Abstract
Lovastatin, an inhibitor of HMG-CoA reductase, was produced by solid state fermentation (SSF) using a strain of Aspergillus terreus UV 1718. Different solid substrates and various combinations thereof were evaluated for lovastatin production. Wheat bran supported the maximum production (1458 ± 46 µg g-1 DFM) of lovastatin. Response surface methodology (RSM) was applied to optimize the medium constituents. A 24 full-factorial central composite design (CCD) was chosen to explain the combined effects of the four medium constituents, viz. moisture content, particle size of the substrate, di -potassium hydrogen phosphate and trace ion solution concentration. Maximum lovastatin production of 2969 µg g-1 DFM was predicted by the quadratic model which was verified experimentally to be 3004 ± 25 μg g-1 DFM. Further RSM optimized medium supplemented with mycological, peptone supported highest yield of 3723.4±49 µg g-1 DFM. Yield of lovastatin increased to 2.5 fold as with compared to un-optimized media.
Keywords: lovastatin, response surface methodology, solid state fermentation, Aspergillus terreus
Introduction
Lovastatin, a potent drug for lowering blood cholesterol, competitively inhibits the rate-limiting enzyme of cholesterol biosynthesis, 3-hydroxy-3methyl glutaryl coenzyme A (HMG-CoA) reductase (1). Besides, it has clear beneficial evidence on stroke and it has shows in vivo tumor suppression by inhibiting the synthesis of non-sterol isoprenoid compounds (9,16). Lovastatin was the first statin to be approved by US FDA as a hypocholestremic drug (13). Although several microorganisms have been reported to produce lovastatin, only strains of A. terreus have been successfully implemented for large-scale production (10) and most of the literature deals with this species (5,7,17). However, Szakacs et al . (20) explored the possibility of lovastatin production from SSF by screening various strains of Aspergillus species for production of lovastatin in SSF and submerged fermentation (SmF). Lian et al . (12) used rice as a substrate for production of lovastatin by SSF. Lovastatin production by SSF using Monscus. purpureus CCRC 31615 (19), M. purpureus NTU 601 (21), M. pilosus M12–69 (6), and M. ruber (22), are also reported in literature.
The application of statistical experimental design techniques in fermentation process development can result in improvement of product yield; reduce process variability, sgive closer confirmation of the output response to nominal and reduce overall costs. Conventional practice of single factor reduce optimization by maintaining other factors involved at an unspecified constant level does not depict the combined effect of all the factors involved. This method is also a time consuming and requires a number of experiments to determine optimum levels, which are unreliable. These limitations can be eliminated by optimizing all the affecting parameters collectively by RSM. RSM can be used to evaluate the relative significance of several factors even in the presence of complex interactions (3,15). There are also some reports on RSM optimization of submerged fermentation using A. terreus (4,11).
There is scarcity of literature on production of lovastatin by SSF using A. terreus . Moreover, SSF is advantageous due to low production cost and high productivity. The present work investigates screening of different solid substrates for the production of lovastatin by SSF followed by optimization of the inoculum size. Subsequently RSM was applied to optimization media components and culture condition.
MATERIALS AND METHODS
Microorganism and media components
Aspergillus terreus UV 1718 is a UV mutant of Aspergillus terreus ATCC 20542, was a gift sample from an Indian pharmaceutical company. A. terreus UV 1718 was one of the mutants from its strain improvement program. Culture was maintained on slants of medium (yeast extract 4 g L-1, malt extract 1 g L-1, dextrose 4 g L-1, agar 2 g L-1), at 4°C and sub-cultured every 15 days.
Yeast extract, malt extract, dextrose and agar were procured from Hi-media Laboratories, Mumbai, India. MgSO4·7H2O, FeSO4·7H2O, K2HPO4, NaCl, ZnSO4·4H2O, glacial acetic acid, HCl, NaOH, MnSO4, boric acid, borax and solvents like HPLC grade acetonitrile, methanol, ethyl acetate, butyl acetate, benzene, chloroform etc. were obtained from Merck India Ltd. Mumbai. All solvents used were of AR grade except HPLC grade acetonitrile. Standard lovastatin was gift from Biocon Ltd. India. Wheat bran, sugar cane bagasse, orange peel, orange pulp, gram bran, corn hull, cottonseed oil cake, groundnut oil cake and rice husk were collected from local market.
Influence of various substrates on lovastatin production
Experiments were performed using different solid substrates like agricultural wastes (wheat bran, corn hull and rice husk), fruit juice wastes (sugarcane bagasse, orange peel and orange pulp) and edible oil industries waste (cottonseed oil cake and groundnut oil) to check their suitability for lovastatin production. Different cake combination of these substrates was also evaluated such as wheat bran and corn hull (1:1), wheat bran and gram bran (1:1), corn hull, and gram bran (1:1) and orange peel and pulp (1:1). All the substrates used were of the same particle size (0.8 – 0.95 mm), except cottonseed oil cake; due to fibrous nature of the cake it was impossible to have a defined particle size. 5 g accurately weighed dry solid substrates were taken in different conical flasks and sealed with cotton plug, and autoclaved After cooling, the flasks were inoculated with 20% v/w of spore suspension (1 x 108 spore mL-1) and subsequently adjusted at 70% moisture content with distilled water of pH 6. After moisture adjustment, each flask was thoroughly mixed with glass rod aseptically and incubated in humidity controlled chamber at 28°C, 80% RH for 10 days.
Production profile of lovastatin
5 g dried wheat bran of particle size (0.8 – 0.95 mm) was transferred to each of conical flasks, sealed with cotton plug, and autoclaved. After cooling, 20% v/w of inoculum (1 x 108 spores mL-1) was inoculated and subsequently adjusted at 70% moisture content with distilled water of pH 6. All other conditions were maintained as previously described. Each day one conical flask was taken and dried at 50°C, 48 h for 10 days. The resultant dried fermented matter was analyzed for lovastatin content.
Determination of optimum inoculum size
A well-sporulated slant of mutant strain of A. terreus UV 1718 was added with sterilized 0.1% tween-80 solution, scratched with a nicrome loop, after which the suspension was filtered aseptically with the sterilized muslin cloth to separate mycelium and the spores. Filtrate obtained was centrifuged at 10,000 rpm and the pellet of the spore was re-suspended in distilled water and mixed using a cyclomixer. Spore count of the suspension was measured using a haemocytometer and adjusted to 1 × 107, 2.5 × 107, 5 × 107, 7.5 × 107 and 1 × 108 spore mL-1. 20% inoculum of each dilution were inoculated to the 5 g wheat bran and adjusted to 70% moisture level, mixed with glass rod and incubated for 3 days. At the end of the fermentation, lovastatin content was analyzed.
Media optimization by response surface methodology (RSM)
Experimental design was formulated according to central composite design of RSM using DESIGN EXPERT software 6.0.10 trial version (Stat-Ease, Minneapolis, USA) for selected four nutrient parameters viz. moisture content, particle size of the substrate, K2HPO4 (2 g L-1) and trace ion solution (0.5 g L-1 MgSO4·7H2O, 0.5 g L-1 NaCl, 0.5 g L-1 MnSO4, 3.4 mg L-1 ZnSO4·4H2O, 5 mg L-1 FeSO4·7H2O, 2 mg L-1 CoCl2·6H2O and 1.6 mg L-1 MnSO4) (Table 1). A set of 30 experiments was required with each variable being at five levels (α = 2). All the flasks were incubated for 3 days. The relation between the coded values and actual values, independent variables and the response were calculated according to equations given by (14).
Table 1.
Value of coded and actual factors for the CCD matrix.
| Nutrient parameter | Levelsa | |||||
|---|---|---|---|---|---|---|
| -2 | -1 | 0 | 1 | 2 | ||
| (A) Moisture content (%) | 50 | 60 | 70 | 80 | 90 | |
| (B) Particle size (mm) | (0.175) | (0.35) | (0.525) | (0.7) | (0.875) | |
| (C) K2HPO4 (% v/w) | 0 | 10 | 20 | 30 | 40 | |
| (D) Trace ion solution (% v/w) | 0 | 10 | 20 | 30 | 40 | |
Values in parentheses are arithmetic mean of particle size.
The relative effects of two variables on response were identified from three-dimensional plots. An optimum value of the variables for maximum production of lovastatin was determined by point prediction tool of the software.
Effect of supplementation of carbon and nitrogen sources
To further enhance the production of lovastatin various carbon (glucose, lactose, sucrose, and soluble starch at a concentration of 1% w/w) and nitrogen sources (yeast extract, soybean meal, mycological peptone and malt extract at a concentration of 1% w/w) supplemented to the RSM optimized medium, properly mixed and incubated for 3 days.
HPLC analysis
Lovastatin was identified by comparison with original standard kindly provided by Biocon Ltd. India. Lovastatin was quantified on a HPLC system (Model Jasko HPLC system) equipped with UV detector and a Hamilton C18 column (250 × 4.6 mm ID, 5 μm) and an eluent comprising acetonitrile and 0.1% phosphoric acid (60:40). The flow rate used was 1 mL min-1; the injection volume was 20 μl. The chromatogram was recorded at 238 nm. Data acquisition and analysis were done on PC based software. For conversion of lovastatin to hydroxy acid lovastatin, 20 mg of lovastatin powder was suspended in 25 mL methanol and 0.025 N NaOH and incubated in orbital incubator shaker at 45°C, 100 rpm for 30 min. After completion of reaction, pH of the solution was adjusted to 7.7 using 0.1 N HCl. By diluting above solution standard plot was prepared by diluting above solution (18). Lovastatin and the corresponding hydroxy acid lovastatin were identified by their retention time. The concentration of lovastatin and hydroxy acid lovastatin were added and reported as yield. Fermented matter was dried in oven at 50–55°C for 48 h, crushed to powder in mortar-pastel. 2 g of the powdered material was taken in 250 mL Erlenmeyer flask containing acetonitrile-water mixture (1:1 v/v) of pH 7 and sonicated for 5 min. After sonication flask was extracted in rotary shaker for 2 h, centrifuged at 10,000 rpm for 10 min. Lovastatin and hydroxy acid lovastatin in the clear supernatant was estimated by HPLC as described earlier.
RESULTS AND DISCUSSION
Influence of various substrates on lovastatin production
The use of inexpensive substrates for the production of lovastatin has combined benefit of utilizing a low-grade substrate while producing a commercially valuable product. Wheat bran yielded maximum lovastatin of 1458 ± 46 µg g-1 of dried fermented matter (DFM) among all substrates used (Fig. 1); this result is in accordance with that of Szakacs et al . (20) who also found wheat bran to be a suitable substrate for production of lovastatin. Gram husk and combination of wheat bran & gram husk also yielded equally good amount of lovastatin of 1305 ± 42 and 1352 ± 41 µg g-1 DFM respectively. Among these substrates, sugarcane bagasse and orange peel & orange pulp (1:1) showed low growth as well as low production of lovastatin 66 ± 12 and 121 ± 12 µg g-1 DFM, respectively.
Figure 1.
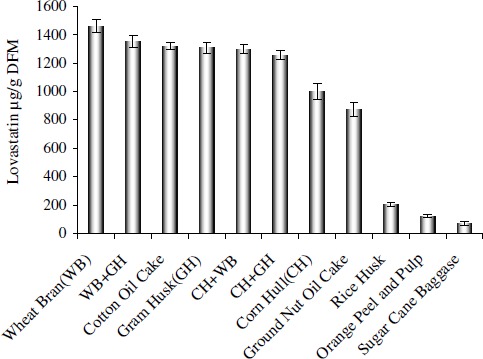
Evaluation of various substrates as SSF matrix for production of lovastatin.
Production profile of lovastatin using wheat bran as substrate
For further study, wheat bran was taken as a substrate for the mutant strain of A. terreus ATCC 20542. Here The production profile of the lovastatin was carried out. Production of the lovastatin was observed even after first day of the incubation. The maximum lovastatin concentration of 1685 ± 72 µg g-1 DFM was obtained just after day 3 of incubation (Fig. 2). There was a rapid rise in lovastatin production between days 2 and 3, after which the lovastatin concentration gradually fell up to day 6 and there after remained almost constant until the day 10 of fermentation.
Figure 2.
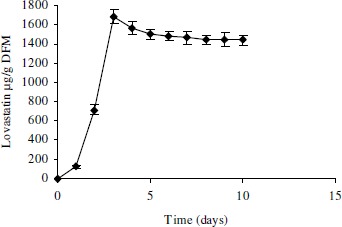
Production profile of lovastatin using wheat bran as a substrate.
Determination of optimum inoculum size
A spore count of 5 × 107spore mL-1 (20% v/w) was found to be optimum with the production of 1845 ± 38 µg g-1 DFM of lovastatin (Fig. 3). Higher inoculum size decreased the lovastatin production marginally.
Figure 3.
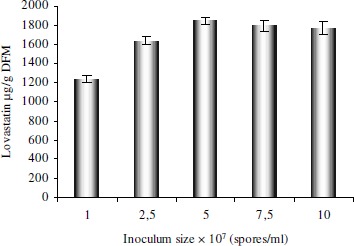
Determination of optimum inoculum size for production of lovastatin by SSF.
Media Optimization by RSM
The individual and interactive effects of these nutrient variables were studied by conducting the fermentation run at randomly selected different levels of all four parameters. The response was measured in terms of lovastatin production. The results of experimental data and predicted by quadratic model are listed in Table 2. The ANOVA of the quadratic model shows the model F-value of 13.89 (Table 3). Model F-value is calculated as ratio of mean square regression and mean square residual. Model P-value (Prob > F) is very low (0.0001). This signifies that the model is significant. The mathematical model is very reliable with a R2 value of 0.928. The closer the R2 value is to 1, the better is the fit of the model is fit to experimental data, the less is the distance between the predicted and the observed values. The P values were used as a tool to check the significance of each of the coefficients, which, in turn are necessary to understand the pattern of the mutual interactions between the test variables. The smaller the magnitude of P , the more significant is the corresponding coefficient. Values of P less than 0.05 indicate model terms are significant. The coefficient estimates and the corresponding P values suggest that, among the test variables used in the study, A (moisture content), B (particle size) and D (trace ion solution) are significant model terms. Particle size and moisture content ( P < 0.0002) had the largest effect on lovastatin production, followed by trace ion concentration ( P < 0.0125). The mutual interaction between any media constituent was also found to be insignificant. Dipotassium hydrogen phosphate concentration was also found to be insignificant. This could be due the fact that wheat bran itself contains phosphates in the form organic salt (2), which might be sufficient for the growth and production of lovastatin from Aspergillus terreus . By analysis on the experimental data, the quadratic model equation is obtained was follows.
Table 2.
Central composite design (CCD) matrix of independent variables in with their corresponding response by experiments and predicted.
| Run | A | B | C | D | Lovastatin yielda (µg g-1 DFM) | |
|---|---|---|---|---|---|---|
| % Moisture content | Particle size (mm) | K2HPO4 (% v/w) | Trace ion conc. (% v/w) | Experimental | Predicted | |
| 1 | 60 | 0.35 | 10 | 10 | 2206 ± 51 | 2532 |
| 2 | 80 | 0.35 | 10 | 10 | 1743 ± 21 | 1916 |
| 3 | 60 | 0.7 | 10 | 10 | 1721 ± 43 | 1736 |
| 4 | 80 | 0.7 | 10 | 10 | 1203 ± 34 | 1353 |
| 5 | 60 | 0.35 | 30 | 10 | 2322 ± 51 | 2248 |
| 6 | 80 | 0.35 | 30 | 10 | 1633 ± 14 | 1941 |
| 7 | 60 | 0.7 | 30 | 10 | 989 ± 54 | 1238 |
| 8 | 80 | 0.7 | 30 | 10 | 1187 ± 23 | 1164 |
| 9 | 60 | 0.35 | 10 | 30 | 2563 ± 39 | 2632 |
| 10 | 80 | 0.35 | 10 | 30 | 1539 ± 45 | 1681 |
| 11 | 60 | 0.7 | 10 | 30 | 2076 ± 44 | 2159 |
| 12 | 80 | 0.7 | 10 | 30 | 1322 ± 60 | 1442 |
| 13 | 60 | 0.35 | 30 | 30 | 2506 ± 21 | 2747 |
| 14 | 80 | 0.35 | 30 | 30 | 2075±35 | 2106 |
| 15 | 60 | 0.7 | 30 | 30 | 2187 ± 31 | 2061 |
| 16 | 80 | 0.7 | 30 | 30 | 1587 ± 22 | 1652 |
| 17 | 50 | 0.525 | 20 | 20 | 1196 ± 47 | 1023 |
| 18 | 90 | 0.525 | 20 | 20 | 263 ± 21 | 262 |
| 19 | 70 | 0.175 | 20 | 20 | 2914 ± 54 | 2525 |
| 20 | 70 | 0.875 | 20 | 20 | 1324 ± 26 | 1276 |
| 21 | 70 | 0.525 | 0 | 20 | 3071 ± 45 | 2751 |
| 22 | 70 | 0.525 | 40 | 20 | 2795 ± 37 | 2678 |
| 23 | 70 | 0.525 | 20 | 0 | 2577 ± 17 | 2233 |
| 24 | 70 | 0.525 | 20 | 40 | 2914 ± 30 | 2821 |
| 25 | 70 | 0.525 | 20 | 20 | 2593 ± 46 | 2472 |
| 26 | 70 | 0.525 | 20 | 20 | 2501 ± 19 | 2472 |
| 27 | 70 | 0.525 | 20 | 20 | 2451 ± 61 | 2472 |
| 28 | 70 | 0.525 | 20 | 20 | 2435 ± 46 | 2472 |
| 29 | 70 | 0.525 | 20 | 20 | 2435 ± 26 | 2472 |
| 30 | 70 | 0.525 | 20 | 20 | 2443 ± 43 | 2472 |
Values are mean ± SD of three or more determinations
Table 3.
Analysis of variance and regression for lovastatin production (quadratic model).
| Source | Sum of squares | Degree of freedom | Mean square | F – value | P > F |
|---|---|---|---|---|---|
| Model error | 12504911 | 14 | 893208 | 13.89 | < 0.0001 |
| Residual error | 964480.2 | 15 | 64298.7 | ||
| Total | 13469391 | 29 |
Coefficient of correlation (R2), 0.928. Coefficient of determination (adjusted R2), 0.861. Coefficient of variance (CV), 12.51%
Lovastatin yield (µg) = 2476.90 – 255.98A – 312.29B – 18.38C + 146.84D – 491.44A2 – 144.04B2 + 59.43C2 + 12.62D2 + 58.26AB + 77.34AC – 83.63AD – 53.52BC + 80.79BD + 99.81CD
Accordingly, with the help of software three dimensional graphs were generated for the pair-wise combination of the four factors, while keeping the other two at their center point levels. Graphs are given here to highlight the roles played by various factors. This model resulted in five response surface graphs. The response surface plots of significant factors for lovastatin production are shown in Fig. 4 (a, b and c). The special features of the RSM tool, “contour plot generation” was analyzed for determining the optimized value of the factors, but it was difficult to analyze all these simultaneously. Hence, point prediction of software was used to determine optimum values of the factors for maximum lovastatin production. Finally the optimum combination values of moisture content 66.8%, particle size 0.35 mm, K2HPO4 30% v/w and trace ion solution 30% v/w were determined. This combination predicted 2969 μg g-1 DFM of lovastatin production. These optimized values of nutrient parameters were validated in a triplicate flask study and an average 3004 ± 25 μg g-1 DFM of lovastatin production was obtained.
Figure 4.
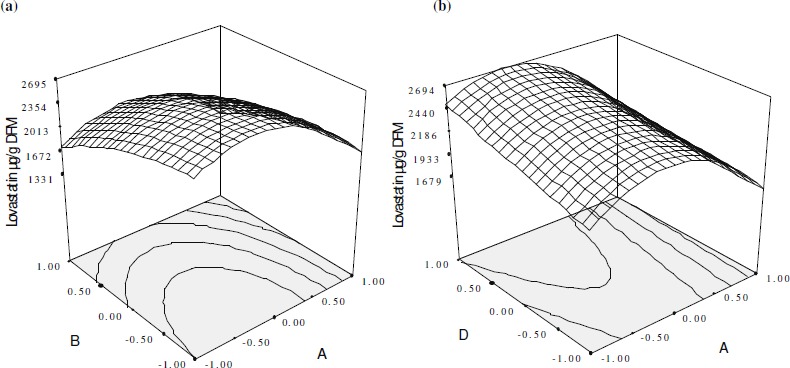
(a and b) Response surface plot showing relative effect of two nutrient parameters on lovastatin production while keeping others at constant level. A, B and D represent % moisture content, particle size and trace ion concentration, respectively.
The proposed model equation illustrates the interaction between two factors; from the equation it was found that % moisture content interacts positively with trace ion and particle size, whereas trace ion, particle size and K2HPO4 showed negative interaction with respect to lovastatin production. From the Fig. 4 (a, b) it is evident that too high and too low % moisture and particle size gave less growth and lower lovastatin production. This phenomenon can be explained by increased voidage volume and decreased surface area with increasing particle size and vice versa . As the moisture content increases, the air present in the void volume decreases, resulting in poor oxygen availability in the processes without forced aeration. Consequently, a certain combination of particle size and moisture content gives the best result in terms of lovastatin yield and biomass. In case of higher particle size, voidage is higher. Hence, the amount of trapped air is also higher but the substrate available is however lesser because the exposed surface area is lower. With lower moisture content (50%), the available oxygen is sufficient, but the water content is not enough to support good metabolic activity and dissipation of the heat generated. This may account for lower lovastatin production and biomass. As the moisture level increased from 60–70%, air present in the void volume was replaced by water, resulting in decrease of available oxygen. Lovastatin content as well as biomass increased as the moisture level increased from 60–70%. This may be because of the adequate amount of oxygen and water present within the substrate bed to support good fungal growth and removal of metabolic heat. However, when the moisture level was increased from 80–90%, biomass as well as lovastatin content decreased. This is presumably due to poor oxygen availability caused by excessive replacement of air by water in the voidage volume. In the present study the optimum particle size was found to be 0.35 mm rather 0.175 mm. At lower particle size the particles are too densely packed with lower voidage volume which retards the oxygen transfer and also increases the localized heat generation in the bed of the solid substrate. This eventually lowers both the growth and lovastatin production.
Effect of supplementation of carbon and nitrogen sources
Nature and type of carbon and nitrogen sources are among the most important factors for any fermentation process. The Organic nitrogen sources are rich in protein and sources of amino acid to the organism. Amino acids are very important for biosynthesis of lovastatin (13). The effect of additional complex organic sources at 1% w/w on lovastatin production is shown in Fig. 5. Mycological peptone and yeast extract yielded higher lovastatin 3723.4±49 and 3405.2±42 µg g-1 DFM, respectively as compared to control. It is indicated that wheat bran alone may not be sufficient to provide nitrogen source. Hence additional nitrogen source was thought to be essential for enhancement of lovastatin production. None of the carbon sources used could increase production of lovastatin. Glucose, lactose and sucrose decreased the yield of lovastatin to 2101±51, 2534±29 and 2435±38 µg g-1 DFM respectively. This could be either due to inhibition of the product formation or catabolic repression, the exact mechanism not being known. This warrants further work to establish the mechanism of inhibition.
Figure 5.
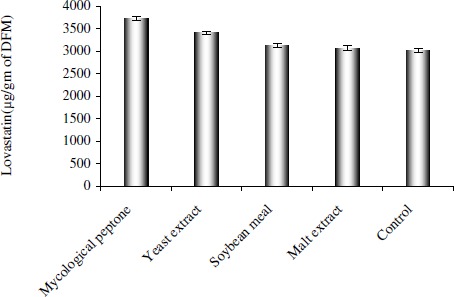
Effect of additional organic nitrogen source at 1% w/w in RSM optimized media on lovastatin production by SSF
The production of lovastatin increased 2.6 fold to 3723.4±49 µg g-1 DFM under optimum conditions as compared to the medium before optimization. This level of production is much higher as compared to lovastatin production by other organisms such as A. terreus , 1.5 mg g-1 DFM (20), A. terreus ATCC 20542, 2.9 mg g-1 DFM (12), M. purpureus CCRC 31615, 0.39 mg g-1 DFM (19), M. purpureus NTU 601, 0.53 mg g-1 DFM (21) and M. pilosus M12–69, 2.52 mg g-1 DFM (6) in SSF.
CONCLUSION
The results obtained in the present paper reveal the potential of SSF as an alterative to the submerged fermentation for the production of lovastatin by using strain of A. terreus UV 1718. To best of our knowledge the yield reported is the highest by strain of A. terreus using SSF.
ACKNOWLEDGEMENTS
The author acknowledge to Department of Biotechnology, Government of India for funding this project. We are also thankful to Biocon Ltd, India for the gift of lovastatin standard.
REFERENCES
- 1.Alberts AW., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E., Patchett A., Monaghan G., Currie S., Stapley E., Alberts-Schonberg G., Hensens O., Hirshfield J., Hoogsteen K., Liesch J., Spinger J. Proc. Natl. Acad. Sci. Vol. 77. USA: 1980. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and cholesterol lowering agents; pp. 3957–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson R.J. The hydrolysis of the organic phosphorus compound of wheat bran by the enzyme Phytase. J. Biol. Chem. 1915;20:483–491. [Google Scholar]
- 3.Box G.E.P., Hunter J.S. Multifactor experimental design for exploring response surfaces. Ann. Math. Stat. 1957;28:195–241. [Google Scholar]
- 4.Casas Lopez J.L., Sanchez Perez J.A., Fernandez Sevill J.M., Acien Fernandez F.G., Molina G.E., Chisti Y. Fermentation optimization for the production of lovastatin by Aspergillus terreus : use of response surface methodology. J. Chem. Technol. Biotechnol. 2004;79:1119–1126. [Google Scholar]
- 5.Casas Lopez J.L., Sanchez Perez J.A., Fernandez Sevilla J.M., Acien Fernandez F.G., Molina G.E., Chisti Y. Production of lovastatin by Aspergillus terreus: effect of the C:N ratio and the principal nutrients on growth and metabolite production. Enzyme Microb. Technol. 2003;33:270–277. [Google Scholar]
- 6.Chen F., Hu X. Study on red fermented rice with high concentration of monacolin K and low concentration of citrinin. Inter. J. Food Microbiol. 2005;103(3):331–337. doi: 10.1016/j.ijfoodmicro.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Hajjaj H., Niederberger P., Duboc P. Lovastatin biosynthesis by Aspergillus terreus in chemically defined medium. Appl Environ Microbiol. 2001;67:2596–2602. doi: 10.1128/AEM.67.6.2596-2602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hebert P.R., Gaziano J.M., Chan K.S., Henneken C.H. Cholesterol lowering with statin drugs, risk of stroke and total mortalityan overview of randomized trails. J. Am. Med. Assoc. 1997;278:313–321. [PubMed] [Google Scholar]
- 9.Jones K.D., Couldwell W.T., Hintor D.R., Su Y.H., He S.K., Anker L., Law R.E. Lovastatin induces growth inhibition and apoptosis in human malignant glioma cells. Biochem. Biophy. Res. Comm. 1994;205:1681–1687. doi: 10.1006/bbrc.1994.2861. [DOI] [PubMed] [Google Scholar]
- 10.Kumar M.S., Jana S.K., Senthil V., Shashanka S., Kumar S.V., Sadhukhan A.K. Repeated fed batch process for improving lovastatin production. Proc. Biochem. 2000;36:363–368. [Google Scholar]
- 11.Lai L.S.T., Pan C.C., Tzeng B.K. The influence of medium design on lovastatin production and pellet formation with a high-producing mutant of Aspergillus terreus in submerged cultures. Proc. Biochem. 2003;38:1317–1326. [Google Scholar]
- 12.Lian W.P., Nan X.Z., Lin C.P. Lovastatin production by Aspergillus terreus in solid-state fermentation. J. Zhejiang Univ. – Science A. 2007;8:1521–1526. [Google Scholar]
- 13.Manzoni M., Rollini M. Biosynthesis and Biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl. Microbiol. Biotechnol. 2002;58:555–564. doi: 10.1007/s00253-002-0932-9. [DOI] [PubMed] [Google Scholar]
- 14.Murat E. Optimization of medium composition for actinorhodin production by streptomyces coelicolor A3(2) with response surface methodology. Proc. Biochem. 2004;39:1057–1062. [Google Scholar]
- 15.Myers R.H., Montgomery D.C. Response surface methodology: process and optimization using designed experiments. 1. Wiley Interscience; 1995. [Google Scholar]
- 16.Newman A., Clutterbuch R.D., Powles R.L., Millar J.L. Selective inhibition of primary acute myeloid leukemia cell growth by lovastatin. Leukemia. 1994;8:2022–2029. [PubMed] [Google Scholar]
- 17.Novak N., Gerdin S., Berovic M. Increased lovastatin formation by Aspergillus terreus using repeated fed-batch process. Biotechnol. Lett. 1997;19:947–948. [Google Scholar]
- 18.Pansuriya R.C., Singhal R.S. J. Food Technol. Biotechnol. In Press; 2009. Supercritical fluid extraction of lovastatin from the wheat bran obtained after solid-state fermentation. [Google Scholar]
- 19.Su Y.C., Wang J.J., Lin T.T., Pan T.M. Production of the secondary metabolites γ-aminobutyric acid and monacolin K by Monascus. J. Ind. Microbiol. Biotechnol. 2003;30:41–46. doi: 10.1007/s10295-002-0001-5. [DOI] [PubMed] [Google Scholar]
- 20.Szakacs G., Morovjan G., Tengerdy R.P. Production of lovastatin by a wild strain of Aspergillus terreus. Biotechnol. Lett. 1998;20:411–415. [Google Scholar]
- 21.Wang J.J., Lee C.L., Pan T.M. Modified mutation method for screening low citrinin-producing strains of Monascus purpureus on rice culture. J. Agriculture Food Chem. 2004;52:6977–6982. doi: 10.1021/jf049783o. [DOI] [PubMed] [Google Scholar]
- 22.Xu B.J., Wang Q.J., Jia X.Q., Sung C.K. Enhanced lovastatin production by solid state fermentation of Monascus ruber. Biotechnol. Biopro. Engg. 2005;10:78–84. [Google Scholar]


