Brasil: Fealq, Piracicaba; 1998. pp. 999–1013. [Google Scholar]
- 15.Hartung J.S., Beretta J., Bralansky R.H., Spisso J., Lee R.F. Citrus Variegated Chlorosis bacterium: axenic culture, pathogenicity, and serological relationships with other strains of Xylella fastidiosa. Phytopathology. 1994;84:591–597. [Google Scholar]
- 16.Hartung J.S. Pierce’s disease and Xylella fastidiosa. In: Goodman R.M., editor. Encyclopedia of Crop and Soil Science. New York: Marcel Dekker, USA; 2004. pp. 928–930. [Google Scholar]
- 17.Hopkins D.L., Purcell A.H. Xylella fastidiosa: Cause of Pirece’s disease of grapevine and other emergent diseases. Plant Dis. 2002;86:1056–1066. doi: 10.1094/PDIS.2002.86.10.1056. [DOI] [PubMed] [Google Scholar]
- 18.Jaramillo J., Borgemeister C., Ebssa L., Gaigl A., Tobón R., Zimmermann G. Effect of combined applications of Metarhizium anisopliae (Metsch.) Sorokin (Deuteromycotina: Hyphomycetes) strain CIAT 224 and different dosages of imidacloprid on the subterranean burrower bug Cyrtomenus bergi Froeschner (Hemiptera: Cydnidae) Biol. Control. 2005;34:12–20. [Google Scholar]
- 19.Kanga L.H.B., Jones W.A., Humber R.A., Boyd JR D.W. Fungal pathogens of the glassy-winged sharpshooter Homalodisca coagulata (Homoptera: Cicadellidae) Fla. Entomol. 2004;87:225–228. [Google Scholar]
- 20.Kava-Cordeiro V., Queiroz M.V., Pizzirani-Kleiner A.A., Azevedo J.L. Pulsed field gel electrophoresis reveals chromosome length and number differences in brazilian strains of Metarhizium anisopliae. Braz. Arch. Biol. Technol. 2005;48:1–6. [Google Scholar]
- 21.Kaya H.K., Dara S.K. San Diego, CA: Proceedings of the Pierce’s Disease Research Symposium; 2004. Microbial control of the glassy-winged sharpshooter with entomopathogenic fungi; p. 349. [Google Scholar]
- 22.Khan A.R., Selman B.J. Nosema spp. (Microspore: Microsporida: Nosematidae) of stored-product Coleoptera and their potential as microbial control agents. Agric. Zool. Rev. 1989;3:193–223. [Google Scholar]
- 23.Messias C.L., Azevedo J.L. Parasexuality in the Deuteromycete Metarhizium anisopliae. Trans. Br. Mycol. Soc. 1980;75:473–477. [Google Scholar]
- 24.Miller C.D., Rangel D., Braga G.U.L., Flint S., Kwon S-I, Messias C.L., Roberts D.W., Anderson A.J. Enzyme activities associated with oxidative stress in Metarhizium anisopliae during germination, mycelial growth, and conidiation and in response to near-UV irradiation. Can. J. Microbiol. 2004;50:41–49. doi: 10.1139/w03-097. [DOI] [PubMed] [Google Scholar]
- 25.Paradela Filho O., Sugimori M.H., Ribeiro I.J.A., Garcia Jr. A., Beretta M.J.G., Harakawa R., Machado M.A., Machado F.F., Rodrigues Neto J., Beriam L.O.S. Primeira constatação em cafeeiro no Brasil da Xylella fastidiosa causadora da clorose variegada dos citros. Laranja. 1995;16:135–136. [Google Scholar]
- 26.Parella P.M., Lewis T. Integrated pest management (IPM) in field crops. In: Lewis T., editor. Thrips as Crop Pests. Wallingford, UK: CAB International; 1997. pp. 599–614. [Google Scholar]
- 27.Riba G., Azevedo J.L., Messias C.L., Silveira W.D., Tuveson R. Studies of the inheritance of virulence in the entomopathogenic fungus Metarhizium anisopliae. J. Invert. Pathol. 1985;46:20–25. [Google Scholar]
- 28.Roberto S.R., Coutinho A., Lima J.E.O., Miranda V.S., Carlos E.F. Transmissão de Xylella fastidiosa pelas cigarrinhas Dilobopterus costalimai, Acrogonia terminalis e Oncometopia facialis em citros. Fitop. Bras. 1996;21:517–518. [Google Scholar]
- 29.Roessler Y. Insecticidal bait and cover sprays. In: Robinson A.S., Hoopher G., editors. Fruit flies: their biology, natural enemies and control. World Crop Pests. The Netherlands: Elsevier, Amsterdam; 1989. pp. 329–336. [Google Scholar]
- 30.Rossetti V., de Negri D. Clorose Variegada dos Citros no Estado de São Paulo. Laranja. 1990;11:1–14. [Google Scholar]
- 31.Simpson A.J., Reinach F.C., Arruda P., Abreu F.A., Acencio M., Alvarenga R., Alves L.M., Araya J.E., Baia G.S., Baptista C.S., Barros M.H., Bonaccorsi E.D., Bordin S., Bove J.M., Briones M.R., Bueno M.R., Camargo A.A., Camargo L.E., Carraro D.M., Carrer H., Colauto N.B., Colombo C., Costa F.F., Costa M.C., Costa-Neto C.M., Coutinho L.L., Cristofani M., Dias-Neto E., Docena C., El-Dorry H., Facincani A.P., Ferreira A.J., Ferreira V.C., Ferro J.A., Fraga J.S., Franca S.C., Franco M.C., Frohme M., Furlan L.R., Garnier M., Goldman G.H., Goldman M.H., Gomes S.L., Gruber A., Ho P.L., Hoheisel J.D., Junqueira M.L., Kemper E.L., Kitajima J.P., Krieger J.E., Kuramae E.E., Laigret F., Lambais M.R., Leite L.C., Lemos E.G., Lemos M.V., Lopes S.A., Lopes C.R., Machado J.A., Machado M.A., Madeira A.M., Madeira H.M., Marino C.L., Marques M.V., Martins E.A., Martins E.M., Matsukuma A.Y., Menck C.F., Miracca E.C., Miyaki C.Y., Monteriro-Vitorello C.B., Moon D.H., Nagai M.A., Nascimento A.L., Netto L.E., Nhani A. Jr., Nobrega F.G., Nunes L.R., Oliveira M.A., Oliveira M.C., Oliveira R.C., Palmieri D.A., Paris A., Peixoto B.R., Pereira G.A., Pereira H.A. Jr., Pesquero J.B., Quaggio R.B., Roberto P.G., Rodrigues V.M., Rosa A.J., Rosa V.E. Jr., Sa R.G., Santelli R.V., Sawasaki H.E., Silva A.C., Silva A.M., Silva F.R., Silva W.A. Jr., Silveira J.F., Silvestri M.L., Siqueira W.J., Souza A.A., Souza A.P., Terenzi M.F., Truffi D., Tsai S.M., Tsuhako M.H., Vallada H., Van Sluys M.A., Verjovski-Almeida S., Vettore A.L., Zago M.A., Zatz M., Meidanis J., Setubal J.C. The genome sequence of the plant pathogen Xylella fastidiosa. Nature. 2000;406:151–157. doi: 10.1038/35018003. [DOI] [PubMed] [Google Scholar]
- 32.Valadares-Inglis M.C., Azevedo J.L. Amylase and protease secretion in recombinant strains of Metarhizium anisopliae var. anisopliae following parasexual crosses. Rev. Braz. J. Genet. 1997;20:171–175. [Google Scholar]
- 33.Varela L.G., Smith R.J., Phillips P.A. Davis: University of California; 2001. Pierce’s Disease. Agriculture and Natural Resources Publication 21600. [Google Scholar]
- 34.Yamamoto P.T., Felippe M.R., Caetano A.C., Sanches A.L., Almeida E.J., Nociti L.A.S. Eficiência de inseticidas neonicotinóides aplicados via tronco no controle de Oncometopia facialis (Signoret) (Hemiptera: Cicadellidae) em mudas de laranjeira ‘Pêra’. Laranja. 2002;23:77–100. [Google Scholar]
- 35.Yamamoto P.T., Felippe M.R., Nociti L.A.S., Montesino L.H., Coelho J.H.C. Uso de acetamiprid no controle da cigarrinha em citros. Laranja. 2003;24:337–351. [Google Scholar]


