Abstract
Enzyme application in biotechnological and environmental processes has had increasing interest due to its efficiency, selectivity and mainly for being environmentally healthful, but these applications require a great volume of enzymes. In this work the effect of different concentrations of ethanol and 2,5-xylidine on growth and production of laccase by Pycnoporus sanguineus was investigated. In a medium containing 200 mg.L-1 of 2,5-xylidine or 50 g.L-1 of ethanol, the maximum activity of laccase was 2019 U.L-1 and 1035 U.L-1, respectively. No direct correlation between biomass and activity of laccase was observed for any of the inducers used during the tests. Ethanol concentrations, larger than or equal to 20 g.L-1, inhibited the radial growth of P. sanguineus. This study showed that ethanol, which has less toxicity and cost than the majority of the studied inducers, presents promising perspectives for laccase production by P. sanguineus.
Keywords: Laccase; Pycnoporus sanguineus; ethanol; 2,5-xylidine
INTRODUCTION
Laccases (benzenediol:oxygen oxidoreductase, EC 1.10.3.2) are multi-cooper oxidases widely distributed among plants, insects and fungi (8, 19). Laccases are enzymes that catalyze the oxidation of a phenolic substrate by coupling it to the reduction of O2 to water, without any harmful intermediate. This makes laccase the best candidate for the environmentally benign process (12). Laccases are currently seen as highly interesting industrial enzymes because of their wide variety of potential substrates. These enzymes are increasingly being investigated for a variety of practical applications including decolorizing and detoxifying effluents, drug analysis, textile dye bleaching, synthesis of polymers, biosensors and bioremediation (8, 13, 15). However, high amounts of the enzyme are needed for these applications, as well as to other studies. In this way, researches have been developed in order to increase laccase production by the use of new sources (9, 18) and by screening for inducers of laccase production by microorganisms, like aminoacids (3), aromatic compounds (2), copper (17) and agro-residues (21). The presence of laccases has been reported in many fungal species, but relatively few researches have been directed towards the laccase produced by members of the genus Pycnoporus. The genus Pycnoporus is of particular interest because it produces laccases as predominant lignolytic enzyme (5). It has been reported that laccase production by members of this genus increased after supplementing the fungal cultures with phenolic substrates such as ferulic acid, 2,5-xylidine, veratryl alcohol or lignosulphonate (5, 10, 6). However, most of these aromatic compounds presents great toxicity and/or high cost. The main objective of this work was to study the effect of 2,5-xylidine, a common inducer used for fungal secretion of laccase, and ethanol, a cheap agro-industrial product, on the growth and the laccase production by P. sanguineus CCT-4518.
MATERIALS AND METHODS
Microorganism maintenance and culture conditions
The white-rot fungus Pycnoporus sanguineus CCT-4518, obtained from Fundação André Tosello, Campinas, São Paulo, Brazil, was maintained on potato-dextrose-agar (PDA, Merck, Darmstadt, Germany) at 4ºC. The fungus was previously grown on plates with PDA at 37ºC (5-7 days) until the mycelium occupies 100% of the medium surface. Five fungal discs measuring 7 mm in diameter taken from the active borders of PDA cultures were transferred as inducers to Erlenmeyer flasks (250mL) containing 50mL of liquid medium containing 12.5 g.L-1 of malt extract (Merck, Darmstadt, Germany), 0.005 g.L-1 of CuSO4.H2O (Cromoline, São Paulo, Brazil), and 50 – 200 mg.L-1 of 2,5-xylidine (SIGMA, St. Louis, USA) or 20 – 50 g.L-1 of ethanol (Isofar, Rio de Janeiro, Brazil). The flasks were incubated at 28°C in the dark and shaken at 140 rpm. In regular intervals, during up to 21 days the mycelium was collected by filtration through filter paper and the culture filtrate was used as source of laccase.
Biomass
Fungal biomass was determined at specific time intervals by vacuum filtering mycelia through filter paper, washed with distilled water and dried to constant weight at 80°C (6).
Enzymatic activity assays
Laccase activity was determined at 30°C using 0.5 mM 2,2'-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS; SIGMA, St. Louis, USA) (ε420 = 3.6×104 M cm-1) (1) . The reaction mixture contained 100 μl substrate, 800 μl of buffer 50 mM sodium acetate (pH 5.0 ) and 100 μL of the culture supernatant (diluted when necessary) for 5 min. The enzymatic extract was incubated with the enzyme catalase (SIGMA, St. Louis, USA) for a period of 30 minutes, before the assay for enzymatic activity, in order to eliminate any endogenous hydrogen peroxide that could be present. One unit of enzyme activity is defined as the amount of enzyme required to oxidize 1 μM ABTS under standard assay conditions. Assays were carried out in triplicate, and standard deviation did not exceed 10% of the average values.
Effect of ethanol on the micelial radial growth of Pycnoporus sanguineus
Four concentrations of ethanol were tested: 0 (reference), 20, 30 and 50 g.L-1. Duplicate Petri dishes of each ethanol concentration were centrally inoculated with one fungal disc measuring 7 mm in diameter taken from the active borders of PDA cultures. The colony radial diameter was measured daily in 4 different positions and determined the average value, for a period of 10 days.
RESULTS AND DISCUSSION
In order to evaluate the effect of the 2,5-xylidine and ethanol on the growth and production of laccase by P. sanguineus, concentrations of 50, 100, 150 and 200 mg.L-1 of 2,5-xylidine and 20, 30, 40 and 50 g.L-1 of ethanol were tested. Laccase activity in the extracellular fluid of P. sanguineus cultures supplemented with the four concentrations tested of 2,5-xylidine presented an increase with time (Fig. 1A). A similar effect was demonstrated by P. cinabarinus in which 2,5-xylidine (10 - 19μM) enhanced laccase activity by about 9-fold reaching approximately 9.60 U.mL-1 (5) and also by P. sanguineus in which the addition of 2,5-xylidine (400 μM) resulted in an increase of 14-fold, reaching approximately 1.09 U.mL-1 (6, 7). The inducer concentrations tested in this study are higher than the normally used, considering the toxicity of the same. However, the P. sanguineus was capable of remaining viable and with very good activity in the presence of these concentrations of the 2,5-xylidine. The production of laccase could be detected from the 2nd day of growth for all the concentrations and was higher when 200 mg.L-1 of this inducer was added to the medium. The maximum production (2019 U.L-1) was reached between the 7th and 9th days of growth (Fig 1A). In the presence of different concentrations of ethanol an increase of the production of laccase was also observed (Fig. 1B). This was considerably higher in the presence of 40 and 50 g.L-1 of the inducer, even so this increase was still smaller than that in the presence of the 2,5-xylidine. It was possible to detect the activity of laccase from the 2nd or 4th days of culture and the best enzymatic production (1035 U.L-1) occurred in the 14th day of growth, when 50 g.L-1 of ethanol was used. In the study carried out by Lomascolo et al. (14), the largest production of laccase by Pycnoporus cinnabarinus was obtained with 35 g.L-1 of the inducer (266600 U.L-1), having a reduction of approximately 50% of the enzymatic activity when the concentration of ethanol was 45 g.L-1.
Figure 1.
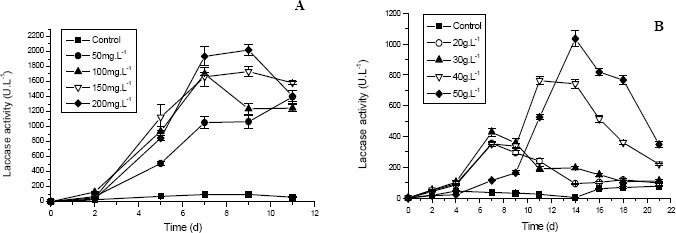
Production of laccase by P. sanguineus in the presence of (A) different concentrations of 2,5-xylidine, and (B) different concentrations of ethanol.
Regarding the biomass, it was observed that the profile was different for the two studied inducers. In the presence of 50 mg.L-1 of 2,5-xylidine, the biomass increased until the 5th day, followed by its decrease and stabilization (Fig. 2A). In the presence of 50 g.L-1 of ethanol a continuous increase of the biomass occurred until 17th day of culture (Fig. 2B). This increase of biomass did not present direct correlation with the production of laccase and this is in agreement with Garcia et al. (6) when the P. sanguineus grew in the presence of 2,5-xylidine. Similar results were found for other fungi as Magnaporthe grisea (11) and Cyathus bulleri (20).
Figure 2.
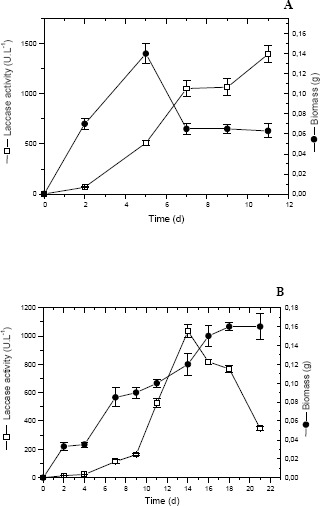
Biomass and laccase activity during growth of P. sanguineus using (A) 50 mg.L-1 2,5-xylidine and (B) 50 g.L-1 ethanol as inducer.
An interesting observed result was the color change occurred in the liquid cultures when P. sanguineus grew in the presence of these inducers. The increase of the laccase production coincided with the synthesis of an orange pigment when 2,5-xylidine was used as inducer. Previous studies (4) indicate that laccase is involved in the process of melanin formation of fungi, e.g. G. graminins var. tritici and probably in other species. A small color change of the liquid cultures was observed when the growth of the P. sanguineus occurred in the presence of ethanol using concentrations larger than 30 g.L-1, which produced higher laccase activity. Although the real influence of ethanol in the level of expression of genes of laccase was not elucidated, the small change in the coloration of the culture medium observed in this study was probably due to the inhibition of the melanin formation (16).
The effects of 20-50 g.L-1 of ethanol on the radial growth of P. sanguineus were evaluated (Fig. 3). It was observed that inhibition of the fungus growth increased with increasing concentration of ethanol added to the medium. After 10 days of growth, comparing itself with the medium without ethanol, was verified a reduction of 40% of the radial growth in the plates when 20 g.L-1 of ethanol was added, and in the presence of 30 g.L -1 and 50 g.L-1 of ethanol the inhibitions of the growth were 50% and 78%, respectively. These results are in agreement with Lomascolo et al. (14). Ethanol concentration equal to 50 g.L-1 inhibited the fungal growth for 6 days (lag time). The effect of ethanol on the micelial growth of P. sanguineus was similar to those observed for the P. cinnabarinus (14).
Figure 3.
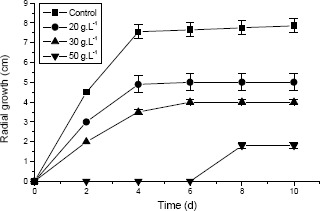
Effect of ethanol concentration on P. sanguineus radial growth.
CONCLUSIONS
The results confirm the important inductive role of the 2,5-xylidine in the production of laccase and present promising perspectives for the use of ethanol as inducer in the production of laccase by P. sanguineus. It is important to consider the economic and environmental advantages of the use of ethanol, when compared to the most used inducers. The possible inhibition of the formation of melanin in the presence of ethanol is another important data to be considered. For example, when using this fungus in studies of discoloration of industrial effluents, the pigment formation may affect the evaluation of the removal of color.
ACKNOWLEDGEMENT
This work was financially supported by the SECTEC/CNPq and the International Foundation for Science, Stockholm, Sweden, through a grant to Dr M.F. Santiago (IFS-W/3433-1).
REFERENCES
- 1.Bourbonnais R., Paice M.G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990;267:99–102. doi: 10.1016/0014-5793(90)80298-w. [DOI] [PubMed] [Google Scholar]
- 2.Cavallazzi J.R.P., Kasuya C.M., Soares M.A. Screening of inducers for laccase production by Lentinula edodes in liquid medium. Braz. J. Microbiol. 2005;36:383–387. [Google Scholar]
- 3.Dhawan S., Kuhad R.C. Effect of amino acids and vitamins on laccase production by the bird’s nest fungus Cyathus bulleri. Bioresour. Technol. 2002;84:35–38. doi: 10.1016/s0960-8524(02)00026-3. [DOI] [PubMed] [Google Scholar]
- 4.Edens W.A., Goins T.Q., Dooley D., Hensoon J.M. Purification and characterization of a secreted laccase of Gaeumannomyces graminins var.tritici. Appl. Environ. Microbiol. 1999;65(7):3071–3074. doi: 10.1128/aem.65.7.3071-3074.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggert C., Temp U., Eriksson K.E.L. The ligninolytic system of white rot fungus Pycnoporus cinnabarinus: Purification and characterization of the laccase. Appl. Environ. Microbiol. 1996;62:1151–1158. doi: 10.1128/aem.62.4.1151-1158.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia T.A., Santiago M.F.S., Ulhoa C.J. Properties of laccases produced by Pycnoporus sanguineus induced by 2,5 xylidine. Biotechnol. Let. 2006;28:633–636. doi: 10.1007/s10529-006-0026-3. [DOI] [PubMed] [Google Scholar]
- 7.Garcia T.A. Brasília: (Ph.D. Thesis. Instituto de Ciências Biológicas, UnB); 2006. Purificação e caracterização das lacases de Pycnoporus sanguineus. p. 90. [Google Scholar]
- 8.Gianfreda S., Xu F., Bollag J.M. Laccases: a useful group of oxidoreductive enzymes. Bioremediation J. 1999;3(1):1–25. [Google Scholar]
- 9.Gochev V.K., Krastanov A.I. Isolation of Laccase Producing Trichoderma Spp. Bulg. J. Agric. Sci. 2007;13:171–176. [Google Scholar]
- 10.Herpöel I., Moukha S., Lesage Meessen L., Sigoillot J., Asther M. Selection of Pycnoporus cinnabarinus strains for laccase production. FEMS Microbiol. Lett. 2000;183(2):301–306. doi: 10.1111/j.1574-6968.2000.tb08975.x. [DOI] [PubMed] [Google Scholar]
- 11.Iyer G., Chatoo B.B. Purification and characterization of laccase from the rice blast fungus, Magnaporthe grisea. FEMS Microbiol. Lett. 2003;227:121–126. doi: 10.1016/S0378-1097(03)00658-X. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y., Cho N.S., Eom T.J., Shiun W. Purification and characterization of a laccase from Cerrena unicolor and its reactivity in lignin degradation. Bull. Korean Chem. Soc. 2002;23(7):985–989. [Google Scholar]
- 13.Leite O.D., Fatibello Filho O., Barbosa A.M. Determination of catecholamines in pharmaceutical formulations using a biosensor modified with a crude extract of fungi laccase (Pleurotus ostreatus). J. Braz. Chem. Soc. 2003;14(2):297–303. [Google Scholar]
- 14.Lomascolo A., Record E., Herpoël Gimbert I., Delattre M., Robert J.L., Georis J., Dauvrin T., Sigoillot J.C., Asther M. Overproduction of laccase by a monokaryotic strain of Pycnoporus cinnabarinus using ethanol as inducer. J. Appl. Microbiol. 2003;94:618–624. doi: 10.1046/j.1365-2672.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- 15.Mayer A.M., Staples R.C. Review – laccase: new functions for an old enzyme. Phytochemistry. 2002;60:551–565. doi: 10.1016/s0031-9422(02)00171-1. [DOI] [PubMed] [Google Scholar]
- 16.Meza J.C., Auria R., Lomascolo A., Sigoillot J.C., Casalot L. Role of ethanol on growth, laccase production and protease activity in Pycnoporus cinnabarinus ss3. Enzyme Microb. Technol. 2007;41(1-2):162–168. [Google Scholar]
- 17.Saparrat M.C.N., Guillén F., Arambarri A.M., Martínez A.T., Martínez M.J. Induction, isolation, and characterization of two laccases from the white rot basidiomycete Coriolopsis rigida. Appl.Environ. Microbiol. 2002;68(4):1534–1540. doi: 10.1128/AEM.68.4.1534-1540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siqueira A.C.R., Garcia T.A. Rio de Janeiro, RJ: VIII Seminário Brasileiro de Tecnologia Enzimática; 2008. Seleção de Trichoderma sp. Produtor de enzimas ligninolíticas. pp. 101–102. [Google Scholar]
- 19.Thurston C.F. The structure and function of fungal laccases. Microbiology. 1994;140:19–26. [Google Scholar]
- 20.Vasdev K., Dhawan S., Kapoor R.K., Kuhad R.C. Biochemical characterization and molecular evidence of a laccase from the bird´s nest fungus Cyathus bulleri. Fungal Genet.Biol. 2005;42:684–693. doi: 10.1016/j.fgb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Vikneswary S., Noorlidah A., Renuvathani M., Sekaran M., Pandey A., Jones E.B.G. Productivity of laccase in solid substrate fermentation of selected agro-residues by Pycnoporus sanguineus. Bioresour. Technol. 2006;97:171–177. doi: 10.1016/j.biortech.2005.02.015. [DOI] [PubMed] [Google Scholar]


