Abstract
The ethanol, methanol and acetone extracts of Aloe vera gel were studied for their antimicrobial activity against four Gram-positive and Gram-negative bacteria using agar well diffusion method. The extracts showed varied levels of antimicrobial activity against the tested pathogens. The ethanol and methanol extracts showed higher activity while acetone extract, showed least or no activity against most of the tested pathogens. Fractions obtained from the extracts by Thin Layer and Column Chromatography were studied for their antagonistic properties using Spot Assay Technique. Compounds with maximum antibacterial activity isolated from the ethanol and methanol extracts were identified as p – coumaric acid (Mol. wt.165), ascorbic acid (Mol. wt.177 ), pyrocatechol (Mol. wt.110 ) and cinnamic acid (Mol. wt.148), on the basis of Gas Chromatography Mass Spectrometry. The study suggests the antimicrobial activity of the A. vera gel extract to be dependant on the synergistic effect of different compounds. With the broad spectral antimicrobial effect of A. vera gel, it could be further recommended in the treatment of various bacterial diseases.
Keywords: Aloe vera, gel, antimicrobial, compounds
INTRODUCTION
Aloe vera is an ornamental and medicinal plant. It is being used therapeutically, since Roman times and perhaps long before (5, 20), different properties being ascribed to the inner colorless leaf gel and to the exudates from the outer layers. Aloe has a history of traditional use by Native Americans for stomach disorders and intestinal disorders including constipation, hemorrhoids, colitis and colon problems. It is said to be a natural cleaner, powerful in penetrating tissues, relieving pain associated with joints and muscles, bactericidal, a strong antibiotic, virucidal when in direct contact with long periods, fungicidal, anti-inflammatory, instrumental in increasing circulation to the area, breaking and digesting dead tissue and moisturizing tissues. The skin absorbs Aloe vera up to four times faster than water, it appears to help pores of the skin open and receive moisture and nutrients of the plants. Additionally, numerous constituents within Aloe vera have demonstrated enhancement of immune system functioning within the body. Aloe also has the ability to stimulate macrophages (6).
To date more than 75 ingredients have been identified from the gel (11, 12) each of which may have a range of mechanism of actions, acting synergistically or individually to explain more than 200 different constituents notably mucopolysaccharides, enzymes, sterols, prostaglandins, fatty acids, amino acids and a wide variety of vitamins and minerals. It contains several potentially active bioactive compounds including salicylates, magnesium lactate, acemannan, lupeol, campestrol, β-sitosterol, aloin A and anthraquinones (10). In addition Aloe vera contains at least seven super-oxide dismutases with antioxidant activity.
The efficacy of Aloe liquid as an antibacterial agent is shown to have a wide range against Gram positive and Gram negative bacteria. The antimicrobial agents of Aloe vera gel was reported to effectively kill or greatly reduce or eliminate the growth of Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pyogenes, Pseudomonas aeruginosa, Escherichia coli, Propionibacterium acne, Helicobacter pylori and Salmonella typhi (16, 23, 24, 27). Whole leaf components are proposed to have direct antibacterial properties include anthraquinones and saponin (24, 27); While polysaccharides have been attributed within direct bacterial activity through the stimulation of phagocytic leucocytes to destroy bacteria (16, 23).Due to the increasing development of antibiotic resistance, the emphasis of the present study is being given on the use of Aloe vera as a natural remedy for the inhibition of various infections and to identify the different compounds.
MATERIALS AND METHODS
Collection of Plant Material
Aloe vera leaves were collected from the Department of Horticulture, Allahabad Agricultural Institute-Deemed University, Allahabad.
Test Organisms
Reference strains viz., Staphylococcus aureus (MTCC 2943), Streptococcus pyogenes (MTCC 442), Bacillus subtilis (MTCC 441), Bacillus cereus (MTCC 1272), Escherichia coli (MTCC 1687), Pseudomonas aeruginosa (MTCC 1688), Salmonella typhi (MTCC 531) and Klebsiella pneumoniae (MTCC 530) were obtained from the IMTECH, Chandigarh, India were used in the study.
Preparation of Aloe vera Gel
The fully expanded leaves of Aloe vera were selected from the plants, washed with distilled water and were subjected to surface sterilization with 70% ethyl alcohol followed by 0.1% HgCl2. The parenchymatous covering of the leaves were peeled and the gel drained out. Slurry was formed with the help of pestle and mortar.
Preparation of the Extracts
For the preparation of ethanol and methanol extracts, fresh leaf gel was dried in the oven at 80 °C for 48 h. and then powdered. Twenty grams of this powder was soaked in 200ml. of each of the solvents namely ethanol and methanol for 24 h. The contents were then filtered through Whattman filter paper no. 1 and the filtrate was evaporated to dryness. This dried extract was further powdered and then dissolved in distilled water. Acetone extract was prepared in a similar manner except that the extracted powder was dissolved in 0.15N NaOH and was further neutralized with 0.15N HCl (22).
Antibacterial Activity of the Aloe vera Gel Extracts
The antibacterial activity of Aloe vera gel extract was tested using Agar Well Diffusion Technique as described by Agarry et al. (1). Wells of 5 mm diameter were cut on sterile nutrient agar plates and swabbed with an overnight broth culture of the organism. About 0.1ml of the A. vera gel extracts were filled into each of the wells and incubated at 37°C ± 0.2°C. Antibacterial activity in terms of zones of inhibition (mm) was recorded after 24 h. of incubation. The antagonistic action of extracts of A. vera gel were tested against test organisms in triplicates.
Fractionization of the extract
The extracts with maximum antibacterial activity were fractionated by using Silica gel thin layer chromatography with different concentrations of MeOH:EtOAc (Table 2). The isolated compounds on the TLC plates were visualized using H2SO4/ Methanol (1:9 v/v) followed by heating at 80°C for 5 min. After deciding the solvent combination the compounds were isolated by eluting through silica gel (60-120 mesh) column.
Table 2.
Major and minor compounds detected in the Aloe vera gel extracts
| Extract | Major compounds | Mobile phase | Rf value | Minor compounds | Mobile phase | Rf value |
|---|---|---|---|---|---|---|
| 1 | MeOH:EtOAc(1:9) | 0.30 | 1 | MeOH:EtOAc(3.5:6.5) | 0.68 | |
| 2 | MeOH: EtOAc( 1.5:8.5) | 0.46 | 2 | MeOH:EtOAc(4.5:5.5) | 0.77 | |
| 3 | MeOH: EtOAc(2.5:7.5) | 0.72 | 3 | MeOH: EtOAc (5:5) | 0.78 | |
| 4 | MeOH:EtOAc (5.5:4.5) | 0.80 | ||||
| Methanol | 5 | MeOH:EtOAc (6:4) | 0.82 | |||
| 4 | MeOH:EtOAc(8.5:1.5) | 0.88 | (6.5:3.5) MeOH:EtOAc (7:3) | |||
| 7 | MeOH:EtOAc (7:3) | 0.85 | ||||
| 8 | MeOH:EtOAc (7.5:2.5) | 0.86 | ||||
| 9 | MeOH:EtOAc (8:2) | 0.87 | ||||
| 1 | EtOH:EtOAc (1:9) | 0.25 | 1 | EtOH:EtOAc (3.5:6.5) | 0.72 | |
| Ethanol | 2 | EtOH:EtOAc (1.5:8.5) | 0.36 | 2 | EtOH:EtOAc (3.5:6.5) | 0.74 |
| 3 | EtOH:EtOAc (2:8) | 0.42 | 3 | EtOH:EtOAc (6:4) | 0.78 | |
| 4 | EtOH:EtOAc (2.5:7.5) | 0.64 | 4 | EtOH:EtOAc (8:2) | 0.83 |
Evaluation of Antibacterial Activity of Different Compounds
The different fractions obtained from the column chromatography were allowed to stand at room temperature till the solvent evaporated. The dried fractions were dissolved in Di-Methyl Sulfoxide (DMSO) and used for screening the antibacterial activity against the selected pathogenic bacteria using Spot Assay Technique as described by Jack et al. (13). For this the overnight broth culture was swab inoculated with the selected pathogenic bacteria and the fractions dissolved in DMSO were spot inoculated. The plates were incubated at 37 °C ± 0.2 °C for 24 h. The presence of zones of inhibition (showing the antibacterial action of the compounds) were recorded after 24 h. of incubation.
Identification of the Antibacterial Compounds
The fractions that showed antibacterial activity against majority of the selected pathogens was further identified using, Gas Chromatography Mass Spectrometry (GCMS) analyzer (Shimadzu QP-2000). ULBON HR-1 Column equivalent to OV-1 fused with silica capillary 0.25mm X 30M with film thickness 0.25 micron was used for this purpose. The initial temperature maintained was 100°C for 6 min. and then heated at the rate of 10°C per minute up to 250°C at 70eV bombardment energy. Carrier gas Helium was used at the rate of 2ml per minute.
Statistical Analysis
The data recorded during the course of investigation were statistically analyzed applying Analysis of Variance (ANOVA), two way classification and F-test at 5% significance level to calculate the significant difference between the organisms, solvents and the replicates.
RESULTS AND DISCUSSION
Antibacterial activity of Aloe vera gel extract against selected pathogenic bacteria
The antibacterial property of Aloe vera gel extracted using different solvents showed varying degree of response towards the selected pathogens (Table 1). Using ethanol extracts the zones of inhibition ranged from 12.66 – 23.33 mm being maximum for B. cereus and minimum for E. coli (p<0.05). Methanol extract exhibited maximum antibacterial activity against B. cereus (22.33 mm) followed by S. pyogenes (15 mm) and least for S. typhi (9.66 mm); the differences being statistically significant (p<0.05). Acetone extract gave lower values of zones of inhibition ranging from 6.00 mm for E. coli to 7.33 mm for S. pyogenes, while no response was observed for P. aeruginosa and S. typhi (p<0.05).
Table 1.
Antibacterial Activity of Aloe vera gel extracts against selected Pathogenic bacteria
| Organisms | Zones of Inhibition (in mm) | Results | ||
|---|---|---|---|---|
| Ethanol Extract | Methanol Extract | Acetone Extract | ||
| Gram-positive | ||||
| B. cereus | 23.33 | 22.33 | 6.66 | S, p< 0.05 |
| B. subtilis | 15.00 | 11.33 | 6.33 | S, p< 0.05 |
| S. aureus | 15.66 | 14.00 | 6.66 | S, p< 0.05 |
| S. pyogenes | 16.00 | 15.00 | 7.33 | S, p< 0.05 |
| Gram-negative | ||||
| E. coli | 12.66 | 14.00 | 6.00 | S, p< 0.05 |
| P. aeruginosa | 23.00 | 10.66 | 0.00 | S, p< 0.05 |
| K. pneumoniae | 22.66 | 14.00 | 6.33 | S, p< 0.05 |
| S. typhi | 14.66 | 9.66 | 0.00 | S, p< 0.05 |
| Results | S, p< 0.05 | S, p< 0.05 | S, p< 0.05 | |
Generally the extracts showed greater antibacterial activity against Gram-positive as compared to Gram-negative bacteria. With respect to individual pathogens, ethanol extract showed greater inhibition than methanol extract while, significantly lower inhibition was observed with acetone extract (p<0.05).
As is in the present study, other workers have reported antibacterial properties of ethanol extracts of A. vera gel against the pathogens selected in the study (1, 24). In the study conducted by Martinez et al. (18) no antimicrobial activity was reported using, aqueous extract of A. vera leaves. As stated by Cowan (4), nearly all of the identified components from plants active against micro-organisms are aromatic or saturated organic compounds and are most often obtained through initial ethanol or methanol extraction. This explains higher antimicrobial activity of ethanol and methanol extracts observed in the study. A lower antimicrobial action against Gram-negative bacteria as compared to Gram-positive organism could be explained to be due to the presence of additional lipopolysaccharide layer in the former.
Major and minor compounds identified in the Aloe vera gel Extracts
Of the various solvent combinations used (Methanol, Ethanol, Acetone, Ethyl acetate, Chloroform, Hexane and Petroleum ether) for the identification of major and minor compounds in A. vera gel extracts, Ethanol and Ethyl acetate, Methanol and Ethyl acetate were found to be most effective in the separation of different compounds. Four major and nine minor compounds were identified on the basis of spots and the retention factor (Rf) values at different combinations of Methanol: Ethyl acetate in the methanol extract. Similarly four major and four minor compounds were identified with different combinations of Ethanol: Ethyl acetate in the ethanol extract (Table 2). The observation of the present study are in contrast with the studies of Speranza et al. (1985, 1986), where only one major spot with different Rf value was obtained through the TLC of the methanol extract of Aloe sp. The variability in the Rf value of the compounds could be due to its dependence on the amount of sample applied, the saturation state of the chamber, relative humidity, temperature and the traveling distance of the solvent front.
Isolation of different compounds in the Aloe vera gel extracts
The different compounds in the methanol and ethanol extracts of A. vera gel were isolated by eluting in the stepwise gradient solvent system determined in TLC, described previously in Table 2. In the methanol extract MeOH:EtOAc solvent systems with increasing polarity (10-85% MeOH in EtOAc, v/v) a total of 22 fractions were obtained. Similarly 16 fractions of ethanol extract were obtained in the EtOH:EtOAc (10-80%, v/v) fractionization. The fractions with similar Rf values were pooled together and labelled accordingly. Further, to distinguish the fractions of methanol and ethanol extract a difference in the numbering system was also followed (Table 3). In a similar study conducted by Cooposamy and Magwa (3) MeOH:EtOAc solvent system (0-30%) was used to elute the different fractions of EtOAc extract. The authors had reported eluting 48 fractions in their study. Further, the fractionization of the ethanol extract reported by Okamura et al. (21) yielded nine fractions, which is comparatively lower than in the present study. The variations in the number of fractions with the present study could be due to the type of extract, solvent system and the technique adopted.
Table 3.
Antibacterial activity of the fractions obtained from the Ethanol and Methanol extracts of Aloe vera gel
| Extract | Fraction No. | Antibacterial activity |
|---|---|---|
| C, D | +++ | |
| Ethanol | E, F, G, H, I, J | ++ |
| A, B, K, L, M, N, O, P | - | |
| 73, 74 | +++ | |
| Methanol | 16, 40, 46, 48, 113, 114, 123, 125, 126, 128 | ++ |
| 80, 83, 94, 95, 100, 103, 104, 105, 117, 127 | - |
* +++ = maximum antibacterial activity, ++ = moderate activity, - = no activity
Antibacterial activity of different fractions obtained in Column Chromatography
A varying degree of antibacterial activity of different extracts of Aloe vera gel against the pathogenic bacteria was observed in the present study. Two fractions viz. C and D obtained from the ethanol extract of A. vera gel showed maximum antibacterial activity against the test organisms (Table 3). Moderate antibacterial activity of six fractions (E-J) were observed in the ethanol extract and no activity was observed with the remaining eight fractions (A, B, K-P). Similarly two fractions (73 and 74) of the methanol extract showed maximum antibacterial activity followed by ten fractions (16, 40, 46, 48, 113, 114, 123, 125, 126 and 128) with moderate activity and the remaining ten fractions (80, 83, 94, 95, 100, 103, 104, 105, 117 and 127) with no activity.
Further, the fractions with maximum antibacterial activity viz. C, D, 73 and 74 showed significant variations in the inhibition zones against the Gram-positive and Gram-negative bacteria tested (P<0.05). Fraction C and D was found to possess maximum antibacterial activity against Gram-positive bacteria in comparison to Gram-negative bacteria (Table 4). The effect of fraction no C was found to be maximum against Bacillus cereus and Bacillus subtilis followed by Streptococcus pyogenes. Among the Gram-negatives Salmonella typhi was observed to be inhibited most in comparison to Pseudomonas aeruginosa and the fraction was ineffective against the remaining Gram-negatives tested. The fraction D was found to be effective against all the Gram-positives except B.subtilis. Conversely, it was found to be effective only against S.typhi among the Gram-negatives.
Table 4.
Antibacterial activity of selected fractions obtained from column chromatography
| Organisms | Zone of Inhibition(mm diameter) | Results | |||
|---|---|---|---|---|---|
| C | D | 73 | 74 | ||
| Bacillus cereus | 10.8μ 0.08 | 10.8μ 0.08 | 0 | 11.1μ 0.08 | S, p< 0.05 |
| Bacillus subtilis | 10.8μ 0.08 | 0 | 0 | 10.1μ 0.08 | S, p< 0.05 |
| Staphylococcus aureus | 0 | 9.2μ 1. 5 | 0 | 10.25μ 0.25 | S, p< 0.05 |
| Streptococcus pyogenes | 8.1μ 0.08 | 8.1μ 0.08 | 0 | 0 | S, p< 0.05 |
| Escherichia coli | 0 | 0 | 8μ 0.25 | 10.1μ 0.08 | S, p< 0.05 |
| Pseudomonas aeruginosa | 7.5μ 0.25 | 0 | 7.8μ 0.08 | 9.1μ 0.08 | S, p< 0.05 |
| Klebsiella pneumoniae | 0 | 0 | 7.1μ 0.08 | 8.8μ 0.08 | S, p< 0.05 |
| Salmonella typhi | 7.8μ 0.58 | 7.5μ 0.25 | 9.1μ 0.08 | 10.1μ 0.08 | S, p< 0.05 |
| Results | S, p< 0.05 | S, p< 0.05 | S, p< 0.05 | S, p< 0.05 | S, p< 0.05 |
Fraction 73 of the methanol extract was found to possess inhibitory effect only against Gram-negative bacteria tested with maximum activity against S.typhi (9.1 mm) and minimum against K. pneumoniae (7.1mm). However fraction 74 showed antibacterial activity against all the bacterial pathogens studied except S. pyogenes (Table 4). Of the four fractions of methanol and ethanol extract, fraction no 4 was observed to possess broad spectrum antibacterial activity. Similar studies were conducted by Cooposamy and Magwa (3) on the antibacterial activity of different fractions of A. vera gel extracts. However, the data could not be compared because the method of evaluation of antibacterial activity was different from the present study.
Antibacterial Compounds from Aloe vera gel extracts
The fractions (73, 74, C and D), exhibiting maximum antibacterial activity, were identified as Pyrocatechol (Fig. 1), Cinnamic acid (Fig. 2), p-coumaric acid (Fig. 3) and Ascorbic acid (Fig. 4).
Figure 1.
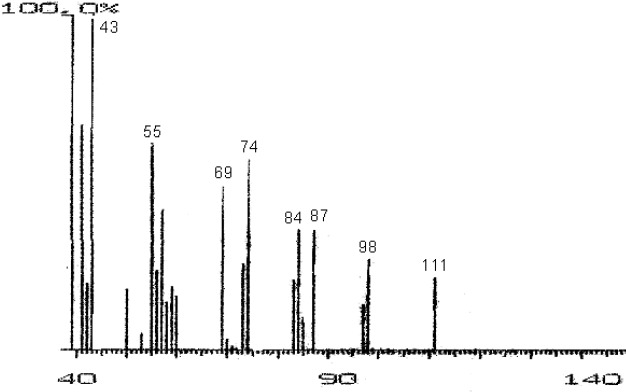
Mass Spectrum of Fraction NO. 73
Figure 2.
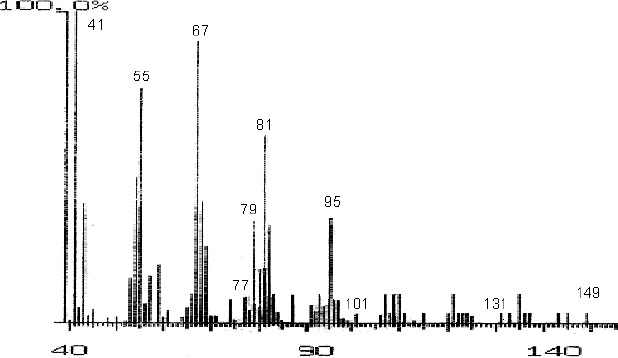
Mass Spectrum of Fraction No. 74
Figure 3.
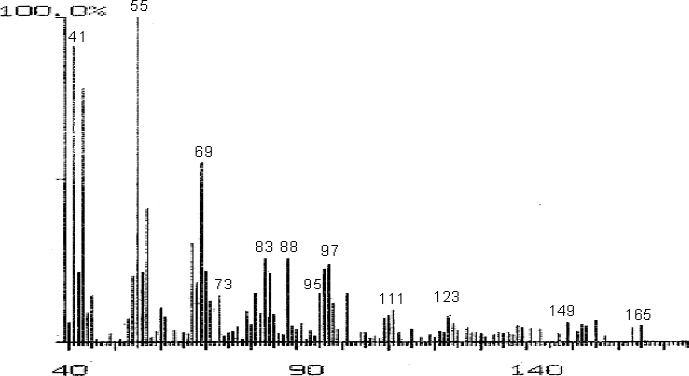
Mass Spectrum of Fraction No. C
Figure 4.
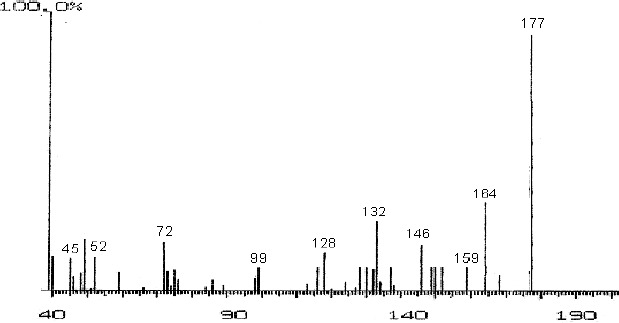
Mass Spectrum of Fraction No. D
The presence of pyrocatechol (Fig. 5) in Aloe vera, already reported by Kametani et al. (14), is comparable with the present study. Further, the antimicrobial action of pyrocatechol was illustrated by Cowan (4). Pyrocatechol is a hydroxylated phenol, known to be toxic to micro-organisms. The site(s) and number of hydroxyl groups on the phenol group are thought to be related to their relative toxicity to micro-organisms and the increase in hydroxylation which further results in increase in toxicity. Further, phenolics also act by denaturing proteins and disrupting cell membranes. They act as a disinfectant, as a tuberculocidal agent, are effective in presence of organic material and remain active on the surfaces even long after application. The presence of cinnamic acid (Fig. 6) in A. vera gel is comparable with Duke’s Phytochemical Databases (7). Further, the antibacterial action of cinnamic acid is comparable with the findings of Kouassi and Shelef (15). They suggested that cinnamic acid inhibits glucose uptake and ATP production in the resting cells of bacteria. The antibacterial activity of p-coumaric acid (Fig. 7) observed in the present study is in agreement with the findings of others studies (2, 19, 29, 30). The compound is reported to increase the lag phase of the micro-organisms (2) and is also able to inhibit the enzymatic activity of the micro-organisms (30). Similarly the presence of ascorbic acid (Fig. 8) in A. vera gel is confirmed with Duke’s Phytochemical Databases (7). The antibacterial activity of ascorbic acid is in agreement with the studies of Vilter (25) and Fite et al. (8). Ascorbic acid may inhibit micro-organisms by interfering with their cell membranes, enzymatic activity, or genetic mechanisms (9).
Figure 5.
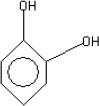
Structure of Pyrocatechol
Figure 6.
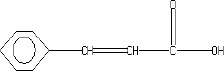
Structure of Cinnamic acid
Figure 7.

Structure of p coumaric acid
Figure 8.
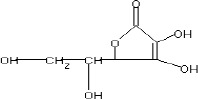
Structure of Ascorbic acid
The unmatched data of various fractions could be due to the effects of the solvents used during the analysis of the sample. Further, slight variations in the peaks are observed and are within the acceptable limits. Moreover the higher range of mismatch in the fraction no. D could be due to the presence of impurities in the sample during handling and processing.
As global antibiotic resistance by bacteria is becoming an interesting public health concern and the race to discover the new antibacterial agent is on, Aloe vera gel along with its identified compounds with promising antibacterial activity could be used as an alternative herbal remedy. Further, these compounds also have been reported to have a number of other advantages on human health along with little side effects in the overdoses. Hence, it can be concluded that the compounds isolated from Aloe vera gel extracts could be recommended for human trials (in proper dosage) against different bacterial pathogens.
REFERENCES
- 1.Agarry O.O., Olaleye M.T., Bello Michael .O. Comparative Antimicrobial Activities of Aloe vera gel and leaf. Afr. J. Biotechnol. 2005;4(12):1413–1414. [Google Scholar]
- 2.Baranowski J.D., Davidson P.M., Nagel C.W., Branen A.L. Inhibition of Saccharomyces cerevisiae by naturally occurring hydroxycinnamates. J. Food Sci. 1980;45(3):592–594. [Google Scholar]
- 3.Coopoosamy R.M., Magwa M.L. Antibacterial Activity of aloe emodin and aloin A isolated from Aloe excelsa. Afr. J. Biotechnol. 2006;5(11):1092–1094. [Google Scholar]
- 4.Cowan M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999;12(4):564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crosswhite .S., Crosswhite C.D. Aloe vera plant symbolism at the threshing floor. Desert plants. 1984;6:43–50. [Google Scholar]
- 6.Davis H.R. New York.: Published by Vantage Press; 1997. Aloe vera: A scientific approach. pp. 3–5. [Google Scholar]
- 7.Duke J.A. Boca Raton, FL.: CRC Press.; 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. pp. 320–340. [Google Scholar]
- 8.Fite A., Dykhuizen R., Litterick A., Golden M., Leifert C. Effects of ascorbic acid, glutathione, thiocyanate and iodide on antimicrobial activity of acidified nitrite. Antimicrob. Agents Chemother. 2003;48(2):655–658. doi: 10.1128/AAC.48.2.655-658.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frazier W.C., Westhoff D.C. Food Microbiology. Fourth edition. Vol. 24. New York, America.: Tata McGraw Hill Publications.; 1995. Food Borne Illness. pp. 434–435. [Google Scholar]
- 10.Ghosh S., Playford R.J. Bioactive natural compounds for the Treatment of Gastrointestinal disorders. Clin. Sci. (Great Britain). 2003;104:547–556. doi: 10.1042/CS20030067. [DOI] [PubMed] [Google Scholar]
- 11.Gur D., Ozalp M., Sumerkan ., Kaygusuz A., Toreci K., Koksal I., Over U., Soyletir G. Relevance of antimicrobial resistance in Haemophilus influenzae, Klebsiella pneumoniae, Moraxella catarrhalis and Streptococcus pyogenes: results of a multicentre study in Turkey. Int. J. Antimicrob. Agents. 2002;19:207–211. doi: 10.1016/s0924-8579(02)00003-1. [DOI] [PubMed] [Google Scholar]
- 12.Hurley J.C. Antibiotic Induced Release of Endotoxin: a reappraisal. Clin.Infect. Dis. 1992;15:840–854. doi: 10.1093/clind/15.5.840. [DOI] [PubMed] [Google Scholar]
- 13.Jack R.W., Tagg J., Ray B. Bacteriocins of Gram-positive bacteria. Microbiol. Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kametani S., Yuasa A.K., Kikuzaki H., Kennedy D.O., Honzawa M., Yuasa M. Chemical constituents of Cape Aloe and their synergistic growth inhibiting effect on Ehrlich Ascites Tumor Cells. Biosci. Biotechnol. Biochem. 2007;71(5):1220–1229. doi: 10.1271/bbb.60659. [DOI] [PubMed] [Google Scholar]
- 15.Kouassi Y., Shelef L.A. Inhibition of Lysteria monocytogenes by cinnamic acid: possible interaction of the acid with cysteinyl residues. J. Food Safety. 1998;18(3):231–242. [Google Scholar]
- 16.Lawless J., Allan J. Aloe vera natural wonder cure. London, United Kingdom.: Thorsons, Publishing Ltd.; 2000. The Clinical Composition of Aloe vera; pp. 161–171. [Google Scholar]
- 17.Marr C., Bent S. An evidence based review of the ten most common herbs. West J. Med. 1999;171:168–171. [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez M.H., Betancourt J., Alonso – Gonzalez N., Jauregui A. Screening of some Cuban medicinal plants for antimicrobial activity. J. Ethnopharmacol. 1996;52:171–174. doi: 10.1016/0378-8741(96)01405-5. [DOI] [PubMed] [Google Scholar]
- 19.Mokbel M.S., Suganuma T. Antioxidant and antimicrobial activities of the methanol extracts from pummelo (Citrus grandis Osbek) fruit albedo tissues. Eur. Food. Res. Technol. 2006;6:217–232. [Google Scholar]
- 20.Morton J.F. Folk uses and commercial exploitation of aloe leaf pulp. Econ. Bot. 1961;15:311–319. [Google Scholar]
- 21.Okamura N., Hine N., Harade S., Fujioka T., Mihashi K., Yagi A. Three chromone components from Aloe vera leaves. Phytochem. 1996;13:495–498. [Google Scholar]
- 22.Pawar V.C., Bagatharia S.B., Thaker V.S. Antibacterial activity of Aloe vera leaf gel extracts against Staphylococcus aureus. Indian J. Microbiol. 2005;45(3):227–229. [Google Scholar]
- 23.Pugh N., Ross S.A., ElSohly M.A., Pasco D.S. Characterization of Aloeride, a new high molecular weight polysaccharide from Aloe vera with potent immunostimulatory activity. J. Agri. Food. Chem. 2001;49(2):1030–1034. doi: 10.1021/jf001036d. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds T., Dweck A.C. Aloe vera leaf gel: a review update. J. Ethnopharmacol. 1999;68:3–37. doi: 10.1016/s0378-8741(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 25.Speranza G., Dada G., Lunazzi L., Gramatica P., Manitto P. A C – Glucosylated 5 Methylchromone from Kenya Aloe. Phytochem. 1986;25(9):2210–2222. [Google Scholar]
- 26.Speranza G., Gramatica P., Dada G., Manitto P. Aloresin C, A Bitter C, O-Diglucoside from Cape Aloe, Phytochem. 1985;24(7):1571–1573. [Google Scholar]
- 27.Urch D. Aloe vera nature’s gift. Bristol, United Kingdom: Blackdown Publication; 1999. Aloe vera the plant. pp. 8–17. [Google Scholar]
- 28.Vilter R.W. Nutritional aspects of ascorbic acid: uses and abuses (Nutrition in Medicine). West. J. Med. 1980;133:485–492. [PMC free article] [PubMed] [Google Scholar]
- 29.Waller G.R., Magiafico S., Ritchey C.R. A Chemical Investigation of Aloe barbadensis miller. Proc. Okla. Acad. Sci. 1978;58:69–79. [Google Scholar]
- 30.Weir T.L., Park S.W., Vivanco J.M. Biochemical and Physiological mechanisms mediated by allelochemicals. Curr. Opinion Plant Biol. 2004;7:472–479. doi: 10.1016/j.pbi.2004.05.007. [DOI] [PubMed] [Google Scholar]


