Abstract
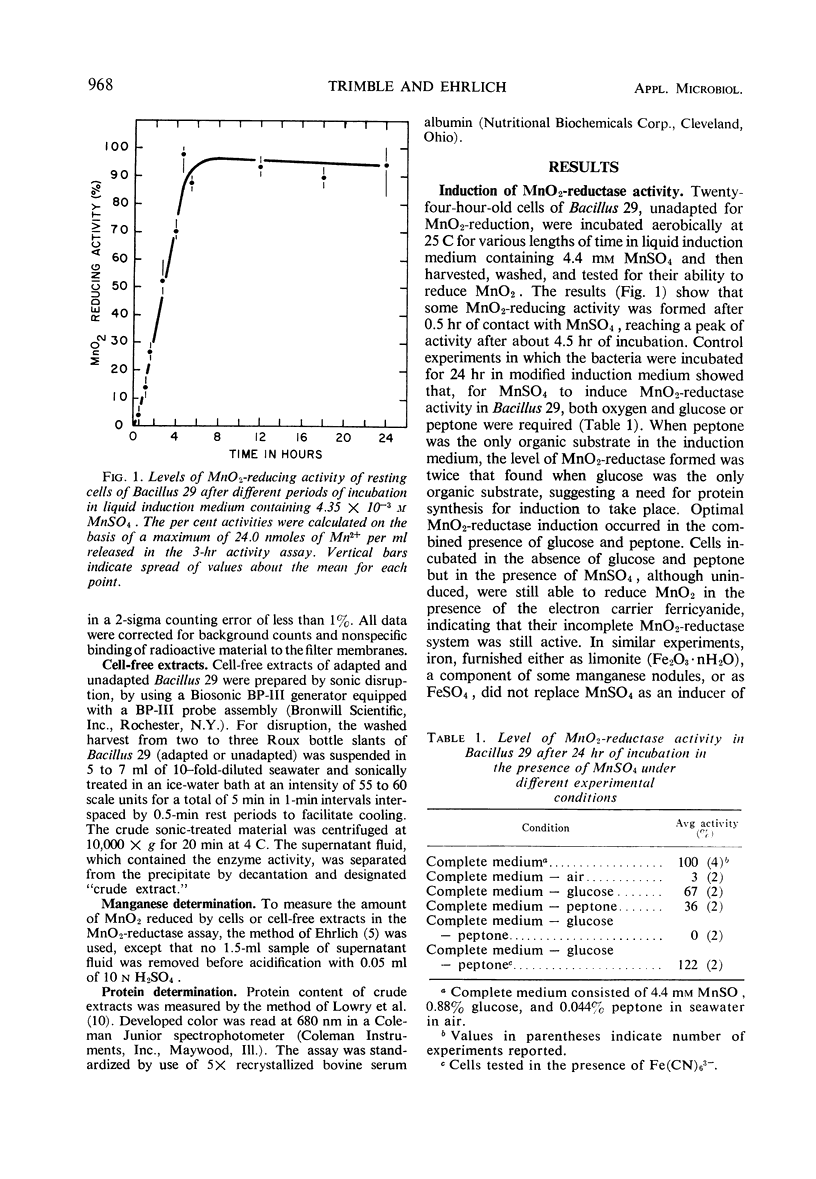
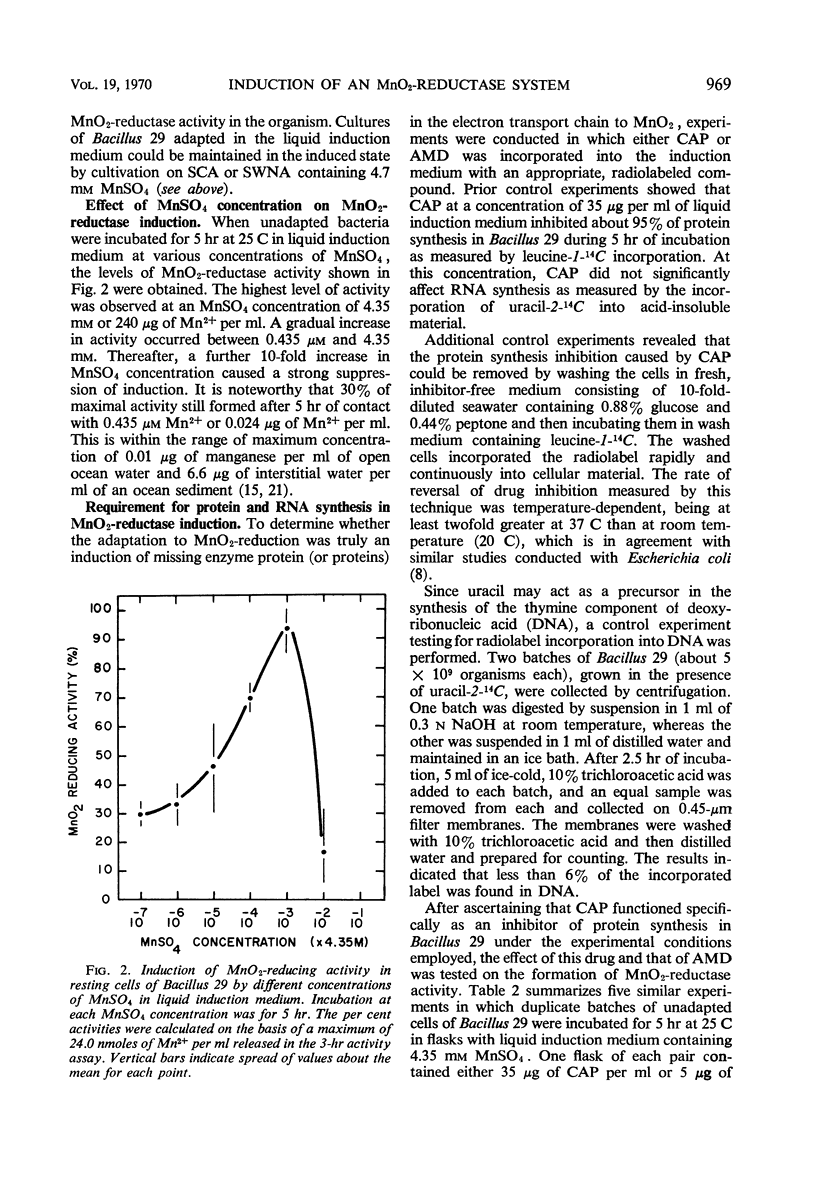
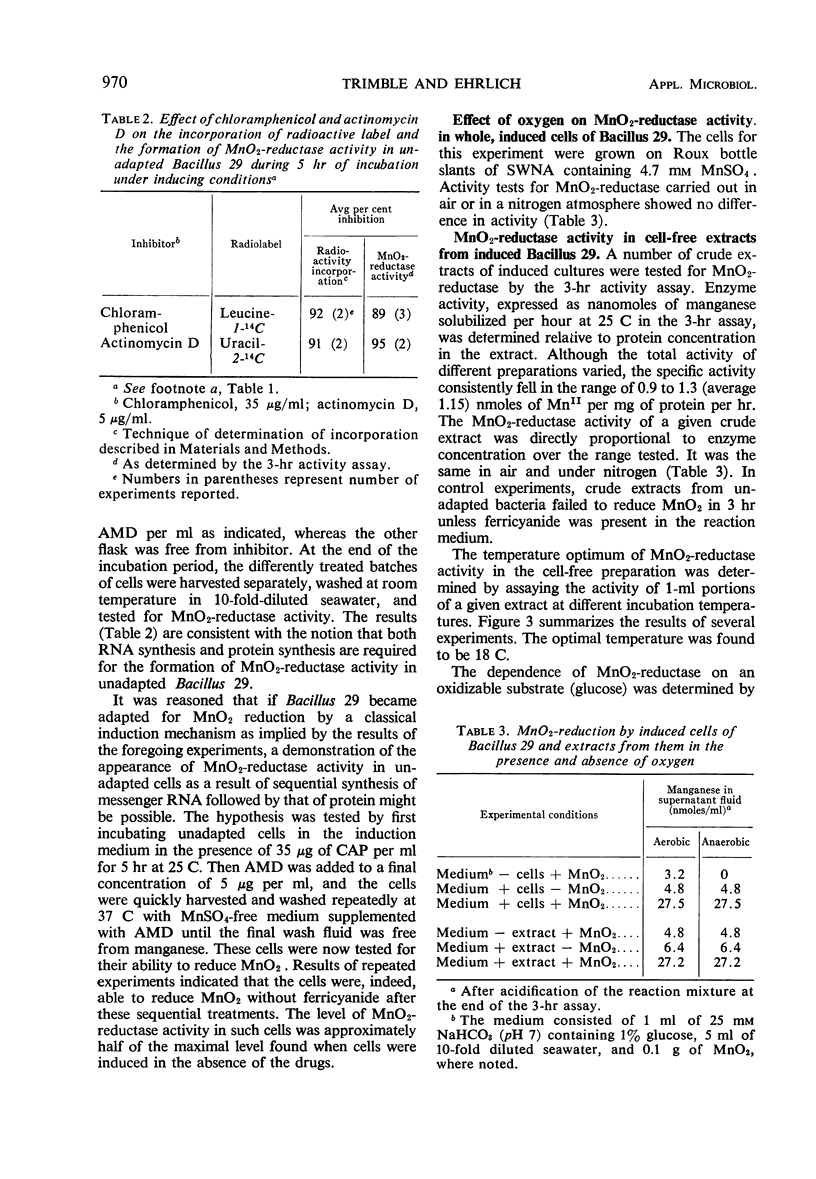
Bacillus 29, isolated from a ferromanganese nodule from the Atlantic Ocean, was shown to possess an MnO2-reductase system which is induced in the presence of manganous ion. Maximal activity of the enzyme system was induced in about 5 hr in the presence of 4.35 mm MnSO4 and was minimally dependent on the presence of either glucose or peptone and oxygen. Induction of optimal activity required the simultaneous presence of glucose and peptone. At least 30% of maximal activity was induced in 5 hr in the presence of 0.4 μm MnSO4. Actinomycin D (5 μg/ml) or chloramphenicol (35 μg/ml), when added to the induction medium, inhibited approximately 90% of MnO2-reductase synthesis and incorporation of uracil-2-14C or leucine-1-14C. Cell-free extracts having MnO2-reductase activity were prepared by sonic disruption of cell suspensions of induced Bacillus 29. Such extracts used glucose metabolism as a source of electrons. They had an average specific activity of 1.15 nmoles of MnII produced per mg of protein per hr at 25 C. They had a temperature optimum of 18 C for reductase activity and retained 50% of their activity at 4 C, the approximate temperature of the natural habitat of the organism. Extracts were stable for several days at 4 C but rapidly lost over 50% of their activity on freezing and thawing. Over 90% of the activity of the extract could be destroyed by heating in a boiling-water bath for 5 min. At a concentration of 1 mm, HgCl2 and atebrine dihydrochloride inhibited MnO2-reductase activity by at least 50%, but sodium azide was ineffective. The MnO2-reductase activity of induced cells and extracts from them was no greater in the absence of oxygen than in its presence, confirming an earlier observation that MnO2 and O2 do not compete as terminal electron acceptors in the respiratory activity of this organism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROMFIELD S. M. Reduction of ferric compounds by soil bacteria. J Gen Microbiol. 1954 Aug;11(1):1–6. doi: 10.1099/00221287-11-1-1. [DOI] [PubMed] [Google Scholar]
- Ehrlich H. L. Bacteriology of Manganese Nodules: I. Bacterial Action on Manganese in Nodule Enrichments. Appl Microbiol. 1963 Jan;11(1):15–19. doi: 10.1128/am.11.1.15-19.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H. L. Bacteriology of manganese nodules. II. Manganese oxidation by cell-free extract from a manganese nodule bacterium. Appl Microbiol. 1968 Feb;16(2):197–202. doi: 10.1128/am.16.2.197-202.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz C., Braun C. B. Temperature-sensitivity of the weak bonds by which chloramphenicol is held in intact cells. Biochim Biophys Acta. 1968 Apr 22;157(2):392–403. doi: 10.1016/0005-2787(68)90093-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NASON A. Symposium on metabolism of inorganic compounds. II. Enzymatic pathways of nitrate, nitrite, and hydroxylamine metabolisms. Bacteriol Rev. 1962 Mar;26:16–41. doi: 10.1128/br.26.1.16-41.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottow J. C., Von Klopotek A. Enzymatic reduction of iron oxide by fungi. Appl Microbiol. 1969 Jul;18(1):41–43. doi: 10.1128/am.18.1.41-43.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postgate J. R. Recent advances in the study of the sulfate-reducing bacteria. Bacteriol Rev. 1965 Dec;29(4):425–441. doi: 10.1128/br.29.4.425-441.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley B. J., Brooks R. R., Kaplan I. R. Manganese and related elements in the interstitial water of marine sediments. Science. 1967 Nov 17;158(3803):906–910. doi: 10.1126/science.158.3803.906. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Methane fermentation. Annu Rev Microbiol. 1967;21:121–142. doi: 10.1146/annurev.mi.21.100167.001005. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Ehrlich H. L. Bacteriology of manganese nodules: III. Reduction of MnO(2) by two strains of nodule bacteria. Appl Microbiol. 1968 May;16(5):695–702. doi: 10.1128/am.16.5.695-702.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLFOLK C. A., WHITELEY H. R. Reduction of inorganic compounds with molecular hydrogen by Micrococcus lactilyticus. I. Stoichiometry with compounds of arsenic, selenium, tellurium, transition and other elements. J Bacteriol. 1962 Oct;84:647–658. doi: 10.1128/jb.84.4.647-658.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]



