Abstract
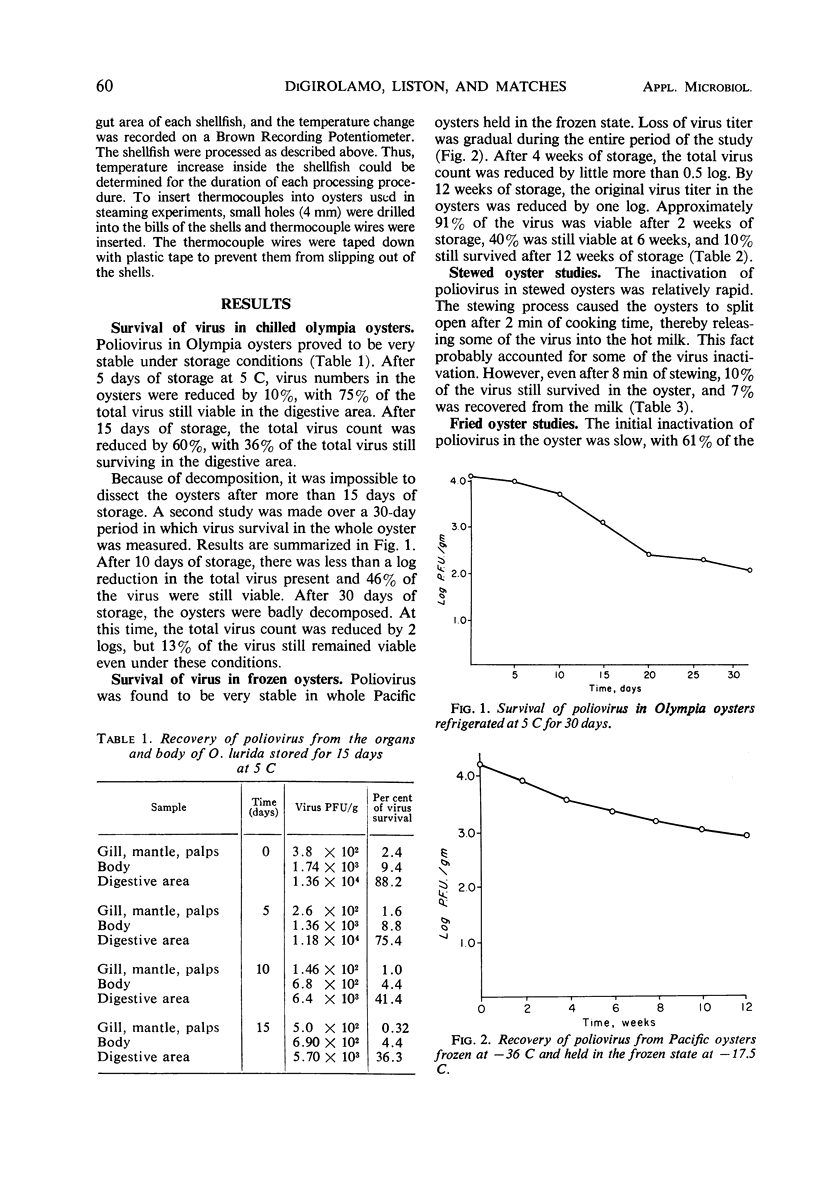
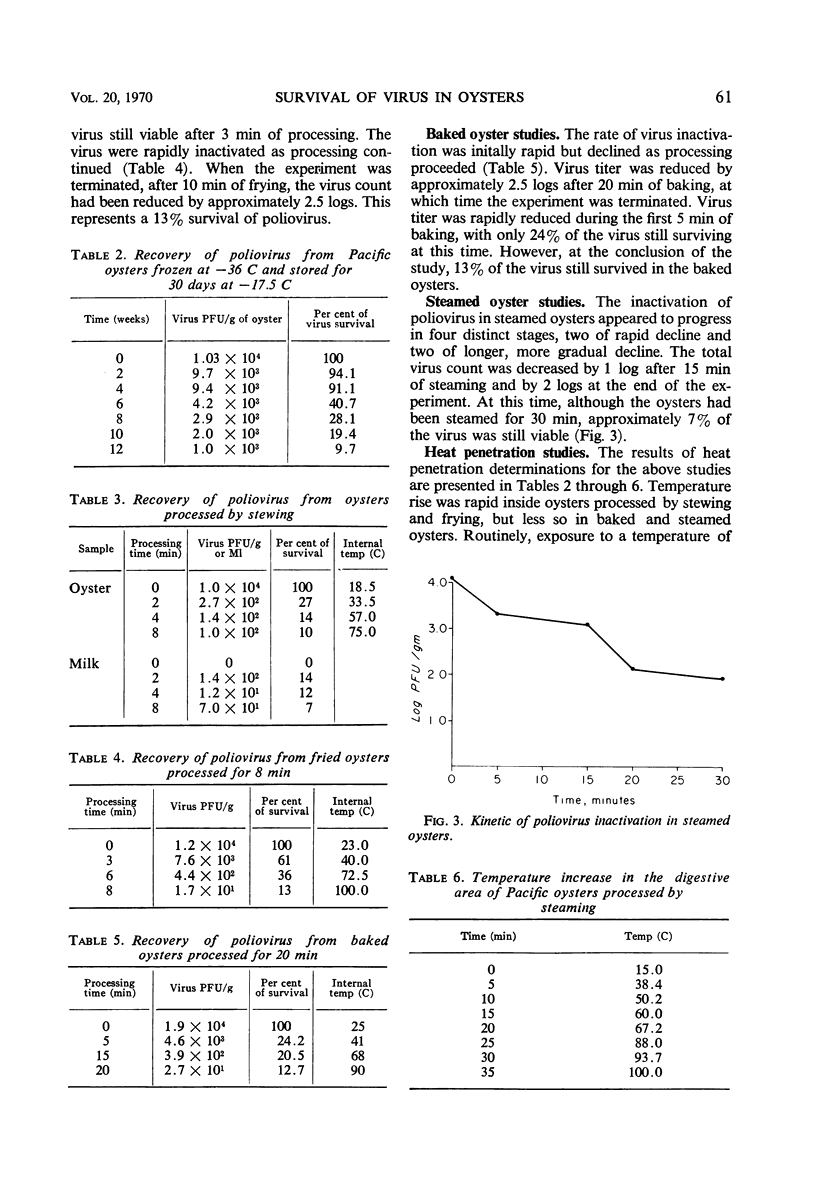
Samples of whole and shucked Pacific and Olympia oysters, contaminated with 104-plaque-forming units (PFU) of poliovirus Lsc-2ab per ml, were held refrigerated at two temperatures, 5 and - 17.5 C. To study the survival of virus in the oysters under these conditions, samples were assayed for virus content at weekly intervals for as long as 12 weeks. Results indicated that poliovirus would survive in refrigerated oysters for a period varying from 30 to 90 days, depending upon temperature. The survival rate varied from 10 to 13%. To study the extent of the hazard presented by oysters contaminated with virus, samples of whole and shucked Pacific oysters contaminated with 104 PFU of poliovirus Lsc-2ab per ml were heat processed in four ways: by stewing, frying, baking, and steaming. Results indicated that virus in oysters withstood these methods of processing. The survival rate varied from 7 to 10% and appeared dependent upon the processing method used. Heat penetration studies showed that the internal temperature in the oyster was not sufficient to inactivate all of the virus present. These results suggest that not only fresh but also refrigerated and cooked oysters can serve as vectors for the dissemination of virus disease if the shellfish are harvested from a polluted area.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DOUGHERTY W. J., ALTMAN R. Viral hepatitis in New Jersey 1960-1961. Am J Med. 1962 May;32:704–716. doi: 10.1016/0002-9343(62)90160-2. [DOI] [PubMed] [Google Scholar]
- Dimmock N. J. Differences between the thermal inactivation of picornaviruses at "high" and "low" temperatures. Virology. 1967 Feb;31(2):338–353. doi: 10.1016/0042-6822(67)90179-1. [DOI] [PubMed] [Google Scholar]
- HSIUNG G. D., MELNICK J. L. Plaque formation with poliomyelitis, Coxsackie, and orphan (echo) viruses in bottle cultures of monkey epithelial cells. Virology. 1955 Dec;1(5):533–535. doi: 10.1016/0042-6822(55)90041-6. [DOI] [PubMed] [Google Scholar]
- Hoff J. C., Becker R. C. The accumulation and elimination of crude and clarified poliovirus suxpensions by shellfish. Am J Epidemiol. 1969 Jul;90(1):53–61. doi: 10.1093/oxfordjournals.aje.a121049. [DOI] [PubMed] [Google Scholar]
- Liu O. C., Seraichekas H. R., Murphy B. L. Fate of poliovirus in northern quahaugs. Proc Soc Exp Biol Med. 1966 Feb;121(2):601–607. doi: 10.3181/00379727-121-30841. [DOI] [PubMed] [Google Scholar]
- Liu O. C., Seraichekas H. R., Murphy B. L. Viral depuration of the Northern quahaug. Appl Microbiol. 1967 Mar;15(2):307–315. doi: 10.1128/am.15.2.307-315.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynt R. K., Jr Survival and recovery of enterovirus from foods. Appl Microbiol. 1966 Mar;14(2):218–222. doi: 10.1128/am.14.2.218-222.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON J. O., McLEAN W. R. Infectious hepatitis traced to the consumption of raw oysters. An epidemiologic study. Am J Hyg. 1962 Jan;75:90–111. doi: 10.1093/oxfordjournals.aje.a120238. [DOI] [PubMed] [Google Scholar]
- METCALF T. G., STILES W. C. THE ACCUMULATION OF ENTERIC VIRUSES BY THE OYSTER, CRASSOSTREA VIRGINICA. J Infect Dis. 1965 Feb;115:68–76. doi: 10.1093/infdis/115.1.68. [DOI] [PubMed] [Google Scholar]
- WALLIS C., YANG C. S., MELNICK J. L. Effect of cations on thermal inactivation of vaccinia, herpes simplex, and adenoviruses. J Immunol. 1962 Jul;89:41–46. [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Thermostabilization and thermosensitization of herpesvirus. J Bacteriol. 1965 Dec;90(6):1632–1637. doi: 10.1128/jb.90.6.1632-1637.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


