Abstract
In a new approach to microbial gallic acid production by Aspergillus fischeri MTCC 150, 40gL−1 of tannic acid was added in two installments during the bioconversion phase of the process (25gL−1 and 15gL−1 at 32 and 44h respectively). The optimum parameters for the bioconversion phase were found to be temperature: 35°C, pH: slightly acidic (3.3–3.5), aeration: nil and agitation: 250 rpm. A maximum of 71.4% conversion was obtained after 71h fermentation with 83.3% product recovery. The yield was 7.35 g of gallic acid per g of biomass accumulated and the fermenter productivity was 0.56 g of gallic acid produced per liter of medium per hour.
Keywords: tannase, gallic acid, tannic acid, Aspergillus fischeri
INTRODUCTION
Gallic acid (3, 4, 5-trihydroxybenzoic acid) is an organic substance occurring in many plants either as a free molecule or as part of tannic acid molecule (Fig. 1). Gallic acid is extensively used as an ingredient of developer in photography and printing inks. It also serves as a precursor for the commercial production of an anti-microbial drug trimethoprim, a food – preservative propyl gallate and some dyestuffs (13). Besides this, gallic acid possesses wide range of biological activities, such as antioxidant, antibacterial, antiviral, analgesic etc. As antioxidant gallic acid acts as an antiapoptotic agent and helps to protect human cells against oxidative damage (17). Gallic acid is also found to show cytotoxic activity against cancer cells, without harming normal cells (11). Because of its several interesting properties and commercial applications, gallic acid is a compound of great interest to both pharmaceutical and chemical industries. Conventionally gallic acid is produced by acid hydrolysis of tannic acid but it has cost, yield and low purity disadvantages. Alternatively, gallic acid can be produced by the microbial hydrolysis of tannic acid by tannase (tannin-acyl-hydrolase EC 3.1.1.20), an inducible enzyme, secreted by microorganisms (12). Microbial production of tannase, especially from fungi, is well documented (1, 2, 4, 5, 8, 11, 17), however, the reports on tannic acid hydrolysis is limited. Mainly Aspergilli have been used for hydrolysis of tannic acid to yield gallic acid (13–15, 18), among bacteria Klebsiella pneumoniae and Corynebacterium sp. have been reported to produce gallic acid from crude extract of tara gallotannin (6).
Figure 1.
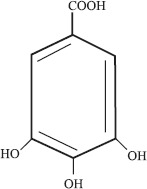
3, 4, 5-trihydroxybenzoic acid.
The current global requirement of gallic acid is around 8,000 tons/year and despite the immense commercial importance of gallic acid little work has been done on development of a process for gallic acid production at fermenter level (13, 18). The main hindrance in the development of a successful bioconversion process is the sensitivity of the microorganisms to tannic acid and the oxidation of the unused tannic acid. This limits the use of high tannic acid concentration during bioconversion process resulting in low productivity. Therefore, the authors have developed a strategy to overcome the above mentioned problems and achieve high percent conversion during bioconversion. The present study proposes a protocol for gallic acid production in a 5 liter fermenter at 40 gL−1 of tannic acid concentration extracted from Quercus infectoria gall nuts using Aspergillus fischeri MTCC 150, a new fungus which has not been reported so far.
A strain of A.fischeri MTCC 150 showing high tannase activity at shake flask level was selected and maintained on PDA slants supplemented with 0.01% tannic acid. Dry powder of tannic acid was obtained by aqueous extraction of Chinese gall nuts (Q. infectoria) in a soxhlet apparatus. The tannin obtained was pentagalloyl glucose. Mineral salt medium was used for raising the inoculum as well as for fermentation. The composition of the medium was (gL−1): NH4NO3, 1.65; KNO3, 1.9; MgSO4.7H2O, 0.37; CaCl2.2H2O, 0.44; KH2PO4, 0.17 and (mg mL−1): H3BO3, 6.2; MnSO4.H2O, 16.9; ZnSO4.7H2O, 8.6; Na2MoO4.2H2O, 0.25; CuSO4.5H2O, 0.025; CoCl2.6H2O, 0.025; FeSO4.7H2O, 5.6 and Na2EDTA, 7.6 supplemented with 5.0g tannic acid l−1 of medium, as an inducer and pH 5.6.
The experiment was performed in a 5 liter capacity, top driven BIOFLO III, NBS fermenter. The bioconversion was divided into two phases: (1) Growth phase: Three liters of growth medium was inoculated with 300 mL of pre-induced, 30 h old culture of A. fischeri MTCC 150. The values of physical parameters during growth phase were – temperature: 30°C, initial pH: 5.6, aeration: 1 vvm and agitation: 300 rpm. (2) Bioconversion phase: During this phase tannic acid was added in two installments of 25 gL−1 and 15 gL−1 after 32 h and 50 h respectively. The values of physical parameters during this phase were- temperature: 35°C, pH: 4.0 to 3.5 (not regulated), aeration: nil, agitation: 250 rpm. The fermentation was stopped after 77 h of fermentation, when there was no significant increase in gallic acid formation. The samples were analyzed at regular intervals for biomass, residual tannic acid and gallic acid formed. Biomass was estimated by dry cell weight method at 105°C, whereas, tannic acid and gallic acid were estimated by following the two wavelengths simultaneous estimation method of Bajpai and Patil (3).
Conc. of tannic acid (mg mL−1) = 34.41 (A293.8) – 6.98 (A254.6)
Conc. of gallic acid (mg mL−1) = 21.77 (A254.6) – 17.17 (A293.8)
The fermentation broth was filtered through the layers of muslin cloth and mycelial mass was washed with minimum quantity of hot water to remove the gallic acid deposited on the mycelium. For recovery of gallic acid from broth, the filtrate and washings were combined, pH was adjusted to 2.0 with HCl and kept at 0°C. Gallic acid becomes unstable at low pH and precipitates out from the broth at 0°C. The precipitate obtained was filtered under vacuum, then dissolved in minimum amount of acidified hot water and recrystallized at 4°C.
The fermentation experiment was performed under the conditions optimized at shake flask level (data not shown) with few variations. The main hindrance in the development of a successful gallic acid production process is the oxidation of tannic acid. Considering the polyphenolic nature of tannic acid and gallic acid, pH of the medium was kept acidic despite the fact that the tannase activity is favored in alkaline pH (11). The aeration was put off few hours after addition of tannic acid to avoid the oxidation of residual tannic acid and gallic acid formed, which is a serious problem and hampers the yield. Further, to prevent the degradation of gallic acid formed by fungus via tricarboxylic acid cycle (16), anaerobic environment in the fermenter was maintained. Earlier experiments have revealed that optimum temperature for growth and tannase activity are -different, therefore the temperature was raised from 30°C to 35°C after the growth phase was over.
Fig. 2 shows the effect of single dose and split dose addition of tannic acid on the pattern of gallic acid accumulation in the culture broth of A. fischeri MTCC 150 in a shake flask. It is clear from the figure that single dose addition of tannic acid had adverse effect on the activity of the fungus and the yield of gallic acid was increased by split dose addition of tannic acid. The low conversion value obtained with single dose addition of tannic acid is a direct reflection of toxic effect of high concentration of tannic acid on fungus, a fact that is supported by other workers also (7). Although fungus shows very high percent conversion (75%) at low substrate concentration (20 gL−1) as compared to 23% conversion obtained at 50 gL−1 tannic acid but for a process to be economically viable it is imperative that the substrate concentration should be as high as possible to achieve high productivity.
Figure 2.
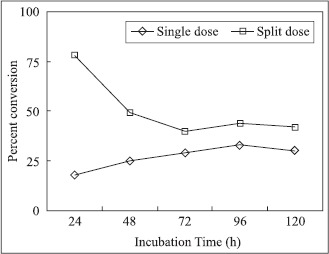
Effect of single dose and split dose addition of tannic acid on percent conversion of tannic acid into gallic acid by A.fischeri MTCC 150.
The time course of pH values and mycelia dry weight during the bioconversion process is depicted in Fig. 3. A continuous increase in the mycelia dry weight was observed up to 35 h of fermentation, which remained constant thereafter. The growth of fungus was arrested after the addition of second installment of tannic acid in the medium. The medium pH showed a steep decline during growth phase indicating the metabolism of carbon source present in the medium and in the latter stages accumulation of gallic acid.
Figure 3.
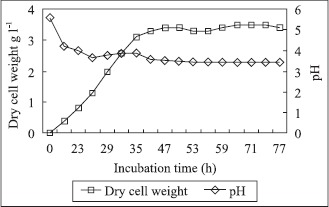
Time course of growth and pH values during bioconversion of tannic acid into gallic acid by A. fischeri MTCC 150 in a 5 liter fermenter.
The estimated concentrations of residual tannic acid and gallic acid formed in the fermentation medium during bioconversion are shown in Fig. 4. A maximum conversion of 71.4% was reached after 37 h of tannic acid addition, which is significantly more as compare to 9.75% conversion obtained by Pourrat et al. (13) at 10% tannic acid concentration. After the addition of first installment of tannic acid (25 gL−1) initially the rate of hydrolysis was slow, similar observation was made after the addition of second installment of tannic acid (15 gL−1). A continuous increase in gallic acid was noted up to 37 h of fermentation and then remained fairly constant. The fermentation was terminated when there was no increase in gallic acid content of the medium. The product recovery was 83.3% and purity of gallic acid was found to be 96%. The yield was 7.35 g of gallic acid per g of biomass accumulated and the fermenter productivity was 0.56 g of gallic acid produced per liter of medium per hour.
Figure 4.
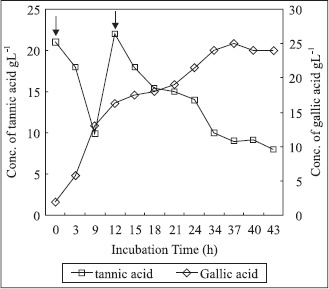
Concentrations of residual tannic acid and gallic acid formed during bioconversion process by A.fischeri MTCC 150 in a 5 liter fermenter. Tannic acid (4%) was added in two installments of 25 gl−1 and 15 gl−1 after 32h and 44h growth respectively. ↓ Indicates addition of tannic acid.
The present study recommends that to increase the yield of gallic acid two strategies should be adopted (i) the tannic acid should be added in installments after the growth phase is over and (ii) the aeration should be switched off after tannic acid addition.
ACKNOWLEDGEMENTS
Financial assistance to one of the author (Bhakti Bajpai) by Council of Scientific and Industrial Research, New Delhi, India is thankfully acknowledged.
RESUMO
Uma nova abordagem para produção microbiana de ácido gálico
Em uma nova abordagem para produção de ácido gálico por Aspergillus fischeri MTCC 150, adiciona-se 40 g.L−1 de ácido tânico em dois momentos da fase de bioconversão do processo (25 g.L−1 e 15 g.L−1 a 32h e 44h, respectivamente). Os parâmetros ótimos para a fase de bioconversão foram: temperatura 35°C, pH levemente ácido (3,3 a 3,5), nenhuma aeração e agitação 250 rpm. Um máximo de 71,4% de conversão foi obtido após 71h de fermentação, com 83,3% de recuperação do produto. O rendimento foi 7,35g de ácido gálico por g de biomassa acumulada e a produtividade do fermentador foi 0,56g de ácido gálico por litro de meio por hora.
Palavras-chave: tanase, ácido gálico, acido tânico, Aspergillus fischeri
REFERENCES
- 1.Aguilar C.N., Augur C., Favela-Torres E., Viniegra-Gonzalez G. Production of tannase by Aspergillus niger Aa-20 in submerged and solid-state fermentation: influence of glucose and tannic acid. J. Industrial Microbiol. Biotechnol. 2001;26(5):296–302. doi: 10.1038/sj.jim.7000132. [DOI] [PubMed] [Google Scholar]
- 2.Aissam H., Errachidi F., Penninckx M., Merzouki M., Benlemlih M. Production of tannase by Aspergillus niger HA37 growing on tannic acid and Olive Mill Waste Waters. W. J. Microbiol, Biotechnol. 2005;21(4):609–614. [Google Scholar]
- 3.Bajpai B., Patil S. Tannin acyl hydrolase (EC 3.1.1.20) activity of Aspergillus, Penicillium, Fusarium and Trichoderma. W. J. Microbiol. Biotechnol. 1996;12(3):217–220. doi: 10.1007/BF00360918. [DOI] [PubMed] [Google Scholar]
- 4.Batra A., Saxena R.K. Potential tannase producers from the genera Aspergillus and Penicillium. Process Biochem. 2005;40(5):1553–1557. [Google Scholar]
- 5.Bradoo S., Gupta R., Saxena R.K. Parametric optimization and biochemical regulation of extracellular tannase from A. japonicus. Process Biochem. 1997;32(2):135–139. [Google Scholar]
- 6.Deschamps A.M., Lebeault J.M. Production of gallic acid from tara tannin by bacterial strains. Biotechnol. Lett. 1984;6:237–242. [Google Scholar]
- 7.Ganga P.S., Nandy S.C., Santappa M. Effect of environmental factors on the production of fungal tannase: Part I. Leather Sci. 1977;24:327–427. [Google Scholar]
- 8.Kar B., Banerjee R. Biosynthesis of tannin acyl hydrolase from tannin rich forest residue under different fermentation conditions. J. Ind. Microbiol. Biotechnol. 2000;25(1):29–38. [Google Scholar]
- 9.Lekha P.K., Lonsane B.K. Comparative titers, location and properties of tannin-acyl-hydrolase produced by Aspergillus niger PKL 104 in solid-state, liquid- surface and submerged fermentations. Process Biochem. 1994;29:497–503. [Google Scholar]
- 10.Madlener S., Illmer C., Horvath Z., Saiko P., Losert A., Herbacek I., Grusch M., Elford H., Krupitza G., Bernhaus A. Gallic acid inhibits ribonucleotide reductase and cyclooxygenases in human HL-60 promyelocytic leukemia cells. Cancer Lett. 2007;245(1–2):156–162. doi: 10.1016/j.canlet.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Mondal K.C., Samanta S., Giri S., Pati B.R. Distribution of tannic acid degrading microorganisms in the soil and comparative study of tannase from two fungal strains. Acta Microbiol. 2001;50(1):75–82. [PubMed] [Google Scholar]
- 12.Pinto G.A.S., Leite S.G.F., Terzi1 S.Z., Couri S. Selection of tannase producing Aspergillus niger strains. Brazilian J. Microbiol. 2001;32:24–26. [Google Scholar]
- 13.Pourrat H., Regerat F., Morvan P., Pourrat A. Production of gallic acid from Rhus coriaria. L. Biotechnol. Lett. 1987;9:731–734. [Google Scholar]
- 14.Pourrat H., Regerat F., Pourrat A., Jean D. Production of gallic acid from tara tannin by a strain of Aspergillus niger. J. Ferment. Biotechnol. 1985;63:401–403. [Google Scholar]
- 15.Seth M., Chand S. Biosynthesis of tannase and hydrolysis of tannins to gallic acid by Aspergillus awamori – optimization of process parameters. Process Biochem. 2000;36(1–2):39–44. [Google Scholar]
- 16.Sohi K.K., Mittal N., Hundal M.K., Khanduja K.L. Gallic acid, an antioxidant, exhibits antiapoptotic potential in normal human lymphocytes: A Bcl-2 independent mechanism. J. Nutr. Sci. Vitaminol. 2003;49(4):221–227. doi: 10.3177/jnsv.49.221. [DOI] [PubMed] [Google Scholar]
- 17.Treviño-Cueto B., Luis M., Contreras-Esquivel J.C., Rodríguez R., Aguilera A., Aguilar C.N. Gallic acid and tannase accumulation during fungal solid state culture of a tannin-rich desert plant (Larrea tridentata Cov.) Bioresource Technol. 2006;98(3):721–724. doi: 10.1016/j.biortech.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Vermeire A., Vandamme E. Fungal conversion of gallotannins into gallic acid. Ferment. Technol. Ind. Appl. 1990:198–203. [Google Scholar]
- 19.Watanabe A. Studies on the metabolism of gallic acid by microorganisms. Part III. On the intermediary metabolism of gallic acid by Aspergillus. niger. Agr. Biol. Chem. 1965;29:20–26. [Google Scholar]


