Abstract
Conidia production is a problem in the study of Alternaria alternata from citrus. Thus, this study aimed to compare existing methodologies for conidial production of A. alternata isolated from Ponkan tangerine (2 isolates), Cravo lemon (1 isolate), Pêra orange (2 isolates) and Murcott tangor (1 isolate). The methodologies used were conidia production with 12 and 24 hours under white fluorescent light, evaluation with 24 and 48 hours after applying fungal mycelium stress technique, cold stress followed by injury of mycelium and evaluation with 24 hours, using healthy vegetable tissue and the use of black fluorescent near ultraviolet (NUV) lamp. Satisfactory result was obtained with A. alternata isolate from Murcott tangor, with the production of 2.8 × 105 conidia mL-1, when fungal mycelium was stressed (Petri dish with 66.66% of fungi growth) and subsequently 24 h of growth. The use of white light (24 h) and black fluorescent NUV lamp also induced expressive conidia production by one isolate of Ponkan tangerine, which produced 17.2 × 105 and 10.1 × 105conidia mL-1 and another of Murcott tangor, which produced 13.9 × 105 and 10.1 × 105 conidia mL-1, respectively. The remaining methodologies analyzed in this study were not able to induce conidia production in satisfactory quantity. The use of both mycelium stress technique and white light (24 h) and black fluorescent NUV lamp allowed the production of enough quantities of conidia to be used in vitro (detection of fungitoxic substances) and in vivo (pathogenicity test) assays, respectively.
Keywords: Alternaria brown spot, mycelium stress technique, Murcott tangor, Ponkan tangerine, black fluorescent NUV lamp
INTRODUCTION
Alternaria brown spot (ABS) is a most serious disease of many tangerines and their hybrids in humid and semiarid areas of citrus cultivation around the world (26). In Brazil, ABS was first found in Rio de Janeiro State (9) and subsequently became widespread in the major citrus area in São Paulo State (15). During the year of 2006, besides those states, this disease was found in many regions of Minas Gerais State, causing injury to Ponkan tangerine and, especially, to Murcott tangor (18). According to Peres and Timmer (16), the disease produces black necrotic lesions on young leaves, twigs, and fruit. On leaves, lesions may expand easily due to the production of a host-specific toxin by the pathogen, resulting in leaf drop and twig dieback, in most cases. On fruits, which are very susceptible to ABS, a dry up occurs and lesions vary from small dark necrotic spots to large sunken pockmarks, reducing their value for the fresh fruit market (31).
The causal agent of ABS was originally described as Alternaria citri Ellis & Pierce, but further molecular studies showed that all small-spored isolates from citrus were similar. As consequence, they were designated as A. alternata f.sp. citri (Fr:Fr) Keissl (14). Thus, one host-specific pathotype causes disease in tangerines and their hybrids, another pathotype is specific to Rough lemon and Rangpur lime (13).
Since conidia production by some A. alternata isolates in culture media requires special techniques (3,30), simple methods to do so are greatly welcome (2,16). Thus, as part of a project aimed to carry out several studies with such fungus, this work was directed towards the establishment of a new methodology to easily obtain A. alternata conidia in a culture medium.
MATERIALS AND METHODS
Fungal isolates
First, fruit peel from symptomatic Murcott tangor, Ponkan tangerine, Pêra orange and Cravo lemon as well as Pêra orange leaves showing symptoms of ABS, were washed in tap water. Then, pieces (10 – 25 mm2) of these materials were subsequently immersed in 70% ethanol (30–60 s), 2% sodium hypochlorite (30–60 s) and distilled water (2 × 30 s). Four fragments of each material were placed in a Petri dish containing 13 mL of Potato-Dextrose-Agar (PDA) (200 g cooked potato, 20 g dextrose and 20 g agar in 1 L distilled water). After seven days at 25°C, under photoperiod of 12 h (19), agar plugs (9 mm) with fungal mycelium were transferred to new PDA Petri dishes for fungus purification. The isolates obtained and used in this study are presented in Table 1.
Table 1.
Isolates of A. alternata from citrus used for the evaluation of conidia production.
| Source of isolate | Part of the plant | Isolate code |
|---|---|---|
| Cravo lemon | Fruit | B-66–01 |
| Murcott tangor | Fruit | A1–03–04 |
| Pêra orange | Fruit | B-52–09 |
| Pêra orange | Leave | A-07–01 |
| Ponkan tangerine | Fruit | B-52–01 |
| Ponkan tangerine | Fruit | B-62–04 |
Pathogenicity assay
Ripe and healthy Murcott tangor fruits, Pêra orange, Ponkan tangerine and Cravo lemon were washed with water and soap and left to dry during 60 min. Under aseptic conditions in a laminar flow chamber, four equidistant points were selected around the point of insertion of the fruit with plant. Four perforations (3 mm deep) were made with a needle on each selected point and agar plugs (6 mm) with fungal mycelium of B-66–01, B-52–09, A-07–01 and B-52–01 isolates was placed on each point in the fruit (7). Isolates A1–03–04 and B-62–04, which were able to produce conidia, were inoculated through 20 μL of an aqueous spore suspension at 106 conidia mL-1 (5). The inoculated fruits were kept in a moist chamber at 25°C, under a 12 h photoperiod. After 12 days, symptoms of ABS were verified and the pathogen was re-isolated. The frequency of plant pathogenic fungi was expressed as the percentage of fruits in which the fungus was isolated and the pathogenicity was confirmed (7). Six fruits were used per isolate and the control treatment comprised six fruits in which no fungal inoculation was applied, but only 1% Tween 80.
Conidia production with 12 and 24 hours of daylight fluorescent lamp
Agar plugs (9 mm) from purified fungus were transferred to PDA plates and after 7 days, under temperature of 25°C and photoperiod of 12 h (Philips daylight fluorescent lamp, 20W, TLT, 75RS), the conidia production was evaluated (A). In (B) the same methodology was applied, but the photoperiod was 24 h of constant light.
Evaluation with 24 and 48 hours after fungal mycelium stress technique
Agar plugs (9 mm) of purified fungal colonies were transferred to plates and after 7 days, under temperature of 25°C and photoperiod of 12 h, the mycelium stress technique was carry out by the introduction of a needle into the colonies at 4 mm deep. Briefly, needle was used to make cuts in the colonies from Petri dish with 66.66% of growth. Thus, a grid with blocks, 7 mm length, was obtained in the mycelium. The Petri dishes were incubated at conditions mentioned before and after 24 h (C) and 48 h (D), conidia production was evaluated.
Cold shock followed by injury of mycelium and evaluation after 24 hours
Fungal cultures produced as in the methodology (A), without the application of mycelium stress technique in the same plate, were grown for 7 days, at 66.66% of dish area. After, Petri dishes were transferred to refrigerator, where were kept by 24 h at 5 -8°C and without light. Immediately after, the dishes were submitted to mycelium stress technique and transferred to another incubator and after 24 h at 25°C and photoperiod of 12 h, they were evaluated regarding conidia production by using a Neubauer chamber (E).
Conidia production on citrus plant tissue
Fungal cultures submitted to methodology (A), without mycelium stress technique, were used to originate agar plugs (9 mm) with fungal mycelium. The agar plugs were transferred to new PDA plates and incubated during 5 days, sufficient for the colonization of 50% of the Petri dish area. After that, pieces from fruits and leaves of autoclaved citrus tissue (1 cm2), of the same species to which the strains was obtained were transferred to PDA plates, next to fungal growth border (F). After 4 days of fungal growth on plant tissues, part of mycelium was removed from the dishes to make microscope mounting using glycerol 50% (v/v) and conidia production was evaluated.
Conidia production by the use of black fluorescent NUV lamp
For the stimuli of conidia production, a methodology developed by Bóveda (3) was used. It was based on the fungus growth on PDA by 7 days, at 25°C and photoperiod of 12 h with daylight fluorescent lamp mixed with black fluorescent NUV lamp (Ecolume/NUV, 20W, FL, T-8/BLB) (G). In addition, the fungal colonies were submitted to mycelium stress technique after 7 days of incubation on black fluorescent NUV lamp, and after 24 h, conidia production was evaluated using a Neubauer chamber (H). The methodology (I) was identical to the (H), excepting incubation time post stress, which was 48 h. A summary of methodologies employed is shown in Table 2.
Table 2.
Methodologies employed to conidia production.
| Methodology | Summary |
|---|---|
| A | Conidia production with 12 hours of daylight fluorescent lamp. |
| B | Conidia production with 24 hours of daylight fluorescent lamp. |
| C | Evaluation with 24 hours after fungal mycelium stress technique. |
| D | Evaluation with 48 hours after fungal mycelium stress technique. |
| E | Cold shock followed by injury of mycelium and evaluation after 24 hours. |
| F | Conidia production on citrus plant tissue. |
| G | Use of black fluorescent NUV lamp during 7 days. |
| H | Use of black fluorescent NUV lamp, followed by mycelium stress and evaluation after 24 hours. |
| I | Use of black fluorescent NUV lamp, followed by mycelium stress and evaluation after 48 hours. |
Evaluation of conidia production on Neubauer chamber
In all methodologies, excepting (F), 10 mL of 1% Tween 80 (g mL-1) autoclaved solution were added and dispersed with glass rod into Petri dishes followed by filtration. The number of conidia was determined with a Neubauer chamber in a light microscope (2). All tests were conducted with 4 replicates.
Light microscopy (LM)
Part of mycelium was removed from dishes to make microscope slides mountings using glycerol 50% (v/v) and the morphology of the fungus was observed using a Leica DME light microscope.
Statistical analysis
Analysis of variance was applied for the data obtained in all methodologies, without any transformation. The Scott-Knott (22) test (P ≤ 0.05) was applied.
RESULTS
Symptoms of Alternaria brown spot were observed in all inoculated fruits (Fig. 1), but not on fruits inoculated only with 1% Tween 80.
Figure 1.
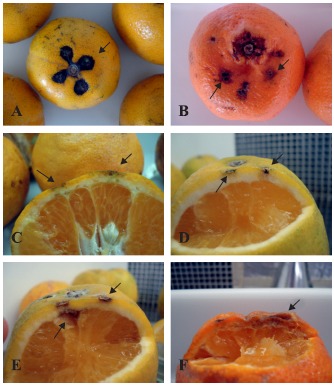
(A – B) Murcott tangor and Ponkan tangerine fruits inoculated with conidia, and (C – F) Ponkan tangerine, Pêra orange and Cravo lemon fruits inoculated with mycelium of Alternaria alternata, evaluated 12 days post-inoculation. (A) Murcott tangor (A1–03–04); (B) Ponkan tangerine (B-62–04). (C) Ponkan tangerine (B-52–01); (D) Pêra orange (B-52–09); (E) Pêra orange (A-07–01); (F) Cravo lemon (B-66–01).
Among the 6 isolates studied, Murcott tangor (A1–03–04) and Ponkan tangerine fruits (B-62–04) produced considerable amounts of conidia when the mycelium stress technique, fluorescent light white lamp (24 h) and black fluorescent NUV lamp were used (Table 3). Also, some conidia production was obtained with the use of thermal shock. Quantity of conidia considered enough to carry out fungi toxic substances identification was obtained with the isolate obtained from Murcott tangor (A1–03–04) under mycelium stress technique conditions, which produced 2.8 – 3.0 × 105 conidia mL-1.
Table 3.
Average number of Alternaria alternata conidia mL-1* and coefficient of variability (CV) after the application of the methodologies (A), (B), (C), (D), (E), (F), (G), (H) and (I) to the stimulation of in vitro conidia production.
| Methodology | Isolate | ||||||
| Cravo lemon fruits | Murcott tangor fruits | Pêra orange fruits | Pêra orange leaves | Ponkan tangerine (B-52–01) | Ponkan tangerine (B-62–04) | CV | |
| A | - | - | - | - | - | - | - |
| B | - | 13.9 × 105 cA | - | - | - | 17.2 × 105 dA | 13.93% |
| C | - | 2.8 × 105 aA | - | - | - | 4.2 × 105 bB | 13.40% |
| D | - | 3.0 × 105 aA | - | - | - | 5.4 × 105 bB | 8.86% |
| E | - | 1.4 × 105 aA | - | - | - | 1.4 × 105 aA | 13.21% |
| F | - | - | - | - | - | - | - |
| G | - | 10.6 × 105 bA | - | - | - | 10.1 × 105 cA | 11.63% |
| H | - | 10.7 × 105 bA | - | - | - | 10.1 × 105 cA | 16.52% |
| I | - | 10.0 × 105 bA | - | - | - | 9.6 × 105 cA | 4.98% |
| CV | - | 17.61% | - | - | - | 12.24% | - |
Means of four replications (4 Petri dishes/treatment, and to each Petri dish, one counting with 8 replications using the Neubauer chamber) with the same small and capital letter in a column and line do not differ significantly (P ≤ 0.05) according to the Scott-Knott (22) calculations, respectively.
The length and width of A. alternata conidia from Murcott tangor (A1–03–04) and Ponkan tangerine (B-62–04) fruits were measured (Table 4). There were few differences in the size of conidia proceeding from both the host tissue and in the methodology (C).
Table 4.
Length and width, beak length, and total length of Alternaria alternata conidia from Murcott tangor and Ponkan tangerine (B-62–04) fruits, after the use of methodology (C), in the in vitro conidia production.
| Variable studied* | Isolated | |
|---|---|---|
| Murcott tangor (A1–03–04) | Ponkan tangerine (B-62–04) | |
| Length (μm) | ||
| Variation | 22.5 – 35.0 | 17.5 – 32.5 |
| Average | 28.2 | 22.8 |
| CV | 3.7% | 3.0% |
| Width (μm) | ||
| Variation | 5.0 – 10.0 | 5.0 – 12.5 |
| Average | 7.8 | 8.0 |
| CV | 1.6% | 1.8% |
| Beak (μm) | ||
| Variation | 2.5 – 7.5 | 2.5 – 9.5 |
| Average | 5.1 | 4.4 |
| CV | 1.7% | 2.0% |
| Total length (μm) | ||
| Variation | 22.5 – 40.0 | 20.0 – 35.0 |
| Average | 31.8 | 25.4 |
| CV | 5.1% | 4.0% |
| Conidia with beak | 70% | 66% |
The key structures of the fungus were measured thirty times; CV: coefficient of variability.
DISCUSSION
The absence of conidia in methodology (A) was not surprising, given that a notable conidia production of A. alternata is difficult without interference of physic factors (3) or an addition of specific synthetic components at culture medium (24). Concerning methodology (F), the literature shows that to some fungi, such as Colletotrichum lindemuthianum and Fusarium spp., the conidia and macroconidia were obtained with insertion of bean pod and carnation leaves into culture medium, respectively (6,10). Similarly, in a specific case, Vakalounakis (29) obtained a high number of Alternaria solani Sorauer conidia through the use of mycelial agar plugs removed from the border of fungal colonies 4 day old and put on Solanaceae leaves. To test this methodology, agar plugs were removed from the border of 4–5 day old colonies, but conidia production was not observed. Also, Timmer et al., (27) observed that Alternaria sp. sporulation was not observed on killed tissues.
The isolates from Cravo lemon (B-66–01), Pêra orange fruits (B-52–09) and leaves (A-07–01) and Ponkan tangerine (B-52–01) did not produce any conidia (Table 3). A possible explanation for these results could be that these isolates are not able to produce conidia in nature (1). The virulence level and growth conditions of the isolate can be related with conidia production, because they affect the potential inoculum of A. alternata (12). These authors obtained the most virulent conidia on PDA medium at 28°C under constant black fluorescent NUV lamp for 4 weeks.
Shahin and Shepard (23) and Teixeira et al., (25) observed that black fluorescent NUV lamp is necessary for growth and sporulation of Alternaria spp. Furthermore, Ungaro (28) classified A. alternata sporulation as small (less than 104 conidia mL-1), medium (104 – 106 conidia mL-1) and abundant (more than 106 conidia mL-1). The methodologies applied to conidia production to be used in Murcott tangor (A1–03–04) and Ponkan tangerine (B-62–04) isolates, including (C), (D) and (E), can be considered as producing a medium quantity of conidia, except (B), (G), (H) and (I), that produced an abundant quantity (Table 3). Thus, all methodologies that resulted in medium and abundant conidia production were considered to be satisfactory, but (C) and (D) produced the ideal quantity of conidia necessary for in vitro assays on ELISA plates, which need 2.6 – 3.0 × 105 conidia mL-1 (21). On the other hand, according with Colturato (5), the methodologies (B), (G), (H) and (I) produced a conidia number adequate for in vivo tests, which need 106 conidia mL-1, such as pathogenicity assay.
It is also worth mentioning that, although without any conidia production, the isolates B-66–01 (Cravo lemon), B-52–09 and A-07–01 (Pêra orange) and B-52–01 (Ponkan tangerine) showed clear symptoms in the pathogenicity assay (Fig. 1). A possible explanation for these results could rely on the fact that in the fields, lesions on fruit appear to require more time to produce conidia than in the leaves (20). Furthermore, Whiteside (32) noted that, in field, sporulation was more abundant on leaves than on fruit. Even without any conidia production, symptoms on fruit can be verified. Low sporulation was observed by Reis and Goes (20), 60 days after lesion development, and lesions may be able to produce a few conidia that would serve as primary inoculum in the spring. Also, these authors verified that the sporulation only began about 10 days after symptom development and continued for about the next 40 days, but about 60 days after symptom appearance, conidia were no longer produced (20).
Surprisingly, the methodology (E) of this work showed that cold shock seems not to be effective in the sporulation. Such behavior is not in accordance with the work of Prasad et al., (17), wherein Alternaria solani showed sporulation under photoperiod of 12 hours and thermal shock at 4°C. However the cultures of Prasad were dipped in cold water (4°C) for 4 min only. In opposition, the objective of thermal shock in this work was to expose the colonies at minimal temperature for longer time and to evaluate the sporulation after subsequently stress of the colonies. Another question relies on temperature, whereas Colturato (5) revealed that the best temperature for A alternata from Murcott tangor to conidia production was 28°C, and not 25°C. Nevertheless high conidia number was obtained at 25°C in this work (Table 3). This author observed that, at 25°C, there is a larger mycelial growth. Although Lukens and Horsfall (11) have reported that the mycelium injury was not benefic to Alternaria spp. conidiophores production, this study showed that the introduction of a needle into PDA culture medium at 4 mm deep aiming to cut the fungal mycelium, was advantageous for conidia production. Today there is not enough information about studies for in vitro conidia production. Thus, Charlton (4) observed that A solani fungal colonies, when not injured, were difficult to remove conidia.
According to the isolate, the methodology applied can be better adjusted to conidia production. An example was the methodologies (C) and (D), which showed higher conidial production to Ponkan tangerine (B-62–04) than Murcott tangor (A1–03–04) (Table 3). This behavior is observed in methodologies that are able to produce lower quantity of conidia.
Regarding length and width of conidia (Table 4), there are not differences among the dimensions of conidia from A. alternata coming from different hosts. It is a confirmation that the isolates are from same species, A. alternata (13). The morphology of conidia, produced under in vitro conditions, is in accordance with Ellis (8), who found out that the length was 20 – 63 μm and the width was 9 – 18 μm for A. alternata.
With the improvements of methodologies for conidia production in A alternata obtained in the present study, it is now possible to obtain conidia at suitable quantity to several purposes, such as to identify fungitoxic substances on ELISA plates and Petri dishes, without using cold shock, black fluorescent NUV lamp and addition of vegetable tissue on the culture medium to stimulate conidia production. Also, these methodologies can serve as alternatives to induce the sporulation of other Alternaria spp. and fungi.
ACKNOWLEDGEMENTS
The authors thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for a master fellowship and the Doctor Cristiano Souza Lima for revision and suggestion to this paper.
RESUMO
Comparaçâo de metodologias para produçâo de conídios por Alternaria alternata do citros
A produção de conídios consiste em problema no estudo de Alternaria alternata do citros. Assim, este estudo objetivou comparar metodologias existentes para a produção de conídios de A. alternata por dois isolados de tangerina Ponkan, um de limão Cravo, dois de laranja Pêra e um de tangor Murcott. As metodologias empregadas foram a produção de conídios com 12 e 24 horas sob luz branca, avaliação com 24 e 48 horas após estressamento do micélio do fungo, choque térmico com imediato estressamento do micélio e avaliação com 24 horas, produção de conídios pelo emprego de tecido vegetal sadio e o emprego de luz negra ultravioleta. Produção satisfatória de conídios foi obtida com o isolado de A. alternata de tangor Murcott, a qual foi de 2,8 × 105 conídios mL-1, mediante emprego da técnica de estressamento da colônia e cultivo do fungo por 24 horas. Os empregos de luz branca (24 h) e negra ultravioleta promoveram expressiva produção de conídios por um isolado de tangerina Ponkan, a qual foi de 17,2 × 105 e 10,1 × 105 conidios mL-1 e por outro de tangor Murcott, a qual foi de 13,9 × 105 e 10,1 × 105 conídios mL-1, respectivamente. As outras metodologias analisadas neste estudo não foram capazes de induzir a produção de conídios em quantidade satisfatória. Com o emprego das técnicas de estressamento do micélio e a utilização de luz branca (24 h) e negra ultravioleta, tornou-se possível obter quantidades de conídios suficientes para serem utilizadas em testes in vitro (detecção de substâncias fungitóxicas) e in vivo (testes de patogenicidade), respectivamente.
Palavras chave: Mancha marrom de alternaria, estressamento do micélio, tangor Murcott, tangerina Ponkan, luz negra ultravioleta.
REFERENCES
- 1.Babu R.M., Sajeena A., Seetharaman K. Solid substrate for production of Alternaria alternata conidia: a potential mycoherbicide for the control of Eichhornia crassipes (water hyacinth) Weed Res. 2004;44(4):298–304. [Google Scholar]
- 2.Balbi-Peña M.I., Becker A., Stangarlin J.R., Franzener G., Lopes M.C., Schwan-Estrada K.R.F. Controle de Alternaria solani em tomateiro por extratos de Curcuma longa e Curcumina – I. Avaliação in vitro. Fitopatol. Bras. 2006;31(3):310–314. [Google Scholar]
- 3.Bóveda R.R.M. Piracicaba: Dissertação de Mestrado – Escola Superior de Agricultura Luiz de Queiroz; 1986. Morfologia, patogenicidade, esporulação e sensibilidade a fungicidas in vitro de Alternaria solani (Ell. e Mart.) Jones e Grout e Alternaria alternata (Fr.) Keissler de solanáceas. 106 p. [Google Scholar]
- 4.Charlton K.M. The sporulation of Alternaria solani in culture. Transactions British Mycol. Society. 1953;36:349–355. [Google Scholar]
- 5.Colturato A.B. Botucatu: Dissertação de Mestrado – Faculdade de Ciências Agronômicas da Universidade Estadual Paulista; 2006. Efeito do meio de cultura, temperatura, fotoperíodo e fungicidas no crescimento micelial e no controle de Alternaria alternata f. sp. citri, causador da mancha marrom do tangor Murcote. 53 p. [Google Scholar]
- 6.Dalla Pria M., Amorim L., Bergamin Filho A. Quantificação dos componentes monocíclicos da antracnose do feijoeiro. Fitopatol. Bras. 2003;28(4):401–407. [Google Scholar]
- 7.Dantas S.A.F., Oliveira S.M.A., Michereff S.J., Nascimento L.C., Gurgel L.M.S., Pessoa W.R.L.S. Doenças Fúngicas Pós-Colheita em Mamões e Laranjas Comercializados na Central de Abastecimento do Recife. Fitopatol. Bras. 2003;28(5):528–533. [Google Scholar]
- 8.Ellis M.B. Dematiaceous Hyphomycetes. Oxon: CAB international; 1993. p. 608. [Google Scholar]
- 9.Goes A., Montes de Oca A.G., Reis R.F. Ocurrencia de la mancha de Alternaria en mandarina Dancy en el estado de Rio de Janeiro. Fitopatol. Bras. 2001;26:386. [Google Scholar]
- 10.Leslie J.F., Summerell B.A. The Fusarium Laboratory Manual. Ames (Iowa, USA): Blackwell Publishing Asia; 2006. p. 388. [Google Scholar]
- 11.Lukens R.J., Horsfall J.G. Process of sporulation in Alternaria solani and their response to inhibitors. Phytopathol. 1973;63:176–182. [Google Scholar]
- 12.Masangkay R.F., Paulitz T.C., Hallett S.G., Watson A.K. Characterization of sporulation of Alternaria alternata f. sp sphenocleae. Biocontrol Sci. Technol. 2000;10(4):385–397. [Google Scholar]
- 13.Peever T.L., Carpenter-Boggs L., Timmer L.W., Carris L.M., Bhatia A. Citrus black rot is caused by phylogenetically distinct lineages of Alternaria alternata. Phytopathol. 2005;95:512–518. doi: 10.1094/PHYTO-95-0512. [DOI] [PubMed] [Google Scholar]
- 14.Peever T.L., Su G., Carpenter-Boggs L., Timmer L.W. Molecular systematics of citrus-associated Alternaria spp. Mycologia. 2004;96:119–134. [PubMed] [Google Scholar]
- 15.Peres N.A.R., Agostini J.P., Timmer L.W. Outbreaks of Alternaria brown spot of citrus in Brazil and Argentina. Plant Disease. 2003;87(8):750. doi: 10.1094/PDIS.2003.87.6.750C. [DOI] [PubMed] [Google Scholar]
- 16.Peres N.A., Timmer L.W. Evaluation of the Alter-Rater model for spray timing for control of Alternaria brown spot on Murcott tangor in Brazil. Crop Protec. 2006;25:454–460. [Google Scholar]
- 17.Prasad B., Dutt B.L., Nagaich B.B. Inducing sporulation in Alternaria solani I. Effect of water treatment. Mycopathol. Mycol. Appl. 1973;49:141–146. doi: 10.1007/BF02050856. [DOI] [PubMed] [Google Scholar]
- 18.Prates H.S. Mancha de alternaria das tangerinas. 2007. [Accessed 27 Feb]. Available at: http://www.cati.sp.gov.br/novacati/tecnologias/doencas_de_plantas/alternaria/mancha_alternaria.htm. [Google Scholar]
- 19.Prusky D., Kobiler I., Akerman M., Miyara I. Effect of acidic solutions and acidic prochloraz on the control of postharvest decay caused by Alternaria alternata in mango and persimmon fruit. Postharvest Biol. Technol. 2006;42:134–141. [Google Scholar]
- 20.Reis R.F., Goes A. Effect of lesion age, humidity, and fungicide application on sporulation of Alternaria alternata, the cause of brown spot of tangerine. Plant Disease. 2005;90(8):1051–1054. doi: 10.1094/PD-90-1051. [DOI] [PubMed] [Google Scholar]
- 21.Saks Y., Barkai-Golan R. Aloe Vera gel activity against plant pathogenic fungi. Postharvest Biol. Technol. 1995;6:159–165. [Google Scholar]
- 22.Scott A.J., Knott M.A. A cluster analyses method for grouping means in the analyses of variance. Biometrics. 1974;30(3):502–512. [Google Scholar]
- 23.Shahin E.A., Sheppard J.F. An efficient technique for inducing profuse sporulation of Alternaria species. Phytopathol. 1979;69:618–620. [Google Scholar]
- 24.Silva C.M.M.S., Melo I.S. Requisitos nutricionais para o fungo Alternaria alternata. Pesq. Agropec. Bras. 1999;34:499–503. [Google Scholar]
- 25.Teixeira H., Arias S.M.S., Chitarra L.G., Machado J.C. Eficiência comparativa de lâmpadas fluorescentes na detecção e quantificação de fungos em sementes. Ciênc. Agrotec. 2002;26(2):259–268. [Google Scholar]
- 26.Timmer L.W., Peever T.L., Solel Z., Akimitsu K. Alternaria diseases of citrus-novel pathosystems. Phytopathol. Mediterr. 2003;42:99–112. [Google Scholar]
- 27.Timmer L.W., Solel Z., Gottwald T.R., Ibanez A.M., Zitko S.E. Environmental factors affecting production, release, and field populations of conidia of Alternaria alternata, the cause of brown spot of citrus. Phytopathol. 1998;88(11):1218–1223. doi: 10.1094/PHYTO.1998.88.11.1218. [DOI] [PubMed] [Google Scholar]
- 28.Ungaro M.R.G. Sensibilidade de Alternaria alternata (Fr.) Keissler a três fungicidas 67 p. Piracicaba: Dissertação de Mestrado – Escola Superior de Agricultura Luiz de Queiroz; 1981. [Google Scholar]
- 29.Vakalounakis D.J. An efficient and simple technique for inducing profuse sporulation in Alternaria solani. Phytopathol. Mediterr. 1982;21(43):89–90. [Google Scholar]
- 30.Verzignassi J.R., Vida J.B., Homechin M. Ocorrência e transmissão de Alternaria steviae e A. alternata em sementes de Stevia rebaudiana (bert.) Bertoni. Rev. Bras. Sementes. 1997;19(2):283–287. [Google Scholar]
- 31.Vicent A., Badal J., Asensi M.J., Sanz N., Armengol J., Garc´ia-Jim´enez J. Laboratory evaluation of citrus cultivars susceptibility and influence of fruit size on Fortune mandarin to infection by Alternaria alternata pv. citri. Eur. J. Plant Pathol. 2004;110:245–251. [Google Scholar]
- 32.Whiteside J.O. Alternaria brown spot of mandarins. In: Whiteside J.O., Garnsey S.M., Timmer L.M., editors. Compendium of citrus diseases. Saint Paul: American Phytopatological Society; 1998. p. 8. [Google Scholar]


