Abstract
In this study we evaluated the ability of Saccharomycopsis schoenii Nadson and Krassiln (UWO-PS 80-91) as biocontrol agent against plant pathogenic filamentous fungi P. expansum Link (UFMG 01-2002), P. italicum Wehmer (LCP 61.1199), and P. digitatum (Pers.: Fr.) (LCP 984263, LCP 68175 and LCP 4354). S. schoenii was able to reduce disease severity in oranges inoculated with all fungi. Among the phytopathogens, P. digitatum LCP4354 was the most virulent whereas P. digitatum LCP 68175 was the most susceptible to predation. The yeast was able to survive for 21 days on the fruit surface and did not produce lesions on oranges. Production of antagonistic substances by S. schoenii was not detected using standard techniques. Our results point to the potential use of S. schoenii to control postharvest phytopathogens in fruits.
Keywords: Biological control, orange, postharvest disease, Penicillium, Saccharomycopsis schoenii
INTRODUCTION
The deterioration of food by fungi results in economical losses ranging from 5 to 20% of the production in developed countries and can be as high as 50% in regions with a tropical climate (7,10,23). It is estimated that up to half of all fruits harvested is lost due to fungal and pests decay worldwide (3). Considerable effort has been done to diminish those losses and the storage time of fruits has increased since the 1960’s mainly due to new technologies for temperature and humidity control, as well as the use of fungicides (22).
The use of fungicides has been efficient in decreasing losses due to deterioration of food, but also generates health and environmental concerns mainly due to the carcinogenic and/or teratogenic properties of the compounds, and by their cumulative toxic effects (10,19). About US$30 billion are spent yearly worldwide in pesticides and recent data show that there are approximately 25 million occupational pesticide poisonings each year among agricultural workers in developing countries (8,25).
In tropical developing countries the loss due to post harvest diseases represents a major economical burden and fungal decay is one of the major factors contributing to loss in stored fruits. It is of uppermost importance to develop new strategies for post harvest disease control, especially due to the increasing restrictions on the use of pesticides by the regulatory agencies and consumer affairs institutions. Biological control is becoming an important alternative in the fight against postharvest disease of fruits and as a result, there is an urgent need for further research in order to develop new and more efficient strategies for biocontrol (21).
Among the microorganisms that can have shown potential as biocontrol agents, yeasts show great potential due to their high colonization rate and ability to survive on the fruit surface for prolonged periods under different environmental conditions (4,5). Different yeast species are being utilized as biocontrol agents; for example, Candida oleophila Montrocher and Pichia membranifaciens Hansen against Botrytis cinerea Pers. Fr. to help prevent postharvest decay in apples (12); Cryptococcus albidus Saito and Pichia anomala (Hansen) Kurtzman against Sclerotinia sclerotiorum (Lib.) de Bary in bean (19); Debaryomyces hansenii (Zopf) Lodder and Kreger-Van Rij against P. digitatum (Pers.: Fr.) Sacc in decaying grapefruit; Pichia guillermondii Wicherham against Botrytis, Rhizopus, Penicillium and Alternaria species in decaying tomato; Cr. laurentii Kufferath and Cr. flavus Saito in the control of Mucor in pears; and Candida sake (Saito and Ota) against B. cinerea and Rhizopus nigricans Ehrenberg, the major contributors of postharvest disease in apples (24).
The goal of this work is to describe the ability of Saccharomycopsis schoenii Nadson and Krassiln to serve as biocontrol agent against orange decay on stored fruit. The predacious yeast S. schoenii was tested for its ability to control phytopathogenic fungi of the genus Penicillium, to persist as viable cells on the surface of oranges without causing pathogenesis, as well as for the production of antagonistic substances. The fungal pathogens were also tested for their virulence to the orange fruit and susceptibility to yeast predation. Yeasts of the genus Saccharomycopsis are known for their ability to predate other fungi (14,15) but to our knowledge, this report is the first attempt to use these yeasts as biocontrol agents.
MATERIAL AND METHODS
Microorganisms
Penicillium expansum Link UFMG 01-2002 was isolated in our laboratory from a decaying orange and characterized by Its D1/D2 domain sequence from the small subunit of ribosomal DNA (13). Penicillium italicum Wehmer LCP 61.1199 also isolated from orange, P. digitatum LCP 68175 and P. digitatum LCP 984263 isolated from lemon and P. digitatum LCP 4354 from an unknown source were gracious gifts from the Museum National d’Histoire Naturelle, Laboratoire de Cryptogamie, Paris, (LCP). The antagonistic yeast S. schoenii UWO - PS 80-91, a predacious species isolated from tree exudates was obtained from Prof. M. A. Lachance, Department of Biology, University of Western Ontario, Canada.
Fruits
The ripe oranges (Citrus sinensis (L.) Osbeck variety Pêra Rio) (approximately 20 cm perimeter) were acquired from a local commercial orchard, and selected for lack of lesions or injuries. The fruits were superficially disinfected by immersion in 1.0% sodium hypochlorite for 3 min, rinsed with sterile water and dried in a sterile chamber. When dry, a wound was made around the entire equatorial region of each orange with a special blade. The wound was approximately 3 mm wide and 1 mm deep. The pathogen and/or the antagonistic yeast were inoculated in these wounds.
Preparation of pathogen and biocontrol agent
The pathogen was prepared from approximately 10 day-old cultures grown on potato dextrose agar - PDA (Difco, USA). Five ml of the suspension containing 104 conidia/ml were prepared in GY broth (glucose 1% and yeast extract 0.01%) supplemented with 500 μl of Tween 20.
The biocontrol agent was prepared from 24 h old cultures on yeast extract-malt extract agar YM (2% glucose, 0.5% peptone, 0.3% malt extract, 0.3% yeast extract, and 2% agar) and the final concentration of the suspension adjusted with haemocytometer to 108 cells/ml in GY broth.
Fruit inoculation
Aliquots of 200 μl of each pathogen suspension were deposited with a sterile pipette on the orange wound immediately after wounding and allowed to dry in aseptic conditions. After pathogen inoculation, 200 μl of a suspension of the putative biocontrol agent was also deposited on the wound. The inoculated fruits were incubated on a 100% humidity chamber at 25°C, to simulate the best conditions for the growth of the pathogens, for 4 to 14 days depending on pathogen virulence. Oranges inoculated with P. digitatum were evaluated 4 days after treatment whereas P. expansum and P. italicum infected oranges were scored 12 and 14 days, respectively, after inoculation. Four fruits arranged in a randomized block design were used per treatment. All treatments were replicated three times.
To test the ability of Saccharomycopsis schoenii to control Penicillium species in oranges, inoculation of each pathogen alone served as a positive control whereas a primary negative control was performed by inoculation of GY without yeast or pathogenic fungus, and a secondary negative control consisted of inoculating yeast alone.
Assessment of lesion
The percentage of disease severity reduction (DSR%) was calculated by the equation: DSR (%) = [(DSc - DSt)/DSc] X 100, Where DSc = average area with lesions on the positive control and DSt = area with lesions on the treated plants (2). Only the mechanically wounded region of the orange was used for the assessment of disease reduction.
Recovery of antagonistic yeast
To assess the ability of S. schoenii to survive on the orange surface, fruits wounded as described above were treated with 200μl of a 2x108 cells/ml suspension of S. schoenii and the persistence of the yeast was determined immediately after application, as well as on fruits stored for 4, 7, 14 and 21 days at 25°C in a 100% humidity chamber. To recover the yeast from fruits, portions of wounds of treated oranges were removed with a 0.5 cm2 diameter cork borer, transferred to tubes containing sterile water, and sequentially diluted to 10-2 and 10-4. A 100 μl aliquot of the resulting solutions were plated in triplicate on YM agar with 200 mg/l of chloramphenicol, incubated at 25°C for at least 24 h, and the number of resulting colonies determined.
Production of inhibitory substances
To test the mechanisms for decay control, an assay to determine the chemical antagonism between S. schoenii and the saprophytic mould strains was performed. The moulds P. digitatum, P. expansum and P. italicum were grown on PDA for 7 days, diluted in sterile water and spread with a sterile swab on the surface of Yeast Extract - Malt Extract - Methylene Blue Agar (YM - MB Agar) buffered with a 0.5 mol citrate buffer to pH 4.2 (26). The yeast tested for mycocinogenic activity was grown on YM for 24 h and inoculated on YM-MB agar plates seeded with the mould strain after 2 hours. Plates were incubated at 22°C for 5 days (1). Concomitantly, filtrates of 24 h old yeast liquid cultures were deposited over PDA plates seeded with molds. The effect of the filtrate on the growth of moulds was observed after 5 days. The yeast Saccharomyces cerevisiae Hansen strain NCYC 1006, normally regarded as being sensitive to killer factors, was used as a control in both preparations.
Predation
In order to observe the events of adhesion and predation, the filamentous fungus P. digitatum LCP 68175 was grown on the top of 0.6 mm diameter discs of GY agar and incubated upside down on glass slides for 3 to 4 days at 25°C in a humid chamber. Next, a 50 μl aliquot of a 102 cell/ml suspension of S. schoenii in GY broth was deposited on the top of the growing mycelium and incubated for 3 to 24 h. Periodic observations of the predation events were made and when necessary, a digital image of the event was recorded on a DP-12 Olympus Digital Camera (Olympus Man., Japan).
Statistical analysis
The experiments of disease severity reduction had been analyzed by the non-parametric test of Mann-whitney with 5% confidence.
RESULTS
Potential for biocontrol
The yeast S. schoenii was capable of reducing the severity and diminished incidence of decay on mature oranges in all tests. P. digitatum LCP 4354 was the most virulent strain since the positive control resulted in almost 100% degradation of the fruits after 4 days of incubation (data not shown). Fig. 1 show the percentage of disease severity reduction (DSR) caused by pathogens in the presence of the antagonist yeast. The highest pathogen control values were observed for the interaction between S. schoenii vs. P. digitatum LCP 68175, with an 86.8% reduction of disease severity when compared to the positive control. The yeast reduced the disease severity against P. expansum by 61.0%. The DSR observed for P. italicum was 35.7%, and the results obtained for P. digitatum LCP 4354 and P. digitatum LCP 984263 were 38.2 and 34.6%, respectively.
Figure 1.
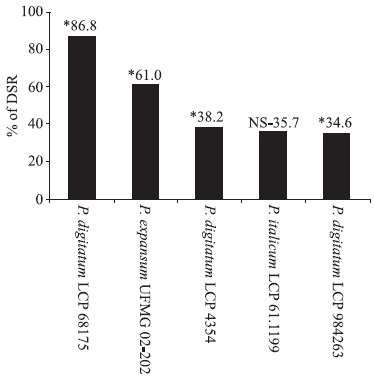
Percentage of disease severity reduction (% DSR) on oranges artificially infected with 104 spores of Penicillium digitatum, P expansum and P. italicum after treatment with 108 cells of Saccharomycopsis schoenii.
* = significant with 5% confidence.
NS = non-significant.
Population dynamics of Saccharomycopsis schoenii on oranges
S. schoenii was re-isolated from treated oranges up to 3 weeks after inoculation (Fig. 2). The yeast exhibits a typical growth curve, where the lag phase can be observed from 0 to 4 days of inoculation, followed by a log phase up until day 7. The stationary phase can be observed until day 15 when the decline begins.
Figure 2.
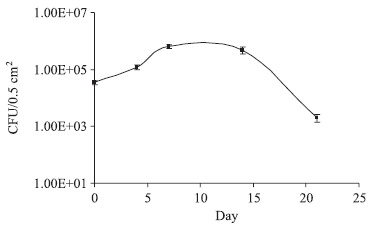
Number of colonies of Saccharomycopsis schoenii recovered from 0.5 cm2 orange surface after inoculation with 108 cells and incubation for 0 to 21 days at 25°C in a 12/12 h photoperiod. The bars represent the standard deviation of the mean (n = 3).
Observation of predation in vitro
Predation events were observed after 3 h of predator and prey co-incubation. Hyphae and spores were preyed upon by the yeast (Fig. 3). The event usually began by the adhesion of the predator cell to the surface of the prey’s hyphae or spore (Fig. 3a). No noticeable changes in the prey cells were observed at this early stage. After adhesion, an external peg was formed from the S. schoenii cells extending into the cytoplasm of the hyphae or spore of P. digitatum. The peg rapidly grew and became a round structure that was defined as an haustorium (Fig. 3b). Some free S. schoenii cells had a structure similar to the external peg (Fig. 3c). After 24 h of incubation, clusters of S. schoenii cells around P. digitatum hyphae were usually observed (Fig. 4), the number and size of which increased over time. When the preparations were washed with sterile saline solution (NaCl 0.85%) and re-mounted on GY medium, the clusters remained present but the number of free S. schoenii cells decreased on the preparation. Further observations (4 h later) of rinsed preparations showed the presence of even bigger clusters, suggesting that the clusters are the result of S. schoenii cells growth and not from the addition of free cells to adhered clusters.
Figure 3.
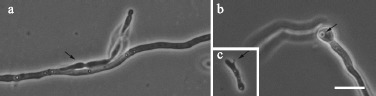
Phase contrast photomicrography of Saccharomycopsis schoenii predation on Penicillium digitatum LCP 68.175. (a) S. schoenii cell (arrow) adhered to P. digitatum hypha 3 h after co-incubation. (b) Haustorium (arrow) of S. schoenii in the interior of germinated spore of P. digitatum 12 h after co-incubation. (c) Infection peg (arrow) of S. schoenii (Bar = 10 μm).
Figure 4.
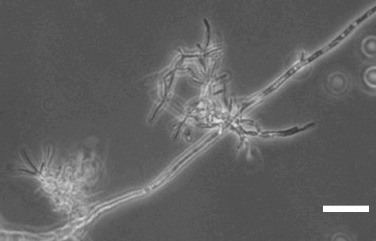
Phase contrast photomicrography of 24 h co-incubated Saccharomycopsis schoenii and Penicillium digitatum cells. Note the yeast cell cluster attached to the hypha of P. digitatum. (Bar = 10 μm).
DISCUSSION
The S. schoenii strain exhibited the ability to reduce disease severity caused by all the moulds tested in our study. The variation in the percentage of reduction in the lesions produced by the different mould strains is probably due to natural differences in pathogen susceptibility to the yeast, pathogen virulence or both of these factors. Lesions were also observed in oranges that were not inoculated with either the pathogen or the yeast and most likely a result of naturally occurring pathogens. No effort was made to identify those naturally occurring pathogens, but filamentous fungi re-isolated from artificially infected fruits were always similar to the pathogen strains used in the experiment. The disease incidence in non-artificially infected oranges was quite variable but after treatment with S. schoenii, disease severity diminished in both artificially infected and naturally infected fruits (data not shown). S. schoenii does not produce symptoms of decay, necrosis or chlorosis on oranges. This is a desirable characteristic in a biocontrol agent, but a feature not frequently observed, some times forcing the use of weak pathogens to control more aggressive ones that could lead to the development of symptoms in the host (6,16,20). Another desirable characteristic of a biocontrol agent is the ability to remain on fruit surfaces for prolonged periods of time. The antagonist S. schoenii strain was present at high concentrations on orange surfaces for at least 3 weeks. This period may be sufficient for the biological control of post harvest disease of oranges.
The yeast cell concentration necessary to achieve disease control was high, as previously demonstrated for other yeasts used in biological control studies (9,17,27). S. schoenii produced efficient control of molds only when inoculated at concentrations of at least 108 cells/ml.
The use of predacious yeasts as biocontrol agents for microbial diseases is a novel approach and may circumvent the difficulties of attempting to control pathogens capable of producing antagonistic substances. The lack of chemical antagonistic effect is interesting because it precludes the contamination of fruits with toxic, allergenic and antibiotic substances. In our study, predation of both hypha and conidia were documented in vitro, and the S. schoenii strain had the ability to control disease inflicted by all moulds tested. Lachance and Pang (14) have observed conidia penetration by Candida sp. 95-697.4 (an anamorph of a not yet described Saccharomycopsis species) in P. chrysogenum Thom. S. schoenii needs organic source of sulfur (15). Probably the predation of fungi is the preferential way by which S. schoenii obtains sulfured compounds which are scarce on the surface of oranges. The interaction of more than one antagonistic event such as predation, competition and other antagonistic interactions is often reported (10,27). As a result, it is possible that the action of S. schoenii arises from more than one of these interactions although it is noteworthy that the production of antagonistic substances was not detected in our study.
Oranges and other citrus fruits are washed before commercialization and the inoculation of an antagonist yeast at this step would be possible, preferentially together with substances that promote the growth of antagonists (11,17,18,19). The storage time of the fruit could be increased by the treatment and securely the economical losses due to decay would diminish. Although further studies are necessary to establish the most efficient means for the application of yeast in commercial facilities, we believe the use of predacious S. schoenii represents one promising strategy for postharvest disease control.
ACKNOWLEDGMENTS
This work was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico of Brazil (CNPq PADCT process number 477528/03-1) and Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG process numbers CBB 1207/98 and CBS - 378/04). We acknowledge Rachel Basques Caligiorne and Marc-Andre Lachance for sending the molds and the yeast strain, respectively.
RESUMO
Controle biológico de Penicillium italicum, P. digitatum e P. expansum com a levedura predadora Saccharomycopsis schoenii em laranjas
Este estudo avaliou a capacidade de levedura Saccharomycopsis schoenii Nadson & Krassiln (UWO-PS 80-91) em controlar o crescimento dos fungos fitopatogênicos Penicillium expansum Link (UFMG 01-2002), P. italicum Wehmer (LCP 61.1199), e P. digitatum (Pers.: Fr.) (LCP 984263, LCP 68175 e LCP 4354). S. schoenii reduziu a severidade da doença em laranjas inoculadas com todos os fitopatógenos testados. Entre estes fitopatógenos, P. digitatum LCP4354 apresentou a maior virulência enquanto que P. digitatum LCP 68175 foi o mais suscetível à predação. A levedura foi capaz de permanecer viável, sem produzir lesões na superfície dos frutos por 21 dias. Outra característica desejável observada foi a ausência de produção de substâncias antagonistas. Sendo assim, este trabalho evidência o potencial de utilização da levedura S. schoenii em protocolos de controle biológico de doenças pós-colheita em laranjas.
Palavras-chave: controle biológico, laranja, doença pós-colheita, Penicillium, Saccharomycopsis schoenii.
REFERENCES
- 1.Abranches J., Morais P.B., Rosa C.A., Mendonça-Hagler L.C., Hagler A.N. The incidence of killer activity and extracellular proteases in tropical yeast communities. Can. J. Microbiol. 1997;43(4):328–336. doi: 10.1139/m97-046. [DOI] [PubMed] [Google Scholar]
- 2.Assis M.P.S., Mariano R.L.R., Michereff S.J., Silva G., Maranhão E.A.A. Antagonism of yeasts to Xanthomonas campestris PV. campestris on cabbage phylloplane in field. Rev. Microbiol. 1999;30(3):191–195. [Google Scholar]
- 3.Burden J., Wills R.B.H., Smith K., Toet A., Shepherd A. Prevention of postharvest food losses: fruits, vegetables and root crops, a training manual. FAO Training Series: no. 17/2, Rome. 1989. Available at: http://www.fao.org/documents/show_cdr.asp?url_file=/docrep/t0073e/T0073E00.htm.
- 4.Droby S., Lischinski S., Cohen L., Weiss B., Daus A., Chand-Goyal T., Eckert J.W., Manulis S. Characterization of an epiphytic yeast population of grapefruit capable of suppression of green mold decay caused by Penicillium digitatum. Biol. Control. 1999;16(1):27–34. [Google Scholar]
- 5.Droby S., Vinokur V., Weiss B., Cohen L., Daus A., Gold-Schmidt E.E., Porat R. Introduction of resistance to Penicillium digitatum in grapefruit by the yeast biocontrol agent Candida oleophila. Phytopathol. 2002;92:393–399. doi: 10.1094/PHYTO.2002.92.4.393. [DOI] [PubMed] [Google Scholar]
- 6.Dugan F.M., Roberts R.G. Etiology of preharvest colonization of Bing cherry fruit by fungi. Phytopathol. 1995;84:1031–1036. [Google Scholar]
- 7.Eckert J.W., Ogawa J.M. The chemical control of postharvest diseases: subtropical and tropical fruits. An. Rev. Phytopathol. 1985;23:421–454. [Google Scholar]
- 8.Fleischer G., Waibel H. The long term consequences of pesticide use in agriculture: Some evidence from Germany. IX annual conference of the European association of environmental and resource economists 1 (EAERE), Oslo. 1999. Available at: http://66.102.1.104/scholar?num=100&hl=pt-BR&lr=&newwindow=1&q=cache:X8XfsQot404J www.ifgb.uni-hannover.de/ppp/I_Fleischer_1999b.pdf+%22The+long+term+consequences+of+pesticide+use+in+agriculture:+Some+evidence+from+Germany.
- 9.Helbig J. Ability of the antagonistic yeast Cryptococcus albidus to control Botrytis cinerea in strawberry. BioControl. 2002;47:85–99. [Google Scholar]
- 10.Janisiewicz W.J., Korsten L. Biological control of postharvest disease of fruits. An. Rev. Phytopathol. 2002;40:411–441. doi: 10.1146/annurev.phyto.40.120401.130158. [DOI] [PubMed] [Google Scholar]
- 11.Janisiewicz W.J., Conway W.S., Glenn D.M., Sams C.E. Integrating biological control and calcium treatment for controlling postharvest decay of apples. HortScience. 1998;33:105–109. [Google Scholar]
- 12.Jijakli M.H., Lepoivre P. Characterization of an exo-α1,3-glucanase produced by Pichia anomala strain K, antagonist of Botrytis cinerea on apples. Phytopathol. 1998;88:335–343. doi: 10.1094/PHYTO.1998.88.4.335. [DOI] [PubMed] [Google Scholar]
- 13.Kurtzman C.P., Robnett C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek. 1998;73:331–371. doi: 10.1023/a:1001761008817. [DOI] [PubMed] [Google Scholar]
- 14.Lachance M.A., Pang W.M. Predacious yeast. Yeast. 1997;13:225–232. doi: 10.1002/(SICI)1097-0061(19970315)13:3<225::AID-YEA87>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 15.Lachance M.A., Pupovac-Velikonja A., Natarajan S., Shlag-Edler B. Nutrition and phylogeny of predacious yeasts. Can. J. Microbiol. 2000;46:495–505. doi: 10.1139/w00-021. [DOI] [PubMed] [Google Scholar]
- 16.Matteson H.M.C., Corral-Garcia M.R., Momol E.A., Burr T.J. Russet of apple fruit caused by Aureobasidium pullulans and Rhodotorula glutinis. Plant Dis. 1997;81:339–342. doi: 10.1094/PDIS.1997.81.4.337. [DOI] [PubMed] [Google Scholar]
- 17.McGuire R.G. Application of Candida guilliermondii in commercial citrus coatings for biological control of Penicillium digitatum on grapefruits. Biol. Control. 1994;4:1–7. [Google Scholar]
- 18.Pusey P.L., Wilson C.L. Postharvest biological control of stone fruit brown rot by Bacillus subtilis. Plant Dis. 1984;68:753–756. [Google Scholar]
- 19.Queiroz B.P.V., Melo I.S. Antagonism of Serratia marcescens towards Phytophthora parasitica and its effects in promoting the growth of citrus. Braz. J. Microbiol. 2006;37:448–450. [Google Scholar]
- 20.Reeleder R.D. The use of yeasts for biological control of the plant pathogen Sclerotinia sclerotiorum. BioControl. 2004;49:583–594. [Google Scholar]
- 21.Rist K.L., Rosenberger D.A. A storage decay of apple fruit caused by a Aureobasidium pullulans. Plant Dis. 1995;79:425–431. [Google Scholar]
- 22.Spadaro D., Gullino M.L. State of the art and future prospects of the biological control of postharvest fuit diseases. Int. J. Food Microbiol. 2004;91:185–194. doi: 10.1016/S0168-1605(03)00380-5. [DOI] [PubMed] [Google Scholar]
- 23.Usall J., Teixido N., Fons E., Vinas I. Biological control of blue mold on apple by a strain of Candida sake under several controlled atmosphere conditions. Int. J. Food Microbiol. 2000;58:83–92. doi: 10.1016/s0168-1605(00)00285-3. [DOI] [PubMed] [Google Scholar]
- 24.Varma J., Dubey N.K. Efficacy of essential oils of Caesulia axillaries and Mentha arvensis against some storage pests causing biodeterioration of food commodities. Int. J. Food Microbiol. 2001;68:207–210. doi: 10.1016/s0168-1605(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 25.Vinas I., Usall J., Sanchis V. Biological control of major postharvest pathogens on apple with Candida sake. Int. J. Food Microbiol. 1998;40:9–16. doi: 10.1016/s0168-1605(98)00009-9. [DOI] [PubMed] [Google Scholar]
- 26.Wesseling C., Corriols M., Bravo V. Acute pesticide poisoning and pesticide registration in Central America. Toxicol. Appl. Pharmacol. 2005;207:697–705. doi: 10.1016/j.taap.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Young T.W. The yeasts: Biology of yeasts. In: Rose A. H., Harrison J. S., editors. vol. 1. London: Academic Press; 1987. [Google Scholar]
- 28.Zhang Hong-yin., Zheng Xiao-dong., Xi Yu-fang. Biological control of postharvest blue mold of oranges by Cryptococcus laurentii (Kufferath) Skinner. BioControl. 2005;50:331–342. [Google Scholar]


