Abstract
The antidepressant drug amitriptyline hydrochloride was obtained in a dry powder form and was screened against 253 strains of bacteria which included 72 Gram positive and 181 Gram negative bacteria and against 5 fungal strains. The minimum inhibitory concentration (MIC) was determined by inoculating a loopful of an overnight peptone water culture of the organism on nutrient agar plates containing increasing concentrations of amitriptyline hydrochloride (0, 10 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL). Amitriptyline hydrochloride exhibited significant action against both Gram positive and Gram negative bacteria at 25-200 μg/mL. In the in vivo studies it was seen that amitriptyline hydrochloride at a concentration of 25 μg/g and 30 μg/g body weight of mouse offered significant protection to Swiss strain of white mice when challenged with 50 median lethal dose (MLD) of a virulent strain of Salmonella typhimurium NCTC 74. The in vivo data were highly significant (p<0.001) according to the chi-square test.
Keywords: Amitriptyline hydrochloride, antimicrobial activity, non antibiotics
INTRODUCTION
The history of development of pharmacological compounds has shown that any agent may possess diverse functions and may therefore have useful activity in the completely different field of medicine. The possible multifunctional nature of most medicinal agents prompted scientists to investigate the antimicrobial properties of compounds classified pharmacologically as psychotropics, tranquilizers, local anesthetics, cardiovascular drugs, anti-inflammatory agents and antihistamines. It was found that chlorpromazine (14, 19), promazine (6), trifluoperazine (16), fluphenazine (7), bromodiphenhydramine (12, 23), tripolidine (22), methdilazine (4), promethazine (3), trimeprazine (10), propranolol (15), methyl DOPA (11), nifedipine (21), amlodipine (2), dobutamine (24), lacidipine (5), procaine and lignocaine (8), diclofenac (1, 9), dicyclomine (13) possess significant antimicrobial activity.
From these studies it was observed that the compounds with two or more benzene rings possess powerful antimicrobial activity. The present paper describes a detailed study on the in vitro and in vivo antimicrobial activity of a tricyclic antidepressant drug: amitriptyline hydrochloride in which two benzene rings are attached to one another by a cycloheptene ring.
MATERIALS AND METHODS
Microorganisms
A total of 253 strains of bacteria from 5 Gram positive and 11 Gram negative genera and 5 fungal strains belonging to 3 genera were used in this study.
Different bacterial and fungal strains used were isolated from patients or obtained from collections at different places. From Kolkata- Gram positive Staphylococcus aureus (ATCC 6538 p, ATCC 25923, ATCC 29737, BDC 1, ML 6, ML 14, ML 17, ML36, ML 37, ML 52, ML 58, ML 81, ML 125, ML 145, ML 149, ML 151, ML 152,ML 159, ML 162, ML174, ML 180, ML 198, ML 264, ML 265,ML 267, ML 269, ML 271, ML 275, ML 276, ML 277, ML 295, ML 311, ML 314, ML 321, ML 322, ML 329, ML 330, ML 333, ML 335, ML 345, ML 351, ML 358, ML 384, ML 394, ML 411, ML 420, ML 422, Bang 44, 3, 15, 17, 40), Staphylococcus saprophyticus VS14, Staphylococcus citreus M1, Staphylococcus lactis 309, Streptococcus faecalis (ATCC 29212, S2), Micrococcus luteus (ATCC 9341, AGD1), Bacillus cereus ATCC 11778 and Lactobacillus sporogenus; Gram negative Shigella flexneri (2a NK 307, 2a 33220, 3a 30903, 5a B 18603, 5a BCH 511, 2b DN 13, 3b NK 331, 6 NK 126, 6 BCH 895, 6 BCH 999, 6E 03429, F20520, F 20570, BDC 1), Shigella sonnei (B 22461, BCH 217, BCH 397, BCH 947, DN 3, DN 9, E 08869, F11001, KS 1, NK 2, NK 29, NK 228, NK840), Shigella boydii 9E16552, Salmonella virchow ATCC3.1, Salmonella derby ATCC 3.2, Salmonella senftenberg ATCC3.4, Salmonella F14669, Vibrio cholerae (ATCC14033,10, 39, 56, 69, 71, 117, 133, 142, 154, 156, Kathmandu 2, 289, 411, 547, 553, 730, 752, 792, 793, 805, 810, 811, 813, 820, 834, 852, 865, 941, 955, 1021, 1023, 1311, 1315, 1342, 1351, 229, Kualalumpur 23, Kualalumpur 37, DN 6, DN 7, DN 16, DN 26, VRC 411, VRC 2080, VRC 423/75, VRC 2002/75, VRC 2004/75, VRC 295/76, VRC 369/76, DN 6, DN 7, DN 16, DN 26), Escherichia coli (ATCC 10536, ATCC 25922, ATCC 25938, 721, 809, 54B, UC 51, 3P/SD, R 224, R 239, 870, 55, 319, TG1, NCTC 10 HD, 424, 868, 871, C1, R122), Klebsiella pneumoniae (1, 725, J14, J/1/4, R114, R119), Pseudomonas aeruginosa (ATCC 25619, ATCC 27853, AMRI 100), Proteus mirabilis (10, 21, 32, C/6/5, C/10/6), Proteus vulgaris SSKM1/01, Providencia spp., Hafnia spp., Enterobacter cloacae and Citrobacter spp. and fungal strains Candida albicans (I, II, ATCC 10231), Cryptococcus spp. and Rhodotorula spp., from New Delhi - Gram negative Pseudomonas aeruginosa (7, 71, 732, 1006, APC1, C/1/5, C/1/7, Kr/12/3), from London - Gram positive Staphylococcus aureus (NCTC 8530, NCTC 8531, NCTC 8532, NCTC 6571), Bacillus brevis NCTC 7096, Bacillus polymyxa NCTC 4747, Bacillus pumilus NCTC 8241, Bacillus licheniformis NCTC 10341 and Bacillus subtilis ATCC 6633; Gram negative Shigella dysenteriae (2 NCTC 566/61, 7 NCTC 519/66, 8 NCTC 599/52, 9 NCTC 7919), Shigella flexneri 1B 67800, Shigella boydii 10 NCTC 386/66, Salmonella typhi (NCTC 59 type2, NCTC 62), Salmonella berta 69, Salmonella choleraesuis 36, Salmonella paratyphi (A 2, B 5), Salmonella viballerup and Salmonella typhimurium (NCTC 11, NCTC 74, NCTC 102), from U.K. - Gram negative Shigella sonnei (2, 17) and Escherichia coli K12 ROW, from Japan - Gram negative Vibrio parahaemotlyticus (732, 734, 916, 4750, 5507, 8742, 8848, 8898, 8942, 9166, 9331, 9369, 9379, 9580, 9601, 9602, 9603, 9606, 9701, 72003, 72006, 72008, 72016, 72040, 72172, P1, P3, P4, P5, P7),from Denmark - Gram negative Escherichia coli K 99 and from USA- Gram positive Staphylococcus aureus UT 0002 and Bacillus subtilis UC 564; Gram negative Escherichia coli V 517 and Pseudomonas Putida 61 were collected.
All the strains are maintained in the Division of Microbiology, Department of Pharmaceutical Technology, Jadavpur University, Kolkata.
Drug
The drug amitriptyline hydrochloride was obtained in pure dry powder form, from Sun Pharmaceuticals Laboratories, Dadra, India.
In vitro screening test against bacteria
The bacteria were grown in peptone water (PW, 1.0 % bacteriological peptone, Difco brand, 0.5% Analar NaCl) for 18 h. An aqueous solution of amitriptyline hydrochloride (1mg/mL) was sterilized by filtration (sintered glass filter, G-5) and stored at 4oC. This was added to molten nutrient agar (Difco brand) at 45oC in varied final concentrations: 0(control), 10, 25, 50, 100, 200 μg per mL of nutrient agar. The final pH of all the media were adjusted to 7.2 to 7.4 before pouring into sterile Petri dishes. The minimum inhibitory concentration (MIC) of amitriptyline hydrochloride was determined by spotting one loopful (internal diameter 2 mm) of a diluted 18 h broth containing 5x105 colony forming units (CFU) on all plates which were incubated at 37oC and examined for growth up to 72 h (20). The test was performed in triplicate for each organism and the experiment was repeated when necessary.
In vitro screening test against fungi
The fungal strains were grown in Sabouraud,s glucose broth (Difco brand). The sterile aqueous amitriptyline hydrochloride solution (1 mg/mL) was added to molten Sabouraud,s glucose agar (SGA) in such concentrations that the final concentrations of amitriptyline hydrochloride were 0 μg/mL (control), 100 μg/mL, 200 μg/mL, 500 μg/mL, 1000 μg/mL. The final pH of SGA media were adjusted to 5.4 before preparing slants in sterile test tubes. The broth of the fungal strain was diluted and adjusted to 0.5 McFarland standard (17) (a turbidity standard prepared by adding 0.5 mL of 1% barium chloride solution to 99.5 mL of 1% H2SO4) and then the slant tubes were inoculated with one loopful (internal diameter 2mm) of this diluted broth, and the tubes were then incubated at 28oC for 7 days. The end points were noted, when growth of colonies of control were clearly visible after incubation for 7 days (25).
Determination of antibacterial activity of amitriptyline hydrochloride
Two milliliters of 18 h broth culture of a bacterium sensitive to amitriptyline hydrochloride were added to 4 mL of fresh nutrient broth (NB) and were incubated at 37oC for 2 h, to reach the logarithmic growth phase. The number of viable organisms (CFU/mL) was determined and amitriptyline hydrochloride was added at this point at a concentration twice of the respective MIC value. The CFU/mL counts were determined up to 6 h at 2 h interval and then after 18 h.
Animal protection test
In vivo experiments were conducted on 50-60 days old male Swiss albino mice weighing 18-20g. They were kept in polypropylene cages containing 5 animals per cage. Mortality experiment with or without amitriptyline hydrochloride were carried out by challenging mice with 50 median lethal dose (MLD) of a passaged virulent strain of Salmonella typhimurium NCTC 74 (corresponding to 0.95x109 C.F.U. suspended in 0.5 mL NB) (7). Reproducibility of the challenge test doses was ensured by standardization of its optical density at 640 nm in a Klett Summerson colorimeter to give the predetermined number of CFU per mL of broth on nutrient agar plates. The MIC of amitriptyline hydrochloride against S. typhimurium NCTC 74 was found to be 200 μg/mL. Three hours before the challenge, amitriptyline hydrochloride was intraperitoneally administered to the animals in doses of 2, 3, 4.5, 6, 10, 20, 25, 30 μg per g body weight of mice in a final volume of 0.1 mL. The control group was injected with 0.1 mL of sterile water, and all the animals were observed up to 100 h. As survival rate is very low for control group, 60 animals were taken in that group for getting statistically significant data.
In a similar experiment 10 mice were divided into 2 groups of 5 each, and all of them were injected with the challenge dose; Group I was given amitriptyline hydrochloride (25 μg/g of mouse) while Group II was given sterile water before the challenge.
All the animals of Group I and Group II were anesthetized by diethyl ether and then were autopsied 18 h after the challenge. Their livers and spleens were removed, homogenized in a sterile glass homogenizer and preserved at -20oC for subsequent determination of viable counts (C.F.U./mL); 0.2 mL to 0.4 mL of heart blood was also collected aseptically at the same time and viable count was determined immediately.
Animal experiments were conducted following the guidelines of the Institutional Animal Ethics Committee.
RESULTS
Determination of antibacterial activity of amitriptyline hydrochloride by in vitro test
Amitriptyline hydrochloride was found to possess significant antibacterial activity against both Gram positive and Gram negative bacteria. From Table 1, it was seen that out of 253 strains of bacteria tested, 28 strains (11%) were inhibited at 25 μg/mL, 16 strains (6%) were inhibited at 50μg/mL, 55 strains (22%) were inhibited at 100 μg/mL and 86 strains (34%) were inhibited at 200 μg/mL of amitriptyline hydrochloride. The drug has significant inhibitory action on Staphylococcus spp., Bacillus spp. and Vibrio cholerae; 39 out of 60 strains (65%) of Staphylococcus spp., 6 out of 7 strains (86%) of Bacillus spp. and 31 out of 50 strains (62%) of Vibrio cholerae were inhibited at 25-100 μg/mL concentration of amitriptyline hydrochloride. Bacillus was the most sensitive amongst all Gram positive organisms tested. The drug has moderate inhibitory action on Shigella, Salmonella, V. parahaemolyticus and E. coli.
Table 1.
In vitro activity of amitriptyline hydrochloride on Gram positive and Gram negative bacteria
| Name of bacteria | No. of strains tested | No. of strains inhibited by amitriptyline hydrochloride (μg/mL) | |||||
|---|---|---|---|---|---|---|---|
| 10 | 25 | 50 | 100 | 200 | >200 | ||
| Staphylococcus spp. | 60 | 9 (15%) | 2 (3%) | 28 (47%) | 16 (27%) | 5 (8%) | |
| Streptococcus faecalis | 2 | 2 (100%) | |||||
| Micrococcus luteus | 2 | 1 (50%) | 1 (50%) | ||||
| Bacillus spp. | 7 | 5 (72%) | 1 (14%) | 1 (14%) | |||
| Shigella spp. | 36 | 1 (3%) | 4 (11%) | 2 (6%) | 18 (50%) | 11 (30%) | |
| Salmonella spp. | 14 | 2 (14%) | 1 (7%) | 2 (14%) | 4 (29%) | 5 (36%) | |
| Vibrio cholerae | 50 | 7 (14%) | 9 (18%) | 15 (30%) | 16 (32%) | 3 (6%) | |
| Vibrio parahaemolyticus | 30 | 2 (7%) | 22 (73%) | 6 (20%) | |||
| Escherichia coli | 23 | 4 (17%) | 8 (35%) | 11 (48%) | |||
| Klebsiella pneumoniae | 6 | 1 (17%) | 5 (83%) | ||||
| Pseudomonas spp. | 12 | 1 (8%) | 1 (8%) | 10 (84%) | |||
| Proteus spp. | 6 | 6 (100%) | |||||
| Citrobacter spp. | 1 | 1 (100%) | |||||
| Providencia spp. | 1 | 1 (100%) | |||||
| Enterobacter cloacae | 1 | 1 (100%) | |||||
| Hafnia spp. | 1 | 1 (100%) | |||||
| Lactobacillus sporogenes | 1 | 1 (100%) | |||||
| Total | 253 | 28 (11%) | 16 (6%) | 55 (22%) | 86 (34%) | 68 (27%) | |
The MIC of amitriptyline hydrochloride against a representative Staphylococcus aureus NCTC 6571 was found to be 100 μg/mL. 200 μg/mL of the drug was added to the NB culture of S. aureus NCTC 6571 at zero hour of the logarithmic growth phase, when CFU/mL count was 4.4x107. After 2 h, 4 h, 6 h the CFU/mL count was 4.4x105, 3.9x105 and 3.9x105 respectively and at the end of the 18 h was 3.5x104, thereby proving the bacteriostatic nature of amitriptyline hydrochloride against Gram-positive S. aureus NCTC 6571 (Fig. 1).
Figure 1.
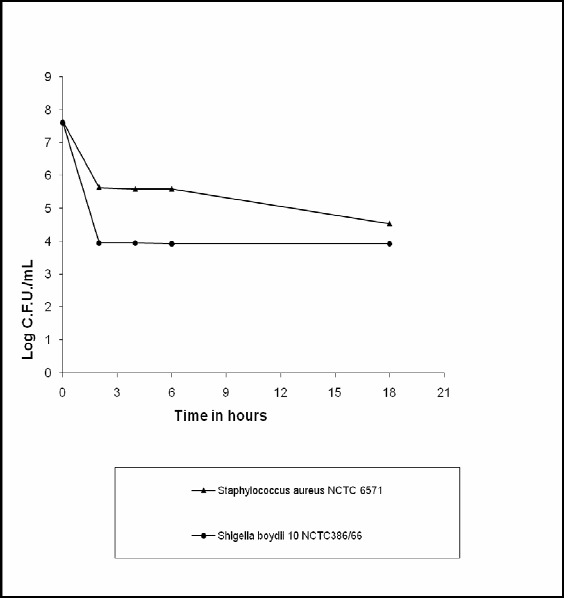
Mode of action of amitriptyline hydrochloride on Staphylococcus aureus NCTC 6571 and Shigella boydii 10 NCTC 386/66
Similarly as the MIC of amitriptyline hydrochloride against a representative Shigella boydii 10 NCTC 386/66 was 25μg/mL, 50μg/mL of the drug was added at the logarithmic growth phase of this culture. The CFU/mL before addition of the drug was 4x107 and it was 9x103 after 2 h, 8.9x103 after 4 h and 8.8x103 after 6 h which did not change further and remained constant at 8.8x103 at the end of the 18 h. So the amitriptyline hydrochloride is also bacteriostatic against Gram negative Shigella boydii 10 NCTC 386/66 (Fig. 1).
Determination of antifungal activity of amitriptyline hydrochloride by in vitro tests
Table 2 shows that Cryptococcus spp. was inhibited at 500 μg/mL of amitriptyline hydrochloride. At 1000 μg/mL concentration of the drug the growth of Candida albicans ATCC 10231 and Candida albicans II was reduced indicating the toxigenic effect of amitriptyline hydrochloride against these two strains.
Table 2.
Inhibitory effect of amitriptyline hydrochloride on different fungal strains
| Name of fungal strain | Growth in SGA containing different concentrations of amitriptyline hydrochloride (μg/mL) | ||||
|---|---|---|---|---|---|
| 0* | 100 | 200 | 500 | 1000 | |
| Candida albicans ATCC 10231 | + | + | + | + | ± |
| Candida albicans I | + | + | + | + | + |
| Candida albicans II | + | + | + | + | ± |
| Rhodotorula spp. | + | + | + | + | + |
| Cryptococcus spp. | + | + | + | – | – |
*, control tube without drug; +, growth; -, no growth, ±, reduced growth.
In vivo experiments
The results presented in Table 3 show that in doses of 30 μg/g and 25 μg/g body weight of mice, only 4 out of 20 mice died in each case, whereas in the control group which received only the drug, no mouse expired. In the control series which received the challenge only, 48 out of 60 mice died. The protection test turned out to be statistically significant (p<0.001 in x2 test) at both 30 μg/g and 25 μg/g doses of amitriptyline hydrochloride, compared to the control (without drug).
Table 3.
Effect of amitriptyline hydrochloride on survival of mice challenged with Salmonella typhimurium NCTC 74
| Group | Dose of Amitriptylinehydrochloride (μg/g mice) | Survival ( live / total ) |
|---|---|---|
| Test (Challenged) | 25 | 16 / 20* |
| 30 | 16/ 20* | |
| Control (Challenged) | – | 12/ 60 |
| (0.1mL of Sterile water) |
P <0.001 according to chi-square test, after elimination of the toxic effects due to the drug alone (test-control). The challenge dose was 0.95 x 109 C.F.U. in 0.5 mL nutrient broth and the survival was recorded up to 100 h after the administration of drug
Amitriptyline hydrochloride at doses of 25 μg/g body weight of mice significantly reduced the bacterial count (CFU/mL) in the organ homogenates of mice 18 h after the challenge compared with the control (p<0.01). The bacterial count in heart blood was also significantly reduced in treated animals (Table 4).
Table 4.
Efficacy of amitriptyline hydrochloride in reducing bacterial counts in different organs of mice challenged with Salmonella typhimurium NCTC 74 for 18 hours
| Group | Drug/g mouse | C.F.U./mL counts in | ||
|---|---|---|---|---|
| Heart blood | Liver | Spleen | ||
| I | Amitriptyline hydrochloride 25 μg | 5.5x103to8.0 x104 | 8.5x103to9.2x104 | 6.4x103 to 5.8x 105 |
| II | Sterile water (control) | 4.9x107to3.8 x108 | 6.2x107to2.4x108 | 2.4x107to4.6x108 |
After drug administration, the animals (5 mice per group) were challenged with 0.95x109 C.F.U./mL of Salmonella typhimurium NCTC 74 and sacrificed 18 h later. Their livers and spleen were removed aseptically and the homogenates were prepared for viable counts. The data were analyzed using Student’s‘t’ test and was found to be significant; p<0.01 in 18 h samples.
DISCUSSION
Amitriptyline hydrochloride, a tricyclic antidepressant drug, has been seen to possess powerful antimicrobial activity both in vitro and in vivo experiments. The sensitive bacterial strains occurred among Staphylococcus spp., Bacillus spp., Vibrio cholerae, Micrococcus spp, Lactobacillus sporogenes and Citrobacter spp. The drug was only moderately active with respect to strains of Shigella spp., Salmonella spp., E. coli, Klebsiella pneumoniae, Vibrio parahaemolyticus and Pseudomonas spp. whereas Streptococcus faecalis, Proteus spp., Enterobacter cloacae, Hafnia spp. and Providencia spp. were resistant to Amitriptyline hydrochloride. Amitriptyline hydrochloride also possesses good antifungal activity against Cryptococcus spp. It possesses moderate antifungal activity against Candida albicans but Rhodotorula spp was resistant to the drug. The drug was found to be bacteriostatic in vitro both against Gram positive and Gram negative bacteria.
In our in vivo experiments we found that amitriptyline hydrochloride at 25 μg/g and 30 μg/g body weights significantly protected the mice. Amitriptyline hydrochloride at doses of 25 μg/g body weight of mice significantly reduced the bacterial count (CFU/mL) in the organ homogenates and heart blood of mice. The high doses required to protect the animals against the challenge of Salmonella typhimurium NCTC 74 may be due to the high in vitro MIC value of amitriptyline hydrochloride against this strain (200 μg/mL). At these doses (25 μg/g and 30 μg/g) the drug showed no toxicity and they were very much below the medial lethal dose of amitriptyline hydrochloride in mice (oral) 350 μg/g (18).
The tricyclic phenothiazines in general possess moderate to powerful antimicrobial activities. The antimicrobial activity of methdilazine (4), trimeprazine (10), fluphenazine (7), trifluoperazine (16) have been reported. Investigations on the structure activity relationship (SAR) suggested that the arrangement of the benzene rings may be responsible for the antimicrobial activity of the drug. Amitriptyline hydrochloride containing two benzene rings attached to one another by a cycloheptene ring may be conceived to mimic a phenothiazine structure, thereby explaining its antimicrobial property. The spectrum of action of amitriptyline hydrochloride is similar to other compounds containing two or more benzene ring (6, 7).
The in vitro and in vivo studies involving amitriptyline hydrochloride suggest that this drug has a remarkable potential for being developed into a potent antimicrobial agent. Further enhancement of its antimicrobial properties can be achieved by synthesizing derivatives of this drug with appropriate structural modifications.
ACKNOWLEDGEMENT
The study was supported financially by Dr. V. Ravichandran Endowment Trust, Jadavpur University, Kolkata (Calcutta), India.
REFERENCES
- 1.Annadurai, S.; Basu, S.; Ray, S.; Dastidar, S.G.; Chakrabarty, A.N. (1998). Antibacterial activity of the anti-inflammatory agent diclofenac sodium. Indian J. Exp. Biol 36, 86-90. [PubMed] [Google Scholar]
- 2.Asok Kumar, K.; Ganguly, K.; Mazumdar, K.; Dutta, N.K.; Dastidar, S.G.; Chakrabarty, A.N. (2003). Amlodipine: a cardiovascular drug with powerful antimicrobial property. Acta Microbiologica Polonica 52 (3), 285-292. [PubMed] [Google Scholar]
- 3.Chakrabarty, A.N.; Acharya, D.P.; Niyogi, D; Dastidar, S.G. (1989). Drug interaction of some non conventional antimicrobial chemotherapeutic agents with special reference to promethazine. Indian J. Med. Res. 89, 233-237 [PubMed] [Google Scholar]
- 4.Chattopadhyay, D.; Dastidar, S.G.; Chakrabarty, A.N. (1988). Antimicrobial properties of methdilazine and its synergism with antibiotics and some chemotherapeutic agents. Arzneimittel-Forschung. 38, 869-872. [PubMed] [Google Scholar]
- 5.Dasgupta, A.; Jeyaseeli, L.; Dutta, N.K.; Mazumder, K.; Karak, P.; Dastidar, S.G.; Motohashi, N.; Shirataki, Y. (2007). Studies on the antimicrobial potential of the cardiovascular drug lacidipine. In Vivo 21, 847-850. [PubMed] [Google Scholar]
- 6.Dash, S.K.; Dastidar, S.G.; Chakrabarty, A.N. (1977). Antimicrobial activity of promazine hydrochloride. Indian J. Exp. Biol 15, 324-326. [PubMed] [Google Scholar]
- 7.Dastidar, S.G.; Chaudhuri, A.; Annadurai, S.; Ray, S.; Mookherjee, M.; Chakrabarty, A.N. (1995). In vitro and in vivo antimicrobial action of fluphenazine. J. Chemother. 7, 201-206. [DOI] [PubMed] [Google Scholar]
- 8.Dastidar, S.G.; Das, S.; Mookerjee, M.; Chattopadhyay, D.; Ray, S; Chakrabarty, A.N. (1988). Antibacterial activity of local anaesthetics procaine and lignocaine. Indian J. Med. Res 87, 506-508. [PubMed] [Google Scholar]
- 9.Dastidar, S.G.; Ganguly, K.; Chaudhury, K.; Chakrabarty, A.N. (2000). The anti-bacterial action of diclofenac shown by inhibition of DNA synthesis. Int. J. Antimicrob. Agents. 14, 249-251. [DOI] [PubMed] [Google Scholar]
- 10.Dastidar, S.G.; Jairaj, J.; Mookerjee, M.; Chakrabarty, A.N. (1997). Studies on antimicrobial effect of the antihistaminic phenothiazine trimeprazine tartarate. Acta Microbiol Immun. Hung 44, 241-247. [PubMed] [Google Scholar]
- 11.Dastidar, S.G.; Mondal, U.; Niyogi, S.; Chakrabaty, A.N. (1986). Antibacterial property of methyl-DOPA and development of cross-resistance in m-DOPA mutants. Indian J. Med. Res. 84, 142-147. [PubMed] [Google Scholar]
- 12.Dastidar, S.G.; Saha, P.K.; Sanyamat, B.; Chakrabarty, A.N. (1976). Antibacterial activities of Ambodryl and Benadryl. J. Appl. Bacteriol 41, 209-214. [DOI] [PubMed] [Google Scholar]
- 13.Karak, P.; Kumar, K.A.; Mazumdar, K.; Mookerjee, M.; Dastidar, S.G. (2003). Antibacterial potential of an antispasmodic drug dicyclomine hydrochloride. Indian J. Med. Res 118, 192-196. [PubMed] [Google Scholar]
- 14.Kristiansen, J.E; Blom, J. (1981). Effect of Chlorpromazine on the ultra structure of Staphylococcus aureus. Acta Path. Microbiol Scand. Sec B. 89, 399-405. [PubMed] [Google Scholar]
- 15.Manna, K.K.; Dastidar, S.G. (1984) .The anti-hypertensive drug propranolol hydrochloride (carditap): its anti-bacterial property. In: Proceedings of the 6th National Congress of the Indian Association of Medical Microbiologists (IAMM).137-141. [Google Scholar]
- 16.Mazumdar, R.; Ganguly, K.; Dastidar, S.G.; Chakrabarty, A.N. (2001). Trifluoperazine: A broad-spectrum bactericide specially active on staphylococci and vibrios Int. J. Antimicrob. Agents 18, 403-406. [DOI] [PubMed] [Google Scholar]
- 17.McFarland J. Nephelometer: an instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J. Am. Med. Assoc. (1907). 49, 176–178. [Google Scholar]
- 18.Merck Index, Fourteenth Edition, p. 485. [Google Scholar]
- 19.Molnar, J.; Mandi, Y.; Kiraly, J. (1976). Antibacterial effect of some phenothiazine compounds and the R-factor elimination by chlorpromazine. Acta Microbiol Acad. Sci. Hung. 23, 45-54. [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards (2003). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 6th ed. NCCLS document M7-A6. NCCLS, Wayne, Pa. [Google Scholar]
- 21.Pal, T.; Dutta, N.K.; Mazumder, K.; Dasgupta, A.; Jeyaseeli, L.; Dastidar, S.G. (2006). Assesement of antibacterial activity of the cardiovascular drug Nifedipine. Oriental Pharmacy and Experimental Medicine 6(2), 126-133. [Google Scholar]
- 22.Roy, K.; Chakrabarty, A.N. (1994). Anti-bacterial activities of anti-histamine triprolidine hydrochloride (actidil) and cross-resistances to antibiotics developed by experimentally derived mutants resistant to this drug. Indian J. Med. Microbiol 12, 9-18. [Google Scholar]
- 23.Saha, P.K.; Dastidar, S.G. (1976). Antimicrobial activity of antihistaminic drugs. Indian J. Med. Res 64, 1677-1679. [PubMed] [Google Scholar]
- 24.Sarkar, A.; Kumar, K. A.; Dutta, N.K.; Chakraborty, P.; Dastidar, S.G. (2003). Evaluation of in vitro and in vivo antibacterial activity of dobutamine hydrochloride. Indian J. Med. Microbiol 21(3), 172-178. [PubMed] [Google Scholar]
- 25.Uchida, K.; Yokota, N.; Yamaguchi, H. (2001). In vitro antifungal activity of posaconazole against various pathogenic fungi. Int. J. Antimicrob. Agents 18(2), 167-172. [DOI] [PubMed] [Google Scholar]


