Abstract
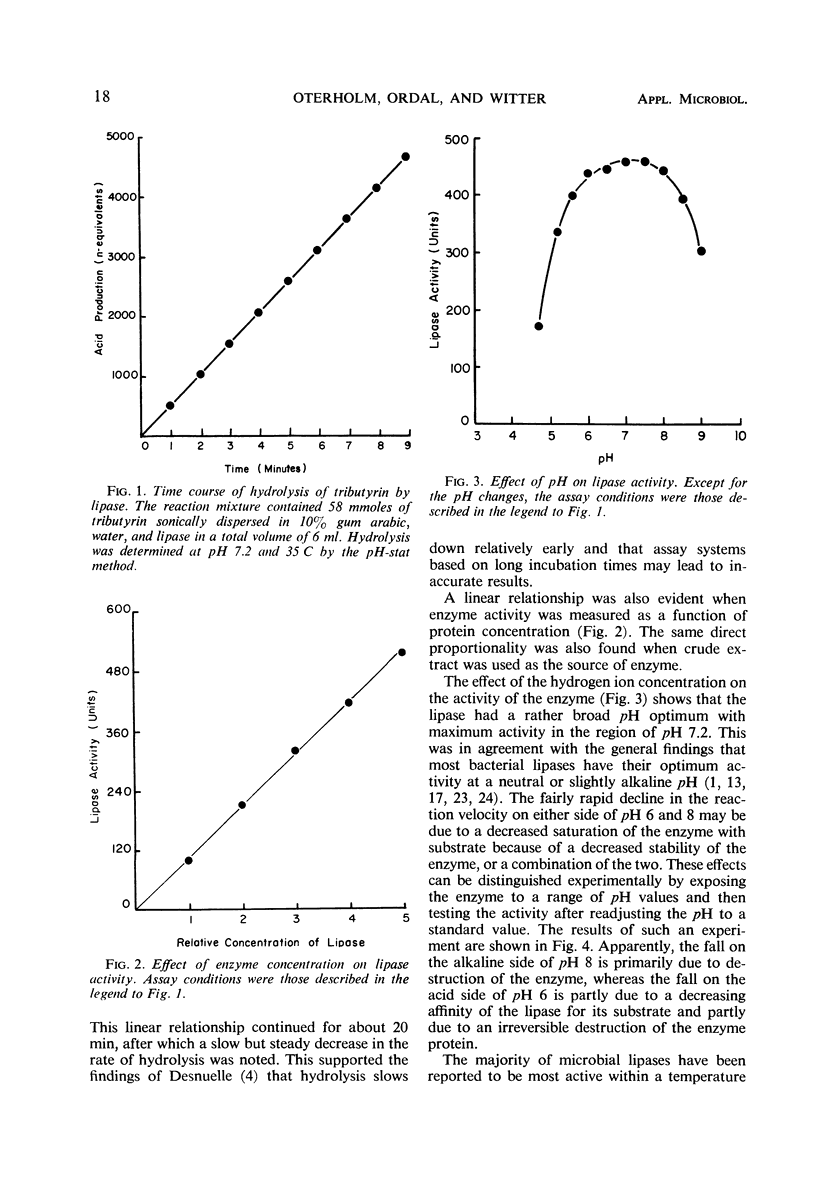
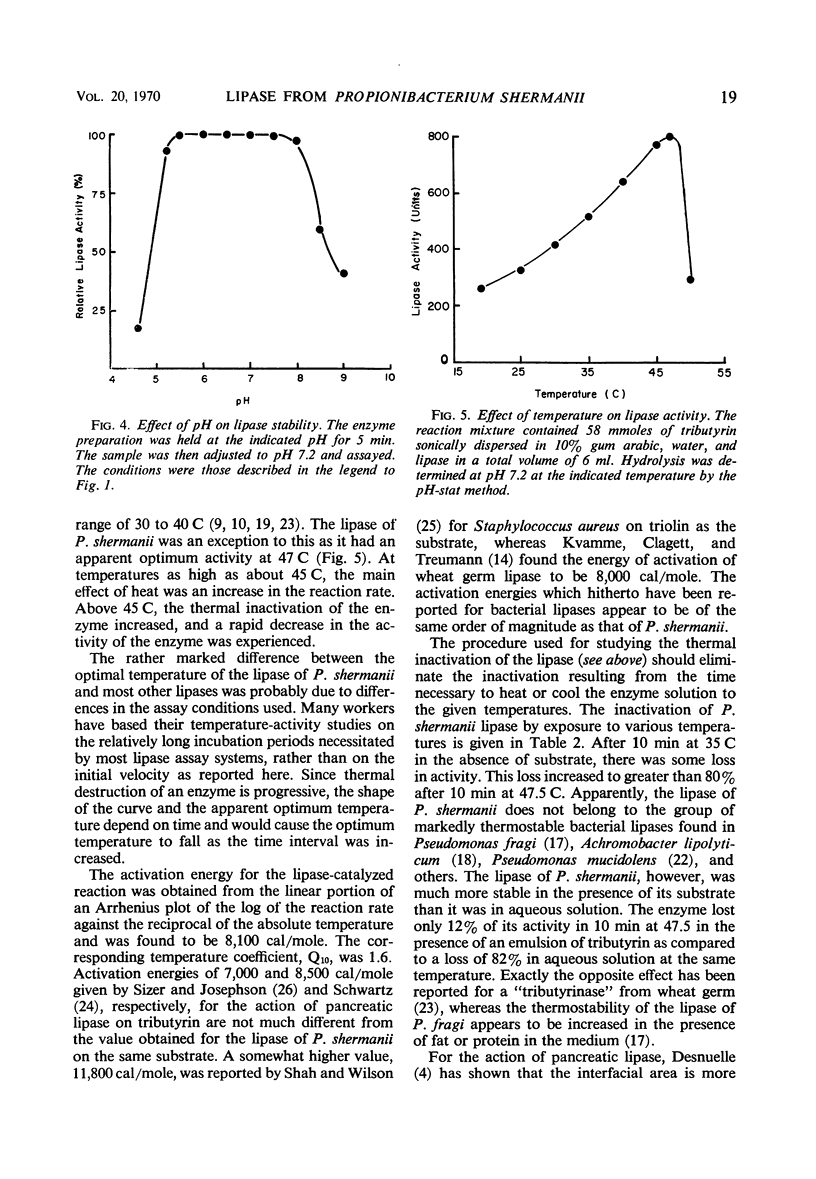
An intracellular glycerol ester hydrolase (lipase) from Propionibacterium shermanii was recovered from cell-free extracts and purified by ammonium sulfate precipitation, gel filtration, and ion-exchange chromatography on diethylaminoethylcellulose. Maximum enzyme activity was observed at pH 7.2 and 47 C when an emulsion of tributyrin was used as substrate. The enzyme was stable between pH 5.5 and 8. Heating the enzyme solution at 45 C for 10 min resulted in a 75% decrease in activity. Maximum rate of hydrolysis of triglycerides was observed on tripropionin, followed in order by tributyrin, tricaproin, and tricaprylin. The lipase was strongly inhibited by mercury and arsenicals, but specific sulfhydryl reagents had little or no inhibiting effect on the enzyme activity. The enzyme also showed some esterase activity, but the hydrolysis of substrates in solution was small as compared to the hydrolysis of substrates in emulsion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN S. H., KELLERMEYER R. W., STJERNHOLM R. L., WOOD H. G. PURIFICATION AND PROPERTIES OF ENZYMES INVOLVED IN THE PROPIONIC ACID FERMENTATION. J Bacteriol. 1964 Jan;87:171–187. doi: 10.1128/jb.87.1.171-187.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford J. A., Pierce D. A., Suggs F. G. Activity of microbial lipases on natural fats and synthetic triglycerides. J Lipid Res. 1964 Jul;5(3):390–394. [PubMed] [Google Scholar]
- DESNUELLE P., CONSTANTIN M. J., BALDY J. Technique potentiométrique pour la measure de l'activité de la lipase pancréatique. Bull Soc Chim Biol (Paris) 1955;37(2-3):285–290. [PubMed] [Google Scholar]
- DESNUELLE P. Pancreatic lipase. Adv Enzymol Relat Subj Biochem. 1961;23:129–161. doi: 10.1002/9780470122686.ch4. [DOI] [PubMed] [Google Scholar]
- DRUMMOND M. C., TAGER M. Enzymatic activity of staphylocoagulase. I. Characterization of an esterase associated with purified preparations. J Bacteriol. 1959 Sep;78:407–412. doi: 10.1128/jb.78.3.407-412.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORBACH G., DEDIC G., LORENZ K. Zur Anreicherung und Wirksamkeitsbestimmung von Bakterienlipasen. Arch Mikrobiol. 1955;21(3):237–247. [PubMed] [Google Scholar]
- KVAMME O. J., CLAGETT C. O., TREUMANN W. B. Kinetics of the action of the sodium salt of 2,4-dichlorophenoxyacetic acid on the germ lipase of wheat. Arch Biochem. 1949 Dec;24(2):321–328. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lu J. Y., Liska B. J. Lipase from Pseudomonas fragi. I. Purification of the Enzyme. Appl Microbiol. 1969 Jul;18(1):104–107. doi: 10.1128/am.18.1.104-107.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'LEARY W. M., WELD J. T. LIPOLYTIC ACTIVITIES OF STAPHYLOCOCCUS AUREUS. I. NATURE OF THE ENZYME PRODUCING FREE FATTY ACIDS FROM PLASMA LIPIDS. J Bacteriol. 1964 Nov;88:1356–1363. doi: 10.1128/jb.88.5.1356-1363.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oterholm A., Ordal Z. J., Witter L. D. Glycerol ester hydrolase activity of lactic acid bacteria. Appl Microbiol. 1968 Mar;16(3):524–527. doi: 10.1128/am.16.3.524-527.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINHEIRO A. J., LISKA B. J., PARMELEE C. E. HEAT STABILITY OF LIPASES OF SELECTED PSYCHROPHILIC BACTERIA IN MILK AND PURDUE SWISS-TYPE CHEESE. J Dairy Sci. 1965 Jul;48:983–984. doi: 10.3168/jds.s0022-0302(65)88372-2. [DOI] [PubMed] [Google Scholar]
- ROTTEM S., RAZIN S. LIPASE ACTIVITY OF MYCOPLASMA. J Gen Microbiol. 1964 Oct;37:123–134. doi: 10.1099/00221287-37-1-123. [DOI] [PubMed] [Google Scholar]
- SHAH D. B., WILSON J. B. EGG YOLK FACTOR OF STAPHYLOCOCCUS AUREUS. II. CHARACTERIZATION OF THE LIPASE ACTIVITY. J Bacteriol. 1965 Apr;89:949–953. doi: 10.1128/jb.89.4.949-953.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLS E. D. The relation of metals and -SH groups to the activity of pancreatic lipase. Biochim Biophys Acta. 1960 Jun 3;40:481–490. doi: 10.1016/0006-3002(60)91389-5. [DOI] [PubMed] [Google Scholar]
- Wills E. D. Lipases. Adv Lipid Res. 1965;3:197–240. doi: 10.1016/b978-1-4831-9939-9.50012-7. [DOI] [PubMed] [Google Scholar]


