Abstract
An alkaliphilic and highly thermostable α-amylase producing Bacillus sp. was isolated from Van soda lake. Enzyme synthesis occurred at temperatures between 25°C and 40°C. Analysis of the enzyme by SDS-PAGE revealed a single band which was estimated to be 66 kDa. The enzyme was active in a broad temperature range, between 20°C and 90°C, with an optimum at 50°C; and maximum activity was at pH 10.5. The enzyme was almost completely stable up to 80°C with a remaining activity over 90% after 30 min pre-incubation. Thermostability was not increased in the presence of Ca2+. An average of 75% and 60°C of remaining activity was observed when the enzyme was incubated between pH 5 and 9 for 1 h and for 2 h, respectively. The activity of the enzyme was inhibited by SDS and EDTA by 38% and 34%, respectively.
Keywords: Bacillus sp, α-amylase, Alkaliphilic, Thermostable, Enzyme
INTRODUCTION
Amylases are one of the most important industrial enzymes. α-Amylase holds the maximum market share of the enzyme sales with major applications in the starch industry, baking, analytical chemistry, automatic dishwashing detergents, textile desizing, medicine, pulp and paper industry (12). Enzymes from organisms grown in habitat characterized by extreme environments have proven to be useful for industrial processes (32). Alkaline environments have drawn the attention for isolation of alkaliphilic bacterium to obtain alkaline enzyme production.
There are two kinds of naturally occuring alkaline environments in the world. One, high Ca2+ environments (ground waters bearing high Ca(OH)2) and two, low Ca2+ environments (soda lakes and deserts dominated by sodium carbonate) (41). Soda lakes represent a specific type of salt lake, which contain an alkaline sodium carbonate/bicarbonate fraction among the dominant salts. They are mostly confined to dry areas with high evaporation rates that facilitate salt accumulation in local depressions. The presence of sodium carbonate in variable combinations with sodium chloride and sodium sulfate creates a unique, buffered haloalkaline habitat appropriate for a stable development of obligately (halo)alkaliphilic microorganisms growing optimally at pH around 10 (39).
Bacillus sp. is one of the dominant genus among the gram-positive isolates from soda lakes (9) and their soil (33). The first alkaline amylase of an alkaliphilic Bacillus strain was reported by Horikoshi (16). Industrial applications of these microorganisms have been investigated extensively and some of their enzymes such as alkaline amylases have been put to use on an industrial scale (18).
Additional considerable interest has been drawn to enzymes of moderately halophilic bacteria and their biotechnological potentials (42). Halophilic enzymes, while performing identical enzymatic functions as their non-halophilic counterparts, have been shown to exhibit different properties such as a requirement for high salt concentrations, increased activity, variable stability etc. (29). Usually, halophilic enzymes not only are able to deal with high ionic strength in their environment but also are able to maintain their function and structure (10).
Therefore the potential of alkaline and halo-alkaline amylases for industrial applications has attracted a search for microbial strains showing relevant activities with those desired properties. Furthermore, along with the increasing importance of the enzymes in biotechnological industry, e.g. biotransformation and biosensors, stable and active proteins in low-water or non-aqueous systems are required (34). Therefore the search for new enzymes with different biochemical properties entails the isolation of the enzyme directly from natural hosts. Recent developments indicate that haloalkaliphilic species are good sources of biomolecules of great industrial interest (20).
The present study deals with the isolation of an alkaliphilic Bacillus sp. from Van Soda lake, and characterization of extracellular a-amylase.
MATERIALS AND METHODS
Organisms and Cultivation Conditions
Bacillus sp.AB68 was isolated from mud samples collected from the shoreline of the Van soda lake, situated on the high plateaus of Eastern Anatolia at about 43 E longitude and 38.5 N latitude in Turkey. Selection of gram positive spore forming bacteria, Bacillus sp., was carried out by pasteurizing the samples at 80°C for 10 min. A total 226 bacterial isolates were screened for amylase production on minimal medium (M9) starch agar plates containing: Na2HPO4 6 g/L, KH2PO4 3 g/L, NaCl 10% (w/v), NH4Cl 1 g/L, MgSO4 x 7H2O 0.24 g/L, CaCl2 0.24 g/L, Pepton 3 g/L, Soluble Starch 1% (w/v) (Merck), Agar agar 15 g/ L. The initial pH was adjusted to 10 after autoclaving with 10% Na2CO3 (30). A total of 88 amylolytic isolates were selected by flooding the agar plates with iodine solution (15). The largest activity showing 5 amylase positive strains were stored at +4°C on agar slope until enzyme production occured.
Enzyme Production
The strain Bacillus sp.AB68 was cultivated in minimal medium (M9) containing 10% NaCl and 1% soluble starch. The pH of medium was adjusted to 10 after autoclaving with 10% Na2CO3. Cultures were grown for 20 hours at 37°C with shaking at 200 rpm. After the removal of cells by centrifugation (Hettich Universal 30 RF) (11 200 g, 20 min) at +4°C, the supernatant was used for further work (28).
Partial Purification of Amylase
The supernatant was subjected to fractionated ammonium sulfate precipitation for enzyme purification. Ammonium sulfate crystals were added to the supernatant to bring the saturation to 40–90% in an ice bath. After for 2 h, the precipitate was collected by centrifugation at 11 200 g, +4°C, for 20 min. The enzyme was recovered by re-suspending the precipitate in 100 mM phosphate buffer at pH 7.6 (28). Then the suspension was dialysed against the same buffer for 3 days with several changes of buffer for desalting.
Enzyme Assay
Amylase activity was assayed by adding 0.5 mL of enzyme to 0.5 mL soluble starch (1% v/v) in 100 mM glycine-NaOH buffer, pH 10.5, and incubating at 50°C for 30 min. The reaction was stopped by the addition of 1 mL of 3,5-dinitrosalicylic acid reagent and absorbance was measured in a Cecil 5500 spectrophotometer (A550) (2). One unit of enzyme activity was defined as the amount of enzyme releasing 1 mmol of reducing sugars per minute under the standard assay conditions.
Effect of pH, Temperature and Salt Concentration on Activity and Stability
Temperature and pH effects on enzyme activity were assayed at different temperatures ranging from 20 to 100°C and at pH values from 4 to 13 for 30 min. The following buffers were used in the reactions: 100 mM Citrate-phosphate buffer (pH 4.0–6.0), 100 mM Na-Phosphate buffer (pH 6.5–8.0), 100mM Glycine-NaOH buffer (pH 8.5–10.5), and 100 mM Borax-NaOH buffer (pH 11.0–13.0). To determine the temperature stability, the enzyme was pre-incubated between temperatures 20°C and 100°C for 30 and 60 min at the optimum pH and the remaining activity was determined under standard enzyme assay conditions. For determination of pH stability, the enzyme was pre-incubated at pH ranging from 6.5 to 12.5 at 50°C for one and two hours. The effect of the NaCl concentration on enzyme activity was assayed in the presence of NaCl concentrations ranging from 3% to 20%, according to the standard enzyme assay condition. On the other hand, enzyme stability was tested by pre-incubating the enzyme with a desired salt concentration at the optimum temperature (50°C) in 100 mM Glycine-NaOH buffer pH 10.5 and then measuring the remaining activity at standard enzyme conditions. The activity of the enzyme stored at +4°C was used as a control for both thermal stability, pH stability and other assays. The experiment was repeated two times and mean values were taken.
Effect of Some Metal Ions and Chemical Agents on Enzyme Activity
The effect of metal ions and some chemicals, including chelating agents and inhibitors on amylolytic activity, was studied by pre-incubating the enzyme in the presence of substances with a final concentration of 3 or 5 mM for 30 min at 50°C, and then performing the assay in the presence of the same substances at the optimum temperature (11,26). All metals used were in the chloride form. The activity in the absence of any additives was taken to be 100%.
PAGE Analysis and Activity Staining
SDS-PAGE (10%) (24) was carried out for determination of homogeneity and molecular mass. After electrophoresis, protein bands were detected by destaining the gel in a methanol-acetic acid-water solution (4:1:5 by volume) after a staining process with 0.1% Coomassie Blue R 250 (4,27). Activity staining was performed on Native-PAGE by soaking the gel in 10 g/L starch in 50 mM Glycine-NaOH at pH 10 and incubating at 50°C for 30 min after electrophoresis. The clear bands after iodine solution treatment indicate α-amylolytic activity (14).
Chromatography of the End Products of Starch Hydrolysis
Soluble starch (amylose) (1%) was digested with amylase in Glycine-NaOH buffer pH 10.5 at 50°C for 45 minutes. Previously chilled ethanol was added to the enzyme and substrate mixture to stop the reaction. The end products were then analysed on silica gel 60 (GF254) (Merck) thin-layer chromatography. After developing the products with a solvent system of butanol-acetic acid-water (3:1:1,by volume), the spots were visualized by spraying it with 20% sulphuric acid in ethanol and baking it in an oven at 120°C for 30 min.
RESULTS
The strain Bacillus sp. AB68 was gram positive, rod shaped, motile, spore forming, and aerobic. The growth observed between pH 7.0 and 12.0 in the presence of NaCl ranged from 3% to 15%, and occurred up to 45°C. Although the enzyme synthesis occurred at temperatures between 25°C and 40°C at optimum pH 9.5, the optimum temperature for growth and enzyme production was 37°C. While maximal growth and enzyme production were obtained in the presence of 3% NaCl, no growth was observed with 20% NaCl. According to Ventosa (42), this strain is moderately halophilic. The supernatant was precipitated using 40 to 90% ammonium sulfate and the active enzyme fraction was recovered from the precipitate obtained at 70–80% ammonium sulfate saturation.
Determination of the Molecular Weight
SDS-PAGE analysis of the enzyme revealed a single band which, based upon linear regression considering the migration of the standard proteins, has an estimated molecular weight around 66 kDa (Fig. 1). The correlation coefficient (R) for the regression analysis is 0.939.
Figure 1.
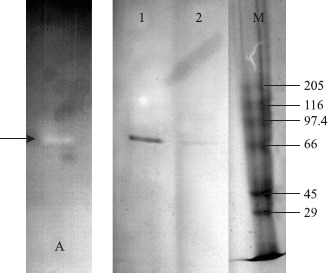
Zymogram and SDS-PAGE analysis of Amylase. Lane A: Amylase was run on native-PAGE, then soaked in 10 g/L of soluble starch in 50 mM Glycine-NaOH pH 10.0 and incubated with shaking (60 rpm) at 50°C for 30 min. Activity zones on gel were then visualised with a solution containing 5 g/L KI and 0.5 g/L I2.The sample was subjected to SDS-PAGE and the protein bands visualized with Coomasie Brillant Blue staining. Lane 1: 30 μL and Lane 2: 15 μL of Amylase. M: SDS-6H High Molecular Weights Standard Mixture (Sigma, Carbonic Anhydrase 29; Ovalbumin 45; Bovine Albumin 66; Phosphorylase 97.4; β-Galactosidase 116 and Myosine 205 kDa).
Properties of the Enzyme
The optimum pH was determined using four different buffer systems. The enzyme presented relative activity over 80% in the pH range of 6.0 to 11.5, with an optimum 10.5. Although the average relative activity between pH 6.0 and 11.5 was 89%, over 90% of relative activity was observed between pH 7.5 and 11.5 (Fig. 2). Although the optimum temperature was observed arround 50°C (Fig. 3), the enzyme presented over 60% activity between 20 and 100°C. Between 20 and 80°C, the enzyme was highly active, with an average of 94%.
Figure 2.

Effect of pH on the activity of Bacillus sp. AB68 Amylase. The reaction mixture contained 0.5 mL substrate (1% soluble starch) and 0.5 mL enzyme. The reaction mixture was incubated at 50°C for 30 min. The buffers were 100 mM Citrate-Phosphate (pH 4.0–6.0), Na-Phosphate (pH 6.5–8.0), Glycine-NaOH (pH 8.5–10.5) and Borax-NaOH (pH 11.0–13.0).
Figure 3.

Effect of temperature on the activity of Bacillus sp. AB68 amylase. The reaction mixture contained 0.5 mL substrate (1% soluble starch in Glycine-NaOH buffer, pH 10.5) and 0.5 mL enzyme. The mixture was incubated for 30 min at temperatures from 20 to 100°C under standard enzyme assay conditions.
The interference of pH on the stability of the enzyme was determined by incubating it at 50°C for one and two hours. Remaining activity was measured by the standard assay methods. An average of 75% remaining activity was observed between pH 5.0 and 9.0, which is a significant stability value for 1 h (Fig. 4). Although the average of 58% of the original activity was preserved for 2 h between pH 4.0 and 9.0, the maximal remaining activity was obtained at pH 8.0 (62.5%). For the thermal stability estimation, the enzyme was pre-incubated at temperatures between 20 and 100°C for 30 min and 60 min at the optimum pH, and the remaining activity was determined (Fig. 5). The enzyme was almost completely active up to 90°C with a remaining average activity of 95%. On the other hand, more than 80% of the original activity was retained up to 80°C after heat treatment for 60 min.
Figure 4.
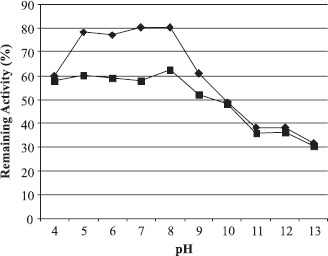
Effect of pH on the stability of Bacillus sp.AB68 Amylase. For determination of pH stability of amylase AB68, the enzyme was pre-incubated in buffers at 50°C for 1 (◆) and 2 hours (■). The buffers used were 100 mM Citrate-Phosphate (pH 4.0–6.0), 100 mM Na-Phosphate (pH 6.5–8.0), 100 mM Glycine-NaOH (pH 8.5–10.5), and 100 mM Borax-NaOH (pH 11.0–13.0).
Figure 5.

Thermal stability of Bacillus sp. AB68 amylase. The enzyme was pre-incubated at temperatures from 20 to 100°C for 30 min (◆) and 60 min (■) at optimum pH, subsequently remaining activity (%) was determined under standard enzyme condition.
To test the effect of salt concentration on amylase activity, NaCl concentrations ranging from 3% to 20% were used (Fig. 6). The enzyme activity was determined according to the standard enzyme assay. The optimal salt concentration for maximal activity (106%) was 5% of NaCl. Although the enzyme activity increased in the presence of 3%, 5%, and 7% of NaCl, only 28% of activity was lost in the presence of 20% NaCl.
Figure 6.

Effect of salt concentration on enzyme activity (•) and stability (■,▲) of Bacillus sp. AB68 amylase. The reaction mixture for enzyme activity contained 0.5 mL substrate (1% soluble starch in Glycine-NaOH buffer, pH 10.5) with NaCl and 0.5 mL enzyme. Enzyme stability was tested by pre incubating the enzyme at 50°C, pH 10.5 for 30 (■) and 60 min (▲).
Amylase stabilty was tested by pre-incubating the enzyme at 50°C, pH 10.5 for 30 min and 60 min within determined salt concentrations. Although the enzyme activity was unaffected by 3% NaCl, there was a gradual decrease in the amylase activity as the salt concentration rose. But, the remaining activity was over 80% up to 15% NaCl for both pre-incubation times (Fig. 6), indicating that the enzyme AB68 is halotolerant.
Effect of Some Metal Ions and Chemicals on Activity
The enzyme was incubated at 50°C for 30 min at different concentrations of metal ions and various chemicals prior to the standard enzyme assay. The activities measured were expressed as residual activity. The activity was inhibited in the presence of EDTA, SDS, ZnCl2 and Urea at 66%, 62%, 66% and 76% respectively (Table 1). The additions of CaCl2 and Triton X–100 did not affect the enzyme activity. On the other hand, a stimulated enzyme activity was observed in the presence of 2-Mercaptoethanol and Na2SO3, around 147% and 105%, respectively. Among the tested chemicals, SDS (1%) was the most inhibitory: the enzyme lost 38% of its original activity within 30 min.
Table 1.
Effect of some metal ions and chemicals on the activity of Bacillus sp. AB68 Amylase.
| Effectors | Concentration | Residual Activity (%) |
|---|---|---|
| Control | None | 100 |
| EDTA | 5 mM | 66 |
| CaCl2 | 5 mM | 99 |
| PMSF | 3mM | 96 |
| 2-Mercaptoethanol | 1% | 147 |
| SDS | 1% | 62 |
| Triton X–100 | 1% | 99 |
| Na2SO3 | 5 mM | 105 |
| ZnCl2 | 5 mM | 66 |
| Urea | 8 M | 76 |
Analysis of the End Products of the Soluble Starch Hydrolysis
After 45 min of incubation of the enzyme with the substrate mixture, maltose was the shortest detectable sugar on thin layer chromatography (Fig. 7). Longer oligosaccharides were also detected, what indicates that the enzyme is an a-amylase.
Figure 7.

Thin layer chromatography of enzyme products from Bacillus sp. AB68. N: 5 mL Untreated soluble starch 1% (w/v); G: 5 μL of Glucose 4% (w/v); M: 5 μL of Maltose, 4% (w/v); AB68: 5 μL of enzyme substrate mixture.
DISCUSSION
Bacillus sp. AB68 produced an extracellular a-amylase in a liquid medium containing 10% NaCl and 1% soluble starch. After extraction from culture supernatant by fractionated ammoniun sulfate precipitation, dialysis, recovery from the precipitate at 70–80% ammonium sulfate saturation, and SDS-PAGE analysis for molecular weight and homogenity determination, a single band was detected and calculated to be 66 kDa. Similar findings between 53 kDa and 70 kDa have been reported earlier (1,13,16,19). These differences of molecular weights of α-amylases result from the genes corresponding to the organism (38).
It has been reported that most alkaline amylases of different species of Bacillus have optimum temperature around 40–70°C (3,7,19,21,25,40). Although the maximal activity was obtained at 50°C, the enzyme AB68 presented activity in a broad temperature range, up to 80°C (Fig. 3) confirming that the enzyme is thermotolerant. Thermostable alkaline amylases have been investigated but only a limited number of these were reported (3,5,37). AB68 presents a highly remarkable thermostability compared to the literature values, maintining around 90% activity up to 80°C. Among the alkaline amylases reported to date, the enzyme AB68 is one of the highest alkaliphilic amylases, having optimum pH around 10.5, just after the amylase from GM8901 with optimum pH 11.0–12.0 (21). Similar results were also announced by Horikoshi (17) and Burhan (5) with an optimum pH 10.5.
The amylases are calcium metalloenzymes (36). The bound calcium amount is between one and ten per enzyme molecule and, usually, the last calcium ion has the greatest effect on enzyme conformation and stability (43). Amylase AB68 must be tightly bound to calcium ions, that must explain why addition of calcium (5 mM) had no increasing effect on enzyme activity (99%). The inhibition of the original activity (by 34%) with EDTA (5mM) treatment also suggests that the enzyme is a metalloenzyme and that the calcium ion plays a role on the thermostability of the enzyme.
The effect of Zn2+ on activity varies among amylases. It could have since potent inhibitory effect (8, 31) to no effect at all (21). AB68 amylase was inhibited by Zn2+ to 66%, as was the amylase from the thermophilic Bacillus sp. TS–23 (25). The inhibiton of amylase AB68 by Zn2+ could be due to competition between the exogenous cations and the protein associated cation, resulting in decreased activity (25). PMSF produced no effect on amylase activity (Table 1). This may be the reason why carboxylic residues are essential for catalysis (25) and similar findings (91%) were also reported (3).
Urea (8M) and SDS (1%) inhibited the amylase by 24.29% and 38.29%, respectively. SDS was the most effective effector as reported earlier (6,23), what shows the proportion of hydrophobic amino acid composition of the enzyme (11). Triton X-100 (1%) had no inhibitory action on the enzyme (Table 1). The increase in the activity (147%) of amylase AB68 indicates that 2-Mercaptoethanol protected enzyme activity. This finding was also stated by Egas (11) for the amylase from Thermus filiformis Ork A2 (132%). Disulfide bonds are commonly found in extracellular proteins and it is widely accepted that they contribute to the stabilization of the native conformation of proteins (35); in spite of the ability of 2-Mercaptoethanol to disrupt the structure of proteins, the enzyme AB68 has not lost its activity (Table 1) at 1% concentration. On the other hand, the presence of sodium sulfite (5 mM) was found to be beneficiary for activity (105.43%) as for B.licheniformis CUMC305 amylase (115%) reported by Krishnan and Chandra (22). If the NaCl effect (Fig. 6) is also considered, the enzyme becomes more stabilized in the presence of Na+ ions.
In conclusion, the enzyme described here presented several features found in other alkaliphilic amylases, including halophilic behavior. Amylase AB68 may not be suitable enough as an additive for detergents if SDS inhibition is considered, but the enzyme demonstrates significant thermostability and alkaliphilic properties, as well as biochemical features for most industrial applications.
ACKNOWLEDGEMENTS
We thank Drs. S.O.Hashim and Emin Ozkose for helpful critical discussions and some material they have supplied. We also thank Mr. Kadir and Mrs. Erika Cakmak for English revising of the manuscript.
RESUMO
α-amilase alcalina termoestável de Bacillus sp AB68 halotolerante-alcalifílico
Bacillus sp AB68 alcalifílico produtor de α-amilase alcalina termoestável foi isolado do lago Van soda. A síntese da enzima ocorreu entre 25°C e 40°C. A análise da enzima por SDS-PAGE revelou uma única banda estimada em 66 kDa. A enzima foi ativa em uma ampla faixa de temperatura, entre 20°C e 90°C, com um ótimo a 50°C. A atividade máxima foi em pH 10,5. A enzima foi estável até 80°C, mantendo 90% de atividade após 30 min de pré-incubação. A termoestabilidade não aumentou na presença de Ca2+. Quando incubada em pH entre 5 e 9 por 1h e por 2h, a enzima manteve 75% e 60% de atividade, respectivamente. SDS e EDTA causaram redução de 38% e 34% na atividade da enzima, respectivamente.
Palavras-chave: Bacillus sp, a-amilase, alcalifílico, termoestável, enzima
REFERENCES
- 1.Ben M.A., Mhiri S., Mezghani M., Bejar S. Purification and sequence analysis of the atypical maltohexaose-forming α-amylase of the B. stearothermophilus US100. Enzyme Microb. Technol. 2001;28:537–542. doi: 10.1016/s0141-0229(01)00294-0. [DOI] [PubMed] [Google Scholar]
- 2.Bernfeld P. Amylases α- and β-methods. Enzymol. 1955;1:149–158. [Google Scholar]
- 3.Bernhardsdotter E.C.M.J., Ng J.D., Garriott O.K., Pusey M.L. Enzymic properties of an alkaline chelator-resistant α-amylase from alkaliphilic Bacillus sp. isolate L1711. Process. Biochem. 2005;40:2401–2408. [Google Scholar]
- 4.Bollag D.M., Rozycki M.D., Edelstein S.J. New York: Willey-Liss Inc; 1996. Protein methods. [Google Scholar]
- 5.Burhan A., Nisa U., Gokhan C., Omer C., Ashabil A., Osman G. Enzymatic properties of a novel thermostable, thermophilic, alkaline and chelator resistant amylase from an alkaliphilic Bacillus sp. isolate ANT-6. Process Biochem. 2003;38:1397–1403. [Google Scholar]
- 6.Chung Y.C., Kobayashi T., Kanai H., Akiba T., Kudo T. Purification and properties of extracellular amylase from the hyperthermophilic archeon Thermococcus profundus DT5432. Appl. Environ. Microbiol. 1995;61:1502–1506. doi: 10.1128/aem.61.4.1502-1506.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordeiro C.A.M., Martins M.L.L., Luciano A.B. Production and Properties of α-Amylase from Thermophilic Bacillus sp. Braz. J. Microbiol. 2002;33:57–61. doi: 10.1590/S1517-838220080001000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demirkan E.S., Bunzo M., Adachi M., Higasa T., Utsumi S. α-Amylase from B.amyloliqefaciens: purification, characterization, raw starch degradation and expression in E. coli. Process Biochem. 2005;40:2529–2636. [Google Scholar]
- 9.Duckworth A.W., Grant W.D., Jones B.E., van Steenbergen R. Phylogenetic diversity of soda lake alkaliphiles. FEMS. Microbiol. Ecol. 1996;19:181–191. [Google Scholar]
- 10.Dym O., Mevarech M., Sussman J.L. Structural features that stabilize halophilic malate dehydrogenase from archaebacterium. Science. 1995;267:1344–1346. doi: 10.1126/science.267.5202.1344. [DOI] [PubMed] [Google Scholar]
- 11.Egas M.C.V., da Costa M.S., Cowan D.A., Pires E.M.V. Extracellular α-amylase from Thermus filiformis Ork A2: purification and biochemical characterization. Extremophiles. 1998;2:23–32. doi: 10.1007/s007920050039. [DOI] [PubMed] [Google Scholar]
- 12.Gubta R., Gigras P., Mohapatra H., Goswami V.K., Chauhan B. Microbial α-amylases:a biotechnological perspective. Process Biochem. 2003;38:1599–1616. [Google Scholar]
- 13.Hamilton L.M., Kelly C.T., Fogarty W.M. Production and properties of the raw starch-digesting α-amylase of Bacillus sp. IMD 435. Process Biochem. 1999;35:27–31. [Google Scholar]
- 14.Hashim S.O., Delgado O., Hatti-Kaul R., Mulaa F.J., Mattiasson B. Starch hydrolysing Bacillus halodurans isolates from a Kenyan soda lake. Biotechnol. Lett. 2004;26:823–828. doi: 10.1023/b:bile.0000025885.19910.d7. [DOI] [PubMed] [Google Scholar]
- 15.Hols P., Ferain T., Garmyn D., Bernard N., Delcour J. Use of expression secretion signals and vector free stable chromosomal integration in engineering of Lactobacillus plantarum for α-amylase and levanase expression. Appl. Environ. Microbiol. 1994;60:1401–1403. doi: 10.1128/aem.60.5.1401-1413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horikoshi K. Production of alkaline amylase by alkalophilic microorganisms II Alkaline amylase produced by Bacillus no A-40–2. Agric. Biol. Chem. 1971;35:1783–1791. [Google Scholar]
- 17.Horikoshi K. Alkaliphiles-from industrial point of view. FEMS Microbiol. Rev. 1996;18:259–270. [Google Scholar]
- 18.Horikoshi K. Alkaliphile: Some application of their products for biotechnology. Microbiol. Mol. Biol. Rev. 1999;6:735–750. doi: 10.1128/mmbr.63.4.735-750.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igarashi K., Hatada Y., Hagihara H., Saeki K., Takaiwa M., Eumura T., Ara K., Ozaki K., Kawai S., Kobayashi T., Ito S. Enzymatic properties of a novel liquefying α-amylase from an alkaliphilic Bacillus isolate and entire nucleotide and amino acid sequences. Appl. Environ. Microb. 1998;64:3382–3389. doi: 10.1128/aem.64.9.3282-3289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito S., Kobayashi T., Ara K., Ozaki K., Kawai S., Hatada Y. Alkaline detergent enzymes from alkaliphilic: enzymatic properties, genetics and structures. Extremophiles. 1998;2:185–190. doi: 10.1007/s007920050059. [DOI] [PubMed] [Google Scholar]
- 21.Kim T.U., Gu B.G., Jeong J.Y., Byun S.M., Shin Y.C. Production and characterization of a maltotetraose-forming alkaline α-amylase from an alkalophilic Bacillus strain, GM8901. Appl. Environ. Microbiol. 1995;61:3105–3112. doi: 10.1128/aem.61.8.3105-3112.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan T., Chandra A.K. Purification and characterization of α-amylase from Bacillus licheniformis CUMC305. Appl. Environ. Microbiol. 1983;46:430–437. doi: 10.1128/aem.46.2.430-437.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laderman K.A., Asada K., Uemori T., Mukoi H., Taguchi Y., Kato I., Anfinsen C.B. α-amylase from the hyperthermophilic archaebacterium Pyrococcus furious. Cloning and sequencing of the gene and expression in. E. coli. J. Biol. Chem. 1993;268:24402–24407. [PubMed] [Google Scholar]
- 24.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Lin L.L., Chyau C.C., Hsu W.H. Production and properties of a raw starch degrading amylase from the thermophilic and alkaliphilic Bacillus sp. TS-23. Biotechnol. Appl. Biochem. 1998;28:61–68. [PubMed] [Google Scholar]
- 26.Lo H.F., Lin L.L., Chen H.L., Hsu H.H., Chang C.T. Enzymatic properties of a SDS-resistant Bacillus sp. TS-23 α-amylase produced by recombinant Escherichia coli. Process Biochem. 2001;36:743–750. [Google Scholar]
- 27.Maniatis T., Fritsch E.F., Sambrook J. Molecular cloning: a laboratory manuel. NY: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 28.McTigue M.A., Kelly C.T., Doyle E.M., Fogarty W.M. The alkaline amylase of the alkalophilic Bacillus sp. IMD 370. Enzyme Microb. Technol. 1995;17:570–573. [Google Scholar]
- 29.Mevarech M., Frolow F., Gloss L.M. Halophilic enzymes: proteins with a grain of salt. Biophys. Chemist. 2000;86:155–164. doi: 10.1016/s0301-4622(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 30.Milner J.A., Martin D.J., Smith A. Two-stage inoculate for the production of alpha-amylase by Bacillus amyloliquefaciens. Enzyme Microb. Technol. 1997;21:382–386. [Google Scholar]
- 31.Nagata N., Yamaguchi K., Shiinoki S. Genetic and Biochemical Studies on Cell-Bound -Amylase in Bacillus subtilis Marburg. J. Bacteriol. 1974;119:425–430. doi: 10.1128/jb.119.2.425-430.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niehaus F., Bertoldo C., Kahler M., Antranikian G. Extremophiles as a source of novel enzymes for industrial application. Appl. Microbiol. Biotechnol. 1999;51:711–729. doi: 10.1007/s002530051456. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen P., Fritze D., Priest F.G. Phylogenetic diversity of alkaliphilic Bacillus strains: proposal for nine new species. Microbiol. 1995;141:1745–1761. [Google Scholar]
- 34.Perez-Pomares F., Bautista V., Ferrer J., Pire C., Marhuenda-Egae F.C., Bonete M.J. α-Amylase activity from the halophilic archaeon Haloferax mediterranei. Extremophiles. 2003;7:299–306. doi: 10.1007/s00792-003-0327-6. [DOI] [PubMed] [Google Scholar]
- 35.Pons J., Planas A., Enrique Q. Contribution of a disulfide bridge to the stability of 1,3–1,4-β-D-glucan 4-glucanohydrolase from Bacillus licheniformis. Protein Eng. 1995;8:939–945. doi: 10.1093/protein/8.9.939. [DOI] [PubMed] [Google Scholar]
- 36.Priest F.G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol. Rev. 1977;41:711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saxena R.K., Dutt K., Agarwal L., Nayyar P. A highly thermostable and alkaline amylase from a Bacillus sp. PN5. Bioresour. Technol. 2007;98:260–265. doi: 10.1016/j.biortech.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Sidhu G.S., Sharma P., Chakrabarti T., Gupta J.K. Strain improvement for the production of a thermostable α-amylase. Enzyme Microb. Technol. 1997;21:525–530. [Google Scholar]
- 39.Sorokin D.Y., Kuenen J.G. Haloalkaliphilic sulfur-oxidizing bacteria in soda lakes. FEMS Microbiol. Rev. 2005;29:685–702. doi: 10.1016/j.femsre.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Teodoro C.E.S., Martins M.L.L. Culture conditions for the production of thermostable amylase by Bacillus sp. Braz. J. Microbiol. 2000;31:298–302. [Google Scholar]
- 41.Ulukanli Z., Digrak M. Alkaliphilic Microorganisms and habitats. Turk. J. Biol. 2001;26:181–191. [Google Scholar]
- 42.Ventosa A., Nieto J.J., Oren A. Biology of Moderately Halophilic Aerobic Bacteria. Microbiol. Mol. Biol. Rev. 1998;62:504–544. doi: 10.1128/mmbr.62.2.504-544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vihinen M., Mäntsälä P. Characterization of a thermostable B. stearothermophilus α-amylase. Biotechnol. Appl. Biochem. 1990;12:427–435. [PubMed] [Google Scholar]


