Abstract
Angkak (red mold rice, red yeast rice, Chinese red rice) is a traditional Chinese medicine produced by solid-state fermentation of cooked non-glutinous rice with Monascus species. The secondary metabolite of Monascus species, monacolin K /lovastatin, has been proven to lower blood lipid levels. In this study, a co-culture of Monascus purpureus MTCC 369 and Monascus ruber MTCC 1880 was used for angkak production. Four medium parameters screened by Plackett-Burman design were optimized by response surface methodology for highest lovastatin production in angkak during solid-state fermentation by the co-culture. Maximum lovastatin production of 2.84 mg g-1 was predicted in solid medium containing 20 g rice and 40 ml liquid nutrients medium (malt extract 9.68 g l-1, dextrose 38.90 g l-1, MnSO4.H2O 1.96 g l-1, and MgSO4.7H2O 0.730 g l-1) by point prediction tool of Design Expert 7.1 software (Statease Inc. USA).
Keywords: Angkak, Co-culture, Monascus purpureus, Monascus ruber, Lovastatin, Response surface methodology
INTRODUCTION
Angkak (red mold rice, red yeast rice, Chinese red rice) is a traditional Chinese medicine produced by solid-state fermentation of cooked non-glutinous rice (Orizae sativa L. Gramineae) with Monascus purpureus, M. ruber, M. anka and M. pilosus (3, 6, 9, 14). The angkak has long been recognized as a folk medicine for improving food digestion and blood circulation and for treatment of muscle bruising and dysentery. The manufacturing process for angkak and its therapeutic applications are well documented in the ancient Chinese pharmacopoeia (Ben-Taso-Gum-Mu) (9, 12). Recent chemical investigation and clinical observation show that angkak contains different secondary metabolites: lovastatin (HMG Co A reductatse inhibitor), also known as monacolin K, which lowers blood lipid levels in animal models and in humans, γ-aminobutyric acid (GABA) which has blood pressure lowering effects, dimerumic acid which is antioxidant, and monascin which has anti-inflammatory effects (6,10,11,24).
A previous study on red mold rice production by Monascus species under monoculture conditions showed that secondary metabolites production is greatly affected by fermentation medium, cultivation conditions and types of Monascus species used in the fermentation process (1, 5, 13, 21, 23). Increasing concentration of lovastatin in angkak was a prime area of research in order to produce high quality bio-nutraceutical or health functional food (4, 11, 22). During process optimization, screening or selection of important parameters influencing fermentation productivity is initially carried out by barrowing method or by Plackett-Burman design, and finally the screened parameters are optimized using different advanced statistical techniques (3, 7, 8, 15). Response surface methodology (RSM) is a widely used technique for optimization of fermentation process parameters. RSM based on Box-Behnken’s design has some advantages over other designs like central composite and full factorial design. RSM requires less experiment runs, and is suitable for multiple factor experiments, search for relationships between factors, and for finding the most suitable condition and prediction of response (8, 19).
In nature, solid-state fermentation (SSF) is carried out by mixed or co-cultures of different fungal species. The co-culture of fungi during fermentation may provide help for better biomass and secondary metabolites production. There are several reports of co-culture of fungal species, and mixed cultures have been found to enhance enzyme and organic acid production (2, 16). However, no reports are available on production of lovastatin (monacolin K) by co-culture of different Monascus species under SSF condition. Therefore, the objective of this study was to produce finest quality angkak containing maximum amount of lovastatin by co-culture of two Monascus species.
Two filamentous fungi, Monascus purpureus MTCC 369 and Monascus ruber MTCC 1880, were used together as inoculum for production of angkak. The effects of medium parameters on lovastatin production were studied and screened by Plackett-Burman design (PBD) and their optimum levels were determined by response surface methodology (RSM).
MATERIALS AND METHODS
Chemicals and raw materials
Pure lovastatin was a gift from Ranbaxy Laboratories (New Delhi, India). HPLC grade acetonitrile was purchased from Merck (Bombay, India). Microbiological media and chemicals were purchased from Himedia Laboratories (Bombay, India) and long nonglutinous rice was purchased from a local market of New Delhi, India.
Microorganisms
Cultures of Monascus purpureus MTCC 369 and Monascus ruber MTCC 1880 were obtained from Institute of Microbial Technology (IMTECH), Chandigarh, India. Fungal cultures were maintained routinely on potato dextrose agar (PDA) medium and subcultured in every 30 d interval (3, 19).
Preparation of mixed seed cultures
Spore suspensions of M. purpureus and M. ruber were prepared separately from actively growing slants in sterile water and diluted to a concentration 5.7 × 103 spores ml-1. Spore counting was carried out using a hemocytometer. Spore suspension (15% v/v) (7.5ml) of M. purpureus was inoculated to conical flasks containing 50ml basal medium (100g dextrose, 10g peptone, 2g KNO3, 2g NH4H2PO4, 0.5g MgSO4.7H2O, 0.1g CaCl2 in 1000 ml distilled water; adjusted to pH 6.0) and incubated at 30oC for 48 h in a shaker incubator at 110 rpm (19,21). For preparation of Monascus ruber seed culture, 15% spore suspension (7.5ml) of M. ruber was inoculated to conical flasks containing 50ml of potato dextrose broth (PDB), and incubated at 30oC for 4 days with shaking at 150 rpm (3). Finally the two seed cultures of M. purpureus and M. ruber were mixed at a ratio of 1:1.
Solid -State Fermentation (SSF)
Long grain, non-glutinous rice was purchased from a local market of New Delhi, India and used as substrate for angkak production under solid-state culture. Initially 20 g of pre-soaked rice was transferred to a 250 ml conical flask to which 40 ml of distilled water containing different nutrients (Table 1) was added. The pH of the medium was adjusted to 6.0 with 0.1M HCl or NaOH and autoclaved for 20min at 121oC. The cooled rice-based medium was inoculated with 5ml of the mixed seed cultures of M. purpureus and M. ruber, incubated for 14 days at 30oC and 70% relative humidity (21).
Table 1.
Concentrations of variables of liquid medium in Plackett-Burman design for solid-state fermentation.
| Designation | Variable | Low level (-)Per liter | High level (+)Per liter |
|---|---|---|---|
| X1 | Peptone | 5.0g | 15.0g |
| X2 | Glucose | 80g | 160g |
| X3 | Glycerin | 16ml | 32ml |
| X4 | NaCl | 2.0g | 8.0g |
| X5 | NH4Cl | 2.0g | 8.0g |
| D1 | Dummy 1 | - | - |
| X6 | MgSO4.7H2O | 0.1g | 0.9g |
| X7 | CaCl2.2H2O | 0.0g | 0.6g |
| X8 | MnSO4.H2O | 0.0g | 1.5g |
| X9 | Malt extract | 2.0g | 8.0g |
| D2 | Dummy 2 | - | - |
Extraction of lovastatin from angkak
Angkak (1g) was suspended in 5 ml ethyl acetate and kept in a shaker incubator at 180 rpm and 70oC for 1.5 h. The mixture was centrifuged at 3000g for 8 min and 10 ml of 1% (v/v) trifluoroacetic acid was added to 1ml of the supernatant for lactonization of the lovastatin. The mixture was concentrated at 80oC, mixed with 1ml with acetonitrile and filtered through 0.45μm filter for HPLC (High Performance Liquid Chromatography) analysis (21).
Quantitative analysis of lovastatin
For HPLC analysis the procedure of Samiee et al. for HPLC, with slight modification, was used. Lovastatin concentration was estimated by HPLC (SHIMADZU, Japan) using a 250 mm x 4.6 mm ID Lichrosper® 100 C18 column containing 5μm sized particles and a 20μl loop injector. The mobile phase was acetonitrile:water acidified to the concentration 0.1% (v/v) with ortho-phosphoric acid (65:35 v/v). The flow rate was 1.5 ml min-1. Detection was carried out by a UV-detector at 235 nm (18, 19).
Screening of nutrient parameters
Peptone, glucose, glycerine, NaCl, NH4Cl, MgSO4.7H2O, CaCl2.2H2O, malt extract, and MnSO4.H2O were the nine medium constituents selected for study. The selection of nutrients was done by barrowing methodology (8, 15). The Plackett-Burman experimental design for eleven variables (Table 2) with nine nutritional components (X1 to X9) (independent variables) and two dummy variables (D1 and D2) were used to evaluate the relative importance of the nutrients for high quality angkak production with high quantity of monacolin K (lovastatin). For each nutrient variable two different concentrations [high (+) and a low (-)] were tested (Table 2). Influence of medium variables on lovastatin production was calculated according to standard analysis procedure of Plackett-Burman experimental design (17).
Table 2.
Plackett-Burman experimental design of 12 trials for eleven variables (9 nutrients + 2 dummy) and observed concentration of lovastatin in angkak samples.
| Trial | X1 | X2 | X3 | X4 | X5 | D1 | X6 | X7 | X8 | X | D2 | Mean lovastatin (mg g-1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | - | + | + | + | - | - | - | + | - | 1.060 |
| 2 | - | + | + | - | + | + | + | - | - | - | + | 0.563 |
| 3 | + | - | + | + | - | + | + | + | - | - | - | 0.764 |
| 4 | - | + | - | + | + | - | + | + | + | - | - | 0.450 |
| 5 | - | - | + | - | + | + | - | + | + | + | - | 1.880 |
| 6 | - | - | - | + | - | + | + | - | + | + | + | 1.990 |
| 7 | + | - | - | - | + | - | + | + | - | + | + | 1.780 |
| 8 | + | + | - | - | - | + | - | + | + | - | + | 0.764 |
| 9 | + | + | + | - | - | - | + | - | + | + | - | 1.760 |
| 10 | - | + | + | + | - | - | - | + | - | + | + | 1.080 |
| 11 | + | - | + | + | + | - | - | - | + | - | + | 0.984 |
| 12 | - | - | - | - | - | - | - | - | - | - | - | 0.534 |
Response surface methodology experimental design
In Plackett-Burman experimental design, various carbon, nitrogen and micronutrients were evaluated for their influence in the production of lovastatin during solid-state fermentation by mixed cultures of M. purpureus and M. ruber. Preliminary data of Plackett-Burman experimental design indicated that lovastatin production was greatly influenced by malt extract, dextrose, MnSO4.H2O and MgSO4.7H2O (Table 3). Therefore these four medium parameters were chosen for further optimization by response surface methodology (3, 19, 20). The various levels of nutrients are summarized in Table 4. An experimental design of 29 runs containing 3 central points (Table 5) was made according to Box-Behnken’s response surface design for selected four parameters using Design Expert 7.1 software of Statease Inc. USA. The response was measured in terms of lovastatin concentration in produced angkak. An optimum value of the factors for maximum production of lovastatin was determined by the point prediction tool of the software.
Table 3.
Influence of medium variables on lovastatin production in angkak samples
| Designation | Variable | ?H | ?L | Mean square | Effect | F Value | % Contribution | P value |
|---|---|---|---|---|---|---|---|---|
| X1 | Peptone | 7.112 | 6.497 | 0.034 | 0.102 | 1.214 | 00.968 | 0.072 |
| X2 | Dextrose | 5.677 | 7.932 | 0.421 | -0.375 | 15.035 | 11.900 | |
| X3 | Glycerine | 7.031 | 6.578 | 0.016 | 0.075 | 0.571 | 00.454 | |
| X4 | NaCl | 6.328 | 7.281 | 0.076 | -0.158 | 2.714 | 02.160 | |
| X5 | NH4Cl | 6.717 | 6.892 | 0.002 | -0.029 | 0.071 | 00.061 | |
| X6 | MgSO4.7H2O | 7.307 | 6.302 | 0.085 | 0.167 | 3.035 | 02.400 | |
| X7 | CaCl2.2H2O | 6.718 | 6.891 | 0.002 | -0.288 | 0.071 | 00.076 | |
| X8 | MnSO4.H2O | 7.828 | 5.781 | 0.345 | 0.341 | 12.321 | 09.710 | |
| X9 | Malt extract | 9.550 | 4.059 | 2.510 | 0.915 | 89.642 | 70.700 |
Table 4.
Levels of nutrient parameters used in Box-Behnken’s response surface design
| Nutrient parameter(g l-1) | Levels | ||
|---|---|---|---|
| -1 | 0 | +1 | |
| Malt extract | 07.00 | 10.00 | 13.00 |
| Dextrose | 20.00 | 40.00 | 60.00 |
| MnSO4.H2O | 01.00 | 01.50 | 02.00 |
| MgSO4.7H2O | 00.50 | 01.00 | 01.50 |
Table 5.
Box-Behnken’s design with actual and predicted lovastatin concentrations
| Run | Malt extract (g l-1)Code A | Dextrose (g l-1)Code B | MnSO4.H2O (g l-1)Code C | MgSO4.7H2O(g l-1)Code D | Lovastatin(mg g-1) | |
|---|---|---|---|---|---|---|
| Actual | Predicted | |||||
| 1 | 7 | 20 | 1.5 | 1 | 1.58 | 1.44 |
| 2 | 13 | 20 | 1.5 | 1 | 0.612 | 0.27 |
| 3 | 7 | 60 | 1.5 | 1 | 0.756 | 0.784 |
| 4 | 13 | 60 | 1.5 | 1 | 0.986 | 0.812 |
| 5 | 10 | 40 | 1 | 0.5 | 2.68 | 2.27 |
| 6 | 10 | 40 | 2 | 0.5 | 2.45 | 1.93 |
| 7 | 10 | 40 | 1 | 1.5 | 0.764 | 0.966 |
| 8 | 10 | 40 | 2 | 1.5 | 0.875 | 0.97 |
| 9 | 7 | 40 | 1.5 | 0.5 | 2.58 | 2.49 |
| 10 | 13 | 40 | 1.5 | 0.5 | 0.765 | 1.01 |
| 11 | 7 | 40 | 1.5 | 1.5 | 0.657 | 0.446 |
| 12 | 13 | 40 | 1.5 | 1.5 | 0.654 | 0.78 |
| 13 | 10 | 20 | 1 | 1 | 0.435 | 0.668 |
| 14 | 10 | 60 | 1 | 1 | 1.78 | 1.86 |
| 15 | 10 | 20 | 2 | 1 | 1.78 | 1.75 |
| 16 | 10 | 60 | 2 | 1 | 0.645 | 0.447 |
| 17 | 7 | 40 | 1 | 1 | 1.76 | 1.78 |
| 18 | 13 | 40 | 1 | 1 | 0.784 | 0.667 |
| 19 | 7 | 40 | 2 | 1 | 0.674 | 1.07 |
| 20 | 13 | 40 | 2 | 1 | 0.783 | 1.04 |
| 21 | 10 | 20 | 1.5 | 0.5 | 1.56 | 1.95 |
| 22 | 10 | 60 | 1.5 | 0.5 | 1.24 | 1.62 |
| 23 | 10 | 20 | 1.5 | 1.5 | 0.645 | 0.543 |
| 24 | 10 | 60 | 1.5 | 1.5 | 0.873 | 0.763 |
| 25 | 10 | 40 | 1.5 | 1 | 2.65 | 2.63 |
| 26 | 10 | 40 | 1.5 | 1 | 2.56 | 2.63 |
| 27 | 10 | 40 | 1.5 | 1 | 2.67 | 2.63 |
| 28 | 10 | 40 | 1.5 | 1 | 2.65 | 2.63 |
| 29 | 10 | 40 | 1.5 | 1 | 2.63 | 2.63 |
RESULTS
Among the nine nutrient components used in the Plackett-Burman Experimental Design, malt extract, dextrose, MnSO4.H2O, and MgSO4.7H2O had contributed to a large extent for lovastatin production. Peptone, glycerine, CaCl2.2H2O, and NH4Cl had little impact, while NaCl contributed moderately in production of lovastatin in angkak under the co-culture system.
The predicted and experimental lovastatin concentrations obtained from Box-Behnken’s response surface design in each run are show in Table 5. Results were analyzed using the software Design Expert 7.1 and fitted into a multiple nonlinear regression model, resulting in the following equation for lovastatin production:
Lovastatin (mg g-1) = +2.63-0.285A-0.0275B-0.0832C-0.568D+0.299AB+0.272AC +0.452AD -0.622BC +0.137BD +0.0853CD -0.924A2 -0.884B2 -0.569C2 -0.529D2
This model resulted in six response graphs and a few representative surface graphs with contours of nonlinear regression model for lovastatin production, shown in Fig. 1a, 1b, 1c and 1d. Analysis of variance of regression is summarized in Table 6. The optimum values of medium parameters for maximum lovastatin production were malt extract 9.68 g l-1, dextrose 38.90 g l-1, MnSO4.H2O 1.96 g l-1, and MgSO4.7H2O 0.730 g l-1. These values predicted 2.84 mg g-1 of lovastatin production in angkak under co-culture of M. purpureus and M. ruber. These optimized values of nutrient parameters were validated by solid-state fermentation and an average 2.75 mg g-1 of lovastatin production in angkak was obtained, indicating a 96.83 % validity of the predicted model.
Table 6.
Analysis of variance of the calculated model for lovastatin production
| Regression | |
| Sum of squares | 17.8 |
| df | 14 |
| Mean squares | 1.27 |
| F Value | 12.3 |
| P | < 0.0001 |
| Residual | |
| Sum of squares | 1.45 |
| df | 14 |
| Mean squares | 0.103 |
| Lack of fit test | |
| Sum of squares | 1.44 |
| df | 10 |
| Mean squares | 0.144 |
| F Value | 81.4 |
| P Value | 0.000354 |
| Correlation coefficient (r2) | 0.925 |
| Coefficient of variation (CV %) | 22.5 |
| Adequate precision value | 10.2 (> 4) |
DISCUSSION
Plackett-Burman experimental design and response surface methodology are powerful tools for screening and optimization of medium parameters for lovastatin production under solid-state fermentation by co-culture of M. purpureus and M. ruber.
Malt extract, dextrose, MnSO4.H2O, and MgSO4.7H2O influenced negatively lovastatin production. The model equation indicated that all medium parameters except dextrose and MnSO4.H2O interacted positively with each other in lovastatin production. The simultaneous effects of two medium parameters on lovastatin production while the other two parameters were kept at optimized levels was depicted in the contours and surface graphs. As shown in Figure 1, reducing malt extract, dextrose, MgSO4.7H2O concentrations below the original search levels and increasing MnSO4.H2O concentration above the original levels stimulated production of lovastatin under co-culture of M. purpureus and M. ruber.
Figure 1.
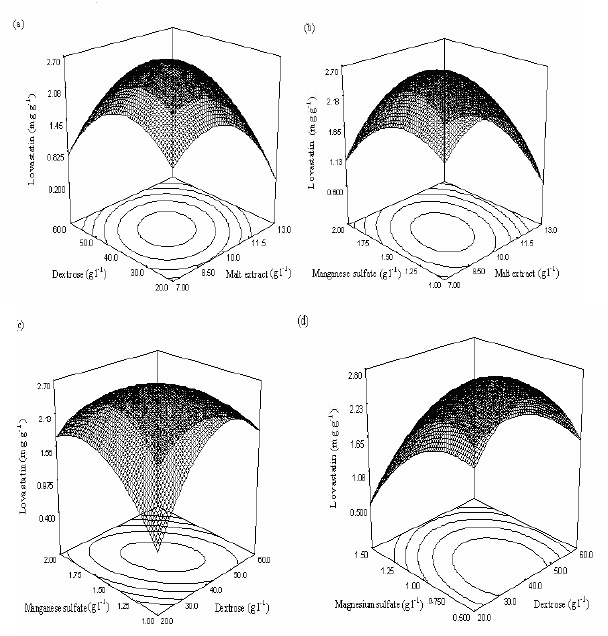
(a-d) Response surface plots showing relative effects of two nutrient parameters on lovastatin production while keeping other two parameters at constant levels.
Co-culture of the two Monascus species resulted in production of 2.75 mg of lovastatin per gram of optimized solid medium in angkak, which is much higher then the lovastatin or monacolin K concentration obtained under mono culture of Monascus pilosus M12-69, Monascus purpureus NTU 601, 301 or Monascus purpureus BCRC 31499, 31504, 31530, 31540, 32966, 32807, 32808, 32809 on different substrates (4). However the lovastatin / monacolin K production in dioscorea by Monascus purpureus NTU 601 was found to be equivalent to lovastatin produced by co-culture of Monascus species in rice (11). This suggests that co-culture or mixed cultures of different Monascus species might result in high quantity of lovastatin or monacolin K in angkak.
REFERENCES
- 1.Babitha, S.; Soccol, C.R.; Pandey, A. (2007). Effect of stress on growth, pigment production and morphology of Monascus sp. in solid cultures. J. Basic Microbiol 47(2), 118-126. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee, R.; Mukherjee, G.; Patra, K.C. (2005). Microbial transformation of tannin–rich substrate to gallic acid through co culture method. Bioresource Technol 96(8), 949-953. [DOI] [PubMed] [Google Scholar]
- 3.Chang, Y.N.; Huang, J.C.; Lee, C.C.; Shih, I.L.; Tzeng, Y.M. (2002). Use of response surface methodology to optimize by Monascus ruber Enzyme Microb. Tech 30(7), 889-894. [Google Scholar]
- 4.Chen, F.; Hu, X. (2005). Study on red fermented rice with high concentration of monacolin K and low concentration of citrinin. Int .J. Food Microbiol 103(3), 331-337. [DOI] [PubMed] [Google Scholar]
- 5.Chiu, C.H.; Ni, K.H.; Guu, Y.K.; Pan, T.M. (2006). Production of red mold rice using a modified Nagata type koji marker. Appl. Microbiol. Biotechnol 73(2), 297-304. [DOI] [PubMed] [Google Scholar]
- 6.Endo, A. (1979). Monacolin K, a new hypocholesterolemic agent produced by a Monascus species. J. Antibiot 32(8), 852-854. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguti, H. Y.; Manrich, E.; Fleuri, L. F.; Sato, H. H. (2005). Production of glucosyltransferase by Erwinia sp. using experimental design and response surface methodology. Braz. J. Microbiol 36 (3), 227-234. [Google Scholar]
- 8.Kennedy, M.; Krouse, D. (1999). Strategies for improving fermentation medium performance: a review. J. Ind. Microbiol. Biotechnol 23(6), 456-475. [Google Scholar]
- 9.Kohama, Y.; Matsumoto, S.; Mimura, T.; Tanabe, N.; Inada, A.; Nakanishi, T. (1987). Isolation and identification of hypotensive principles in red-mold rice. Chem. Pharm. Bull 35(6), 2484-2489. [DOI] [PubMed] [Google Scholar]
- 10.Lee, C.L.; Wang, J.J.; Kuo, S.L.; Pan, T.M. (2006). Monascus fermentation of dioscorea for increasing the production of cholesterol-lowering agents – monacolin K and antiinflammation agent – monascin. Appl. Microbiol. Biotechnol 72(6), 1254-1262. [DOI] [PubMed] [Google Scholar]
- 11.Ma, J.; Li, Y.; Ye, Q.; Li, J.; Hua, Y.; Ju, D.; Zhang, D.; Cooper, R.; Chang, M. (2000). Constituents of red yeast rice, a traditional Chinese food and medicine. J Agric. Food Chem 48(11), 5220-5225. [DOI] [PubMed] [Google Scholar]
- 12.Miyake, T.; Mori, A.; Kii, T.; Okuno, T.; Usui, Y.; Fumihiro, S.; Sammoto, H.; Watanabe, A.; Kariyama, M. (2005). Light effects on cell development and secondary metabolism in Monascus J. Ind. Microbiol. Biotechnol 32(3), 103-108. [DOI] [PubMed] [Google Scholar]
- 13.Miyake, T.; Uchitomo, K.; Zhang, M.Y.; Kono, I.; Nozaki, N.; Sammoto, H.; Inagaki, K. (2006). Effects of the principle nutrients on lovastatin production by Monascus pilosus. Biosci. Biotechnol. Biochem 70(5), 1154-1159. [DOI] [PubMed] [Google Scholar]
- 14.Panda, B.P.; Javed, S.; Ali, M. (2007). Fermentation process optimization. Res. J Microbiol 2 (3), 201-208. [Google Scholar]
- 15.Pandey, A.; Selvakumar, P.; Socool, C.R.; Nigam, P. (1999). Solid state fermentation for production of industrial enzymes. Curr. Sci77 (1), 149-162. [Google Scholar]
- 16.Plackett, R.L.; Burman, J.P. (1946). The design of optimum multi factorial experiments. Biometrika 33(4), 305-325. [Google Scholar]
- 17.Samiee, S.M.; Moazami, N.; Haghighi, S.; Mohseni, F.A.; Mirdamadi, S.; Bakhtiari, M.R. (2003). Screening of lovastatin production by filamentous fungi. Iran Biomed. J 7(1), 29-33. [Google Scholar]
- 18.Sayyad, S.A.; Panda, B.P.; Javed, S.; Ali, M. (2007). Optimization of nutrient parameters for lovastatin production by Monascus purpureus MTCC 369 under submerged fermentation using response surface methodology. Appl. Microbiol Biotechnol73 (5), 1054-1058. [DOI] [PubMed] [Google Scholar]
- 19.Seth, M.; Chand, S. (2000). Biosynthesis of tannase and hydrolysis of tannins to gallic acid by Aspergillus awamori – optimization of process parameters. Process Biochem 36(1): 39-44. [Google Scholar]
- 20.Su, Y.C.; Wang, J.J.; Lin, T.T.; Pan, T.M. (2003). Production of secondary metabolites, γ-amino butyric acid and monacolin K by Monascus J. Ind. Microbiol Biotechnol 30(1): 41-46. [DOI] [PubMed] [Google Scholar]
- 21.Tsai, T.Y.; Wang, J.J.; Pan, T.M. (2006). In vivo hypolipidemic effects and safety of low dosage Monascus powder in hamster model of hyperlipidemia. Appl. Microbiol. Biotechnol 70(5), 533-540. [DOI] [PubMed] [Google Scholar]
- 22.Wang, J.J.; Lee, C.L.; Pan, T.M. (2003). Improvement of monacolin K, γ-amino butyric acid and citrinin production ratio as a function of environmental conditions of Monascus purpureus NTU 601. J. Ind. Microbiol. Biotechnol 30(11), 669-676. [DOI] [PubMed] [Google Scholar]
- 23.Wang, J.J.; Lee, C.L.; Pan, T.M. (2004). Modified mutation method for screening low citrinin-producing strains of Monascus purpureus on rice culture. J. Agric Food Chem 52(23), 6977-6982. [DOI] [PubMed] [Google Scholar]
- 24.Yu, C.C.; Lee, C.L.; Pan, T.M. (2006). A novel formulation approach for preparation of nanoparticulate red mold rice. J. Agric. Food Chem 54 (18), 6845-6851. [DOI] [PubMed] [Google Scholar]


