Abstract
A cadmium (8 mM) resistant Pseudomonas aeruginosa strain KUCd1 exhibiting high Cd accumulation under in vitro aerobic condition has been reported. The isolate showed a significant ability to remove more than 75% and 89% of the soluble cadmium during the active growth phase from the growth medium and from Cd-amended industrial wastewater under growth supportive condition. Transmission electron microscopy (TEM) and energy dispersive X-ray spectroscopy (EDXS) suggest the presence of Cd in the cells from mid stationary phase. The cell fractionation study revealed membrane and periplasm to be the major accumulating site in this strain. The chemical nature of the accumulated Cd was studied by X-ray powder diffraction analysis.
Keywords: Pseudomonas aeruginosa, Cadmium, Bioremediation
INTRODUCTION
Among the metals or metalloids arsenic, cadmium, mercury, lead and chromium have been known to be extremely toxic at low concentration (29) although they have no significant biological function so far reported. Cadmium causes reduced growth rate, long lag phase, lower cell density and may even cause death of bacteria at levels below 1 ppm (2,14,15,19,24). Bacteria detoxify heavy metals in a variety of different ways. Some bacteria employ plasmid mediated efflux system that energetically drives out Cd, Zn and many other toxic metal ions (9,18,26). Whereas many bacteria adsorb heavy metals to their cell wall (7,8,17,23,32) or bind metal ions to proteins like metallothioneins, metal-peptide etc (10). Microbes often do precipitate heavy metals in the form of insoluble phosphate, sulfide or others to reduce their bioavailability (2,3,12,28).
In the present perspective of metal pollution research, the urgency lies in studying metal resistance mechanisms in microbes, which might shed light to develop a potential candidate for bioremediation (6,31). Here we report a Cd resistant Pseudomonas aeruginosa KUCd1, which could effectively accumulate Cd rendering the strain as a possible effective candidate for reclamation of environment. The cellular localization of accumulated cadmium and its chemical nature were analyzed to find out the possible mechanism of Cd detoxification in this test strain.
MATERIALS AND METHODS
Bacterial strain
The isolation and characterization of the strain Pseudomonas aeruginosa KUCd1, used in this study has previously been done (27) by biochemical tests and 16S rDNA sequence (GenBank Accession No. EF 644569 at NCBI database) analysis. This gram-negative rod-shaped motile bacterial strain was found to remarkably resistant to other heavy metals as well. The order of toxicity of the metals to the bacterium on both solidified and liquid medium was found to be Cr>Co>Cu>Cd>Ni>Zn>Mn. It has also showed cadmium-induced siderophore production (27). The culture is being maintained in nutrient agar media (pH 7) supplemented with 2 mM cadmium as CdCl2.
Cadmium tolerance ability of KUCd1
The Cd tolerance ability of the bacterial isolate was tested with increasing concentration of Cd (1 to 10 mM) in undefined nutrient agar medium and compared that in the defined Tris minimal medium (21) supplemented with 0.8% glucose (TMMG).
Cadmium accumulation and removal assay
Twelve hours grown cell suspension from TMMG (~ 4 log number of CFU.ml-1) was inoculated to same medium having 3 mM of Cd and incubated on a shaking incubator at 37°. Cells were harvested at 24, 36, 48, 72 and 96 h of incubation by centrifugation (11000 x g for 10 minutes at 4°). Because of less cell mass production by the strain at the maximum tolerance level of Cd (8 mM), in this experiment 3 mM Cd was used to have substantial biomass yield. The pH change in the medium was also monitored in both the Cd-added and Cd-free sets. The metal content in the medium supernatants were analyzed using an atomic absorption spectrophotometer (Perkin Elmer AAnalyst 400, USA).
Percentages of Cd removal by the cell mass from the culture were calculated by taking difference between the initial metal content in the culture media and that in the time of sampling. Cell-free sets were maintained for each treatment regime to determine the artifacts might arise due to the metal sorption on the glass surface of the culture container.
Survival of the strain in industrial wastewater
Industrial wastewater that contained little Cd (0.01 mM) and nutrients was collected locally, pH was adjusted to 7.2, sterilized and Cd was added as sterilized CdCl2 solution to it for having final concentration of 0.6 mM and 0.4 mM of Cd separately in two different conical flasks. Lower concentrations of Cd were applied in this experiment to get a substantial bacterial cell mass under nutrient limited condition. One ml of 12 h grown cell suspension (~ 4 log number of CFU.ml-1) culture grown in TMMG was added to the experimental sets containing 50 ml wastewater with 0.6 mM and 0.4 mM Cd, and to a control set without added Cd. The three experimental sets were then kept at 30° on an incubator shaker at 110 rpm. At every 24 h of interval, sample was drawn to count colony forming units (CFU) by dilution plating on nutrient agar plates having 3 mM of Cd.
Effect of nutrient supplementation on growth in wastewater and Cd removal
Sterilized industrial wastewater (pH 7.2) was supplemented with analytical grade of NH4Cl (300 mg.L-1), Glucose (0.5 g.L-1) and KH2PO4 (200 mg.L-1) as N, C and P sources respectively to provide sufficient conditions for growth. One ml of 12 h grown cell suspension (~ 4 log number of CFU.ml-1) in TMMG was added to the 250 ml conical flask containing 50 ml of wastewater with Cd (0.6 mM) or without added Cd. The flasks were kept at 30° on shaking incubator for 96 h. The samples were drawn at 24 h interval to determine the viable cell numbers for each set by dilution plate technique on a nutrient agar medium having 3 mM Cd. Culture suspension was withdrawn from each set at 24 h intervals and centrifuged at 11000 x g for 15 min. The Cd content in the supernatant was analyzed and control sets were maintained as described earlier. Cd removal (%) was calculated by taking difference of the metal content of the cultures at time zero and at the time of sampling.
TEM and EDXS study
Bacterial cells grown for 48 h in Cd-added (3 mM) TMMG medium were harvested by centrifugation and were repetitively washed with phosphate buffer saline (pH 7.2). The cells were fixed by treating with equal volume of 3% glutaraldehyde in the same buffer solution for 1 h, post-fixed with 1% osmium tetroxide (31). The cells were then gradually dehydrated in ethanol and finally embedded in SPURR’s resin. Thin sections were cut with a Dupont diamond knife in an LKB Ultramicrotome, and were loaded in formvar carbon-coated grid and observed in TEM (JEOL 100 CX, Japan) at varied magnifications. EDXS analysis of the sample was done with EX-64165 JMU, JEOL (Japan).
X-ray powder diffraction study
The X-ray diffraction pattern of the Cd treated (3 mM) or untreated lyophilized cells after 48 h of growth of the test strain were recorded in a Seifert 3000P Powder Diffractometer using monochromatic Cu Kα1 radiation (λ = 1.540598 Å) The diffraction spectra were recorded in a range between 10° to 90° (2θ) with a step length of 0.02° (2θ). To identify the cadmium salt, the experimental X-ray diffraction pattern was compared with powder diffraction standards.
Distribution pattern of accumulated Cd in the sub-cellular fractions
For localizing the site of intracellular Cd accumulation in the bacterial strain, Cd (3 mM) exposed cells after 48 h of growth were fractionated into cellular, total membrane and periplasmic parts (11). Forty milliliter each of Tris-HCl buffer (0.2 M, pH 7.1) and sucrose (40%, w/v) were used to suspend metal loaded cell pellets and kept for equilibration (30 min). Then the cells were centrifuged and quickly suspended in 80 ml MgCl2 (0.5 mM) in an ice bath to release the periplasmic fraction. The post-shock cells were disrupted by sonication and the homogenate was centrifuged (90,000 g for 1 h) to separate total membrane components and the cytosol. Amount of Cd retained in the sub-cellular fractions was quantified as described before following acid digestion.
Sulfide production test
The bacterial strain was inoculated to Sulfide Indole Motility (SIM) medium (30 mg.ml-1 peptone, 3 mg.ml-1 beef extract, 0.2 mg.ml-1 ferrous ammonium sulfate, 0.025 mg.ml-1 sodium thiosulfate) agar tube and incubated at 30° for observing the black color along the line of stab inoculation.
Protein quantification
Total cellular protein or in the sub-cellular fractions was estimated by a standardized method (5) using bovine serum albumin as standard.
RESULTS AND DISCUSSION
The strain could tolerate as high as 8 mM of Cd in the nutrient agar medium at pH 7.2. However, the strain was found to tolerate maximum of 4 mM Cd concentration in defined TMMG medium (data not shown). The discrepancy in Cd resistance showed by the strain in the two different media may be explained by the fact that complexation of Cd with complex ingredients in the undefined NA medium might have lowered Cd availability around and therefore its toxicity (20). Nevertheless, the level of Cd tolerance of this strain under aerobic conditions is significant in comparison to that of the earlier reported pseudomonad strains (30,31).
Bioaccumulation of Cd by bacteria have been studied by many workers (4,8), among those Pseudomonas aeruginosa CW-96-1 (31) has shown to remove Cd most efficiently from medium having 1 mM Cd, however, the strain used in this study could remove significantly from medium having 3 mM Cd under aerobic condition. Maximum Cd removal by KUCd1 strain occurred after the mid stationary phase. Since growth phase is a biotic variable that can affect metal uptake by a bacterial population, the metal removal capacity was investigated during the different growth phases of the test strain. The pH of the medium was found to be progressively alkalized in both the Cd added and control medium. However, in the Cd added medium the pH change observed to be more (pH 7 to pH 8.5) than the Cd-free set (pH 7 to pH 7.5) after 96 hours of growth. It seems increase in pH is likely to be linked with transformation of Cd to an insoluble species within the cell or around, a phenomenon observed in other bacterial strain as well (4). For better understanding, the contribution of physiological and intracellular oxidation states (1) of the cell on Cd detoxification warrants further investigation. More than 75% soluble cadmium was removed from the culture medium after 96 hours i.e. during stationary phase (Fig. 1). Cell mass dependency on Cd removal was found during active growth phase, however, steady removal of Cd in the stationary phase was found to be independent of viable cell mass increment which might be due to the physical adsorption significantly by the dead cell mass as interpreted earlier (13). In cell free controls abiotic precipitation or complexation was taken into consideration and found insignificant.
Figure 1.
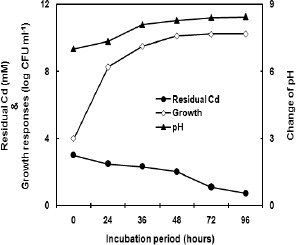
Variation of pH and Cd content in the medium along incubation period in response to the growth of KUCd1 under aerobic condition in TMMG medium having 3 mM of Cd.
Effective bioremediation of heavy metals from the polluted site depends on the survival of the organisms and availability of nutrients under such conditions. The bacterial strain was subjected to grow in the industrial wastewater added with Cd. The data (Fig. 2) showed that the viable cell number decreased comparably in the entire test regimes with the progress of time. Such declining in survival ability might be due to the nutritional scarcity. Survival efficiency and consequently cell mass of the bacterial strain were increased significantly in the wastewater when additional nutrients were supplied (Fig. 2). Accordingly, Cd removal capacity of the strain from wastewater having 0.6 mM Cd was evaluated in nutrient supplemented condition and without adding nutrient. Considering level of Cd content in the industrial wastewater, and to maintain the survival of microflora and also to ensure adequate contact between the biomass and the pollutant metals, in this experiment 0.6 mM of Cd was used to evaluate Cd removal ability of this strain. It was observed (Fig. 3) that under nutrient sufficient condition the bacterial strain removed almost 89% of the Cd from the wastewater after 96 hours. But when the strain was grown in nutrient deficient condition it removed less than 20 % of Cd from the wastewater. It might be due to the less cell mass production, both viable and dead cell mass, under nutrient depleted condition.
Figure 2.
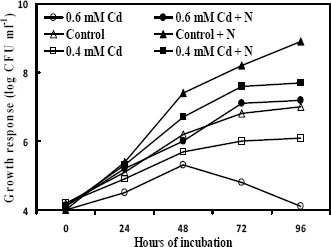
Survival of KUCd1 in the industrial wastewater having 0.6 mM or 0.4 mM Cd with (+N) or without supplemented extra nutrient. Controls are without added Cd. Colony forming units (CFU) were enumerated on nutrient agar supplemented with 3 mM of Cd.
Figure 3.
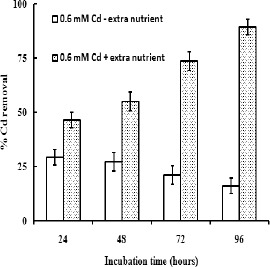
Cadmium removal by KUCd1 from wastewater with or without added nutrient with the function of time (hour of incubation). Data are the mean of five replications with standard error bars.
To localize the intracellular site of accumulated Cd, cells of KUCd1 were observed under TEM. The transmission electron micrograph (Fig. 4) showed electron dense grains in the cytosol and towards the cell envelope. For further analysis of the electron dense bodies, EDXS study was performed. The data showed the peak for Cd in the treated cells (Fig. 5). The strain could produce sulfide when grown on SIM agar tube as indicated by black coloration along the line of stab inoculation. This production of sulfide might confer Cd resistance in this strain for its survival under Cd stress and it detoxifies Cd by converting it into insoluble CdS as reported earlier (25, 30, 31). So biochemical and EDXS data predict that the test strain might convert cadmium into CdS under Cd stress condition. The powdered X-ray diffraction data of the Cd loaded cells revealed the chemical nature of the accumulated Cd within the cell (Fig. 6). The broadened nature of the peaks in the diffractogram indicates the existence of amorphous phase(s) in the sample. However, from the intensity distribution pattern of the diffractogram, existence of a peak at 2θ = 31.54 can readily be understood. Based on the d value, the peak at 2θ = 31.54 might be assigned as CdS. In the Cd untreated set there was no such conspicuous peak for CdS. This observation supports the earlier findings in the present study that the strain tolerated such high level of Cd by depositing insoluble CdS. However, the processes of physical adsorption or complexation of Cd by the dead cell mass could not be eliminated in this strain because of higher amount of Cd removal during stationary phase and warrant further investigation.
Figure 5.
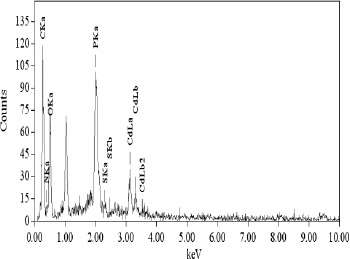
Energy dispersive X-ray spectra of Pseudomonas aeruginosa KUCd1 cells grown in TMMG medium with Cd (3 mM) for 48 h showing intracellular presence of cadmium.
Figure 6.
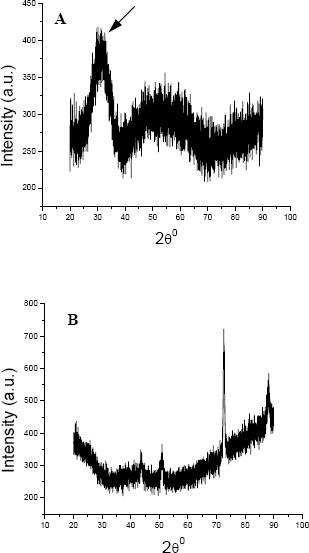
X-ray diffraction spectra of dried powdered Pseudomonas aeruginosa KUCd1 cells grown for 48 h in TMMG medium with 3 mM Cd (A) or Cd-free control (B). Arrow shows the peak of CdS.
Figure 4.
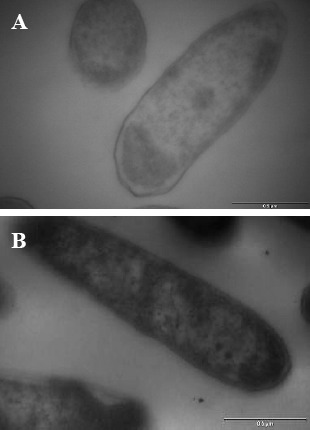
TEM analysis of the bacterial cells grown for 48 h in TMMG medium without (A) or with 3 mM of Cd (B) showing electron dense grains distributed within the cell (Bar = 0.5 μm).
The distribution of Cd in the cell was further investigated through sub-cellular fractionation coupled to its quantitative estimation by atomic absorption spectroscopy. The result showed that a major fraction (84.43%) of total intracellularly accumulated Cd was found to be associated with the envelope with a maximum (55.98%) by the periplasm followed by membrane component (27.69%). The cytoplasm accounted for very low Cd deposition (15.57%) of the total uptake (Table 1). This observation is supportive of earlier findings on membrane-associated accumulation of Ni in Ps. aeruginosa (22) and of Cd in E. coli (16), where the membrane retained more than 50% of the total accumulated metal. Nevertheless, the strain exhibits diversified strategies to detoxify Cd, it might be both due to cytosolic or periplasmic acquisition and physical adsorption on the surface by dead or live cells.
Table 1.
Intracellular distribution of accumulated cadmium in KUCd1 grown for 48 h in TMMG medium supplemented with 3 mM Cd as CdCl2.
| Cell fraction | µg Cd per mg proteina |
|---|---|
| Total cell | 5.020 (± 0.860) |
| Periplasm | 2.810 (± 0.263) |
| Total membrane | 1.390 (± 0.680) |
| Cytoplasm | 0.782 (± 0.026) |
data are the mean of 5 sets with standard error values.
This strain shows promise for successful microbiological detoxification of Cd for both in situ or ex situ bioremediation of Cd-contaminated sites having appropriate growth sufficient conditions. The strain showed its possibility as well to be applied in the Cd contaminated agricultural land as a good bioremediation agent (27), although the extent of Cd removal under actual metal-polluted site conditions warrants further study.
ACKNOWLEDGEMENTS
The work was supported by the grant received from the University of Kalyani. The authors also acknowledge the help received from Indian Institute of Chemical Biology, Kolkata, India for TEM and EDXS studies; and Indian Association for Cultivation of Science, Kolkata, India, for X-ray diffraction study.
RESUMO
Pseudomonas aeruginosa KUCd1, um possível candidato para biorremediação de cádmio
Descreve-se a cepa Pseudomonas aeruginosa KUCd1 resistente a cádmio (8mM) que apresenta elevado acumulo de cádmio em condições de aerobiose in vitro. Durante a fase ativa de multiplicação, a cepa apresentou capacidade de remover mais de 75% e 89% de cádmio solúvel do meio de cultura e da água residual industrial contendo Cd. A microscopia eletrônica de transmissão (TEM) e espectroscopia de raio X-energia dispersiva indicaram a presença de Cd nas células na fase estacionária intermediária. O fracionamento celular indicou serem a membrana e o periplasma os pontos de maior acúmulo. A natureza química do Cd acumulado foi avaliada através de análise de difração de Raios X.
Palavras-chave: Pseudomonas aeruginosa, cádmio, biorremediação
REFERENCES
- 1.Adamis Paula D.B., Panek Anita D., Leite Selma G.F., Eleutherio Elis C.A. Factors involved with cadmium absorption by a wild-type strain of Saccharomyces cerevisiae. Braz. J. Microbiol. 2003;34(1):55–60. [Google Scholar]
- 2.Aiking H., Kok K., Heerikhuizen H.V., Riet J.V.T. Adaptation to cadmium by Klebsiella aerogenes growing in continuous culture proceeds mainly via formation of cadmium sulfide. Appl. Environ. Microbiol. 1982;44:938–944. doi: 10.1128/aem.44.4.938-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiking H., Stijnman A., Garderen C.V., Heerikhuizen H.V., Riet J.V.T. Inorganic phosphate accumulation and cadmium detoxification in Klebsiella aerogenes NCTC 418 growing in continuous culture. Appl. Environ. Microbiol. 1984;47:374–377. doi: 10.1128/aem.47.2.374-377.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreoni V., Colombo M., Colombo A., Vecchio A., Finoli C. Cadmium and zinc removal by growing cells of Pseudomonas putida strain B14 isolated from a metal-impacted soil. Anna. Microbiol. 2003;53:135–148. [Google Scholar]
- 5.Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Castro-Silva M.A., Lima A.O.S., Gerchenski A.V., Jaques D.B., Rodrigues A.L., Souza P.L., Rörig L.R. Heavy metal resistance of microorganisms isolated from coal mining environments of Santa Catarina. Braz. J. Microbiol. 2003;34(1):45–47. [Google Scholar]
- 7.Gadd G.M. Heavy metal accumulation by bacteria and other microorganisms. Experientia. 1990;46:834–840. [Google Scholar]
- 8.Goomes N.C.M., Mendonca Hagler L.C.S., Sawadis I. Metal bioremediation by microorganisms. Braz. J. Microbiol. 1998;29:85–92. [Google Scholar]
- 9.Hassan M.E.T., van der Lelie D., Springael D., Römling N., Ahmed N., Mergeay M. Identification of a gene cluster, czr, involved in cadmium and zinc resistance in Pseudomonas aeruginosa. Gene. 1999;238:417–425. doi: 10.1016/s0378-1119(99)00349-2. [DOI] [PubMed] [Google Scholar]
- 10.Higham D.P., Sadler P.J., Scawen M.D. Cadmium-resistant Pseudomonas putida synthesizes novel cadmium proteins. Science. 1984;225:1043–1046. doi: 10.1126/science.225.4666.1043. [DOI] [PubMed] [Google Scholar]
- 11.Kazy S.K., Sar P., Asthana R.K., Singh S.P. Copper uptake and its compartmentalization in Pseudomonas aeruginosa strains: Chemical nature of cellular metal. W. J. Mirobiol. Biotechnol. 1999;15:599–605. [Google Scholar]
- 12.Keasling J.D., Hupf G.A. Genetic manipulation of polyphosphate metabolism affects cadmium tolerance in Escherichia coli. Appl. Environ. Microbiol. 1996;62:743–746. doi: 10.1128/aem.62.2.743-746.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurek E., Czaban J., Bollag J. Sorption of cadmium by microorganisms in competition with other soil constituents. Appl. Environ. Microbiol. 1982;43:1011–1015. doi: 10.1128/aem.43.5.1011-1015.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Les A., Walker R.W. Toxicity and binding of copper, zinc, and cadmium by the blue-green alga, Chroococcus paris. Water Air Soil Pollut. 1984;23:129–139. [Google Scholar]
- 15.Mitra R.S., Bernstein I.A. Single-strand breakage in DNA of Escherichia coli exposed to Cd2+. J. Bacteriol. 1978;133:75–80. doi: 10.1128/jb.133.1.75-80.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitra R.S., Gray R.H., Chin B., Bernstein I.A. Molecular mechanisms of accommodation in E. coli to toxic levels of Cd+2. J. Bacteriol. 1975;121:1180–1188. doi: 10.1128/jb.121.3.1180-1188.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullen M.D., Wolf D.C., Ferris F.G., Beveridge T.J., Fleming C.A., Bailey G.W. Bacterial sorption of heavy metals. Appl. Environ. Microbiol. 1989;55:3143–3149. doi: 10.1128/aem.55.12.3143-3149.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nies D.H. Resistance to cadmium, cobalt, zinc, nickel in microbes. Plasmid. 1992;27:17–28. doi: 10.1016/0147-619x(92)90003-s. [DOI] [PubMed] [Google Scholar]
- 19.Rachlin J.W., Warkentine B., Jensen T.E. The growth responses of Chlorella saccharophila, Navicula incerta, and Nitzschia closterium to selected concentrations of cadmium. Bull. Torrey Bot. Club. 1982;109:129–135. [Google Scholar]
- 20.Ramamoorthy S., Kushner D.J. Binding of mercuric and other heavy metal ions by microbial growth media. Microb. Ecol. 1975;2:162–176. doi: 10.1007/BF02010436. [DOI] [PubMed] [Google Scholar]
- 21.Sadouk A., Mergeay M. Chromosome mapping in Alcaligenes eutrophus CH34. Mol. Gen. Genet. 1993;240:181–187. doi: 10.1007/BF00277055. [DOI] [PubMed] [Google Scholar]
- 22.Sar P., Kazy S.K., Singh S.P. Intracellular nickel accumulation by Pseudomonas aeruginosa and its chemical nature. Lett. Appl. Microbiol. 2001;32:257–261. doi: 10.1046/j.1472-765x.2001.00878.x. [DOI] [PubMed] [Google Scholar]
- 23.Scott J.A., Palmer S.J. Site of cadmium uptake in bacteria used for biosorption. Appl. Microbiol. Biotechnol. 1990;33:221–225. doi: 10.1007/BF00176529. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro N., Keasling J.D. The recA gene and cadmium toxicity in Escherichia coli K-12. Microbios. 1996;86:23–26. [PubMed] [Google Scholar]
- 25.Sharma P.K., Balkwill D.L., Frenkel A., Vairavamurthy M.A. A new Klebsiella planticola strain (Cd-1) grows anaerobically at high cadmium concentrations and precipitates cadmium sulfide. Appl. Environ. Microbiol. 2000;66:3083–3087. doi: 10.1128/aem.66.7.3083-3087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silver S., Misra T.K. Plasmid mediated heavy metal resistances. Ann. Rev. Microbiol. 1988;42:717–743. doi: 10.1146/annurev.mi.42.100188.003441. [DOI] [PubMed] [Google Scholar]
- 27.Sinha S., Mukherjee S.K. Cadmium–induced siderophore production by a high Cd resistant bacterial strain relieved Cd-toxicity in plants through root colonization. Curr. Micobiol. 2008;56:55–60. doi: 10.1007/s00284-007-9038-z. [DOI] [PubMed] [Google Scholar]
- 28.Tebo B.M. Metal precipitation by marine bacteria: potential for biotechnological applications. Genet. Eng. 1995;17:231–263. [Google Scholar]
- 29.Waalkes M.P., Coogan T.P., Barter R.A. Toxicological principles of metal carcinogenesis with special emphasis on cadmium. Crit. Rev. Toxicol. 1992;22:175–201. doi: 10.3109/10408449209145323. [DOI] [PubMed] [Google Scholar]
- 30.Wang C.L., Maratukulam P.D., Lum A.M., Clark D.S., Keasling J.D. Metabolic engineering of an aerobic sulfate reduction pathway and its application to precipitation of cadmium on the cell surface. Appl. Environ. Microbiol. 2000;66:4497–4502. doi: 10.1128/aem.66.10.4497-4502.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C.L., Michels P.C., Dawson S.C., Kitisakkul S., Baross J.A., Keasling J.D., Clark D.S. Cadmium removal by a new strain of Pseudomonas aeruginosa in aerobic culture. Appl. Environ. Microbiol. 1997;63:4075–4078. doi: 10.1128/aem.63.10.4075-4078.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilde E.W., Benemann J.R. Bioremoval of heavy metals by the use of microalgae. Biotechnol. Adv. 1993;11:781–812. doi: 10.1016/0734-9750(93)90003-6. [DOI] [PubMed] [Google Scholar]


