Abstract
Nosocomial infections caused by methicillin-resistant staphylococci (MRSA) pose a serious problem in many countries. This study aimed to determine the antibacterial susceptibility patterns of methicillin sensitive and resistant Staphylococcus aureus isolates from the hospitalized patients. Totally 356 isolates of Staphylococcus aureus (S. aureus) including 200, 137 and 19 corresponding to MSSA, MRSA, and intermediate MRSA strains, respectively were isolated. Antibacterial susceptibility patterns of the isolates to 14 antibiotics were examined using Kirby-Bauer method. MICs of 15 antibiotics to 156 MRSA isolates were determined by E test method. Cross-resistances of MRSA isolates (137+19) to the other tested antibiotics were also determined. S.aureus with high frequencies were isolated from the blood, sputum and deep wound samples. All of 200 MSSA isolates were sensitive to oxacillin, vancomycin, tecoplanin, rifampin, linezolid, quinupristin/dalfopristin, mupirocin and fusidic acid. A gradient of reduced susceptibility of MSSA to cephalexin, co-trimoxazole, ciprofloxacin, clindamycin, tetracycline, erythromycin and gentamicin were evident. MRSA isolates were sensitive to vancomycin, tecoplanin, linezolid, quinupristin/dalfopristin, mupirocin and fusidic acid, while reduced susceptibility of them to rifampin, co-trimoxazole, clindamycin, cephalexin, tetracycline, ciprofloxacin, erythromycin and gentamicin were observed. MRSA isolates exhibited a high range of cross-resistance to the eight tested antibiotics. Overall, co-trimoxazole, ciprofloxacin, clindamycin, tetracycline, erythromycin and gentamicin showed low activity against MSSA and MRSA isolates which may indicate they are not suitable to be used in clinical practices. To preserve the effectiveness of antibiotics, rational prescription and concomitant application of preventive measures against the spread of MRSA are recommended.
Keywords: MRSA, minimum inhibitory concentration, empirical therapy
INTRODUCTION
Staphylococcus aureus is a major nosocomial pathogen that causes a range of diseases such as endocarditis, osteomyelitis, toxic-shock syndrome, food poisoning, carbuncles, and boils (21). With the discovery of β-lactam antibiotics in the late 1930s, remarkable hope for the treatment of infectious disease appeared. However, in the early 1950s, acquisition and spread of β-lactamase producing plasmids thwarted the effectiveness of penicillin for treating S. aureus infections. In 1959, methicillin, the synthetic penicillin, was introduced while by 1960, methicillin-resistant S. aureus strains were identified. It was the direct result of S. aureus acquiring the mecA gene, which encodes for an altered penicillin binding protein gene (PBP2a) (2). By the early 1960s, European hospitals were reporting outbreaks of MRSA infections, and subsequently MRSA clones spread to health-care institutions around the world (27). In the United States, MRSA is responsible for approximately 25% of nosocomial infections, and reports of community acquired MRSA infections are increasing (28). The multidrug resistant phenotypes of MRSA strains and their intrinsic β-lactam resistance make them difficult and costly to treat (5, 24). In some medical institutions such as New York city hospitals, MRSA accounts for approximately 29% of the nosocomial infections and 50% of the associated deaths (25). Similarly, the high prevalence of MRSA in Shiraz and other parts of Iran have been reported previously (8, 12, 20). Controlling MRSA remains a primary focus of the most hospital infection control programs (3). Unfortunately, the clinical management of the MRSA infected patients has been complicated by the increasing proportion of infections, a problem that was initially limited to the hospital-acquired strains but has now extended to the community-associated strains (24,28). Attributable mortality rates for S. aureus bacteremia (SAB) in the developed world have risen up to 30% (7,10,15), and the costs of treating nosocomial infections, screening for MRSA carriage, and changes in prescribing practice to include the coverage for MRSA are considerable. The availability of over-the-counter antibiotics in many developing countries is likely to be a major driver of drug resistance.
This study seeks to determine the prevalence of MRSA, MSSA and to analyze qualitative and quantitative aspects of antibiotics susceptibility patterns of the isolated bacteria in hospitalized patients. Furthermore, the cross-resistance of MRSA isolates to the tested antibiotics will be determined. Availability of such data have clinical implications for selecting suitable empirical antibiotic therapy, reducing length of stay in the hospital, as well as reducing the mortality/morbidity rates in the patients with infectious diseases. Furthermore, findings of the above regional studies could be comparatively developed to other parts of the world.
MATERIALS AND METHODS
Isolation of staphylococci
Totally 356 consecutive non-duplicate Staphylococcus aureus were isolated from the blood, urine, sputum, deep wound, nasal washing, skin lesion, nose swab, abscess, CSF, joint fluids, eye discharge, pleural fluid, toe web and ascitic fluid of patients from May 2006 to March 2007 in Nemazee hospital, affiliated with Shiraz University of Medical Sciences, Shiraz, Iran. This hospital is tertiary setting facilities with one thousand beds. The isolates were identified as staphylococci based on gram stain, morphology, catalase, bacitricin disc sensitivity, coagulase and DNAse activities using commercial media and reagent (catalase test with hydrogen peroxidase 3%:coagulase test with fresh rabbit plasma; bacitracin sensitivity with Mast antibiotic disc; UK and DNAse activity with DNAse test agar supply from Merck company, Germany).
Susceptibility patterns of the isolates
Qualitative assay: Qualitative antibacterial susceptibility of 156 MRSA and 200 MSSA isolates were determined according to the standard disk diffusion (Kirby-Bauer) method using Mast Co (Mast Co, Merseyside, UK). S. aureus (ATCC 25923) were used as controls for the antibiotic susceptibility determination. Antibacterial susceptibility pattern was interpreted as recommended by Clinical Laboratory Standards Institute (CLSI, 22). Susceptibilities of MRSA and MSSA to the panel of antibiotics including gentamicin (GM; 10µg), cephalexin (CN; 30µg), co-trimoxazole, (SXT; 25µg), rifampin (RF;5µg), ciprofloxacin (CIP;5µg), vancomycin (V;30µg), tecoplanin (TEC;30µg) oxacillin (OX;1µg), erythromycin (E;15µg), quinupristin/dalfopristin (QD;15µg), tetracycline (Te;30µg), clindamycin (CD;2µg), linezolid (LZD;30µg), mupirocin (MUP; 10 µg), and fusidic acid (FUS; 10 µg) were determined.
Quantitative assay: MICs of the above 15 antibiotics to the 156 isolates of MRSA were determined by the E test method (AB Biodisk, Sweden). American Typing Collection (ATCC 25923) of S. aureus was used as a control strain in antibacterial susceptibility testing. Following overnight incubation, the MICs breakpoints were interpreted according to the manufacturer’s instructions.
Cross-resistance profile
To carefully characterize the patterns of antibiotic resistance of MRSA, cross-resistance of the isolates to 8 antibiotics with reduced activities were calculated by SPSS version 11.5 software.
RESULTS
S. aureus with high frequencies were isolated from the blood, sputum and deep wound samples, while low frequencies of this bacterium were isolated from ascetic fluid and perinea samples (Table 1). All of the 200 MSSA isolates were found sensitive to eight antibiotics and a gradient of reduced susceptibility of the MSSA to seven antibiotics were noticed (Table 2). Based on MICs determination, all the 156 MRSA were sensitive to vancomycin, tecoplanin, linezolid, quinupristin/dalfopristin, mupirocin and fusidic acid and a reduced gradient of susceptibility of MRSA to the rest of antibiotics were observed (Table 2). To interpret antibiotic resistance patterns of MRSA more precisely, cross-resistance of these isolates to the eight antibiotics with reduced anti-MRSA activities were calculated and presented in Table 3. MRSA isolates showed high range of cross-resistance to the eight tested antibiotics including co-trimoxazole (93–100 %), clindamycin (84–100%), cephalexin (83–100 %), tetracycline (78–100%), rifampin (65–100%), ciprofloxacin (82–99%) erythromycin (76-99%) and gentamicin (70–87%). Similarly, high range of MIC50 and MIC90 values for MRSA isolates were noticed. Values of MIC50 and MIC90 for the tested antibiotics against the MRSA isolates are collected in Table 4. Co-trimoxazole, ciprofloxacin, clindamycin, tetracycline, erythromycin and gentamicin showed low activities against all the isolates of S.aureus (MRSA, MSSA). Antibiotics sensitivity patterns of all MSSA and MRSA isolates were illustrated in Figure 1.
Table 1.
Source of MRSA and MSSA.
| Source | MRSA (%) | MSSA (%) | Total (%) |
|---|---|---|---|
| Blood | 54 (15.1) | 55(15.3) | 109 (30.6) |
| Sputum | 25 (7.0) | 25 (7.0) | 50 (14.0) |
| Wound | 22 (6.2) | 26 (7.3) | 48 (13.5) |
| Urine | 8 (2.2) | 18( 5.0) | 26(7.3) |
| Nasal swab | 10 (2.9) | 13 (3.6) | 23(6.4) |
| Skin lesion | 6 (1.7) | 15 (4.2) | 21 (5.9) |
| Nose discharge | 6 (1.7) | 13 (3.6) | 20(5.6) |
| Abscess | 8 (2.2) | 7 (1.9) | 15 (4.2) |
| CSF | 8 (2.2) | 10 (2.9) | 18(5.0) |
| Joint fluids | 4 (1.1) | 3 (0.9) | 7 (1.9) |
| Eye discharge | 1 (0.3) | 4(1.1) | 5 (1.4) |
| Pleural fluid | 2 (0.6) | 4(1.1) | 5 (1.4) |
| Toe web | 1 (0.3) | 3 (0.9) | 4 (1.1) |
| Ascitic fluid | 1 (0.3) | 2 (0.6) | 3 (0.9) |
| Perinea sample | - | 2(0.6) | 2 (0.6) |
| Total | 156 (43.8) | 200(56.2) | 356 (100) |
Table 2.
Susceptibility patterns of MSSA and MRSA isolates to antibiotics with reduced sensitivity.
| Antibiotics | ||||||||
|---|---|---|---|---|---|---|---|---|
| SXT(%) | CN(%) | CIP(%) | CD(%) | TE(%) | E(%) | GM(%) | ||
| MSSA | S=188(94) | S=189(94.5) | S=154(77) | S=142(71) | S=140(70) | S=96(48) | S=38(19) | |
| MSSA | R=8(4) | R=5(2.5) | R=7(3.5) | R=11(5.5) | R=18(9) | R=14(7) | R=100(50) | |
| MSSA | IR=4(2) | IR=6(3) | IR=39(19.5) | IR=47(23.5) | IR=42(21) | IR=90(45) | IR=62(31) | |
| MRSA | RF (%) | SXT (%) | CD (%) | CN (%) | TE (%) | CIP (%) | E (%) | GM (%) |
| S=139(89.1) | S=48(30.7) | S=37(23.7) | S=33(21.1) | S=27(17.3) | S=25(16.0) | S=14(10.9) | S=1(0.6) | |
| R=15(9.6) | R=101(64.8) | R=105(67.3) | R=115(73.7) | R=123(78.8) | R=119(76.2) | R=117(75) | R=142(91.0) | |
| IR=2(1.3) | IR=7(4.5) | IR=14(9.0) | IR=8(5.2) | IR=6(3.9) | IR=12(7.8) | IR=22(14.1) | IR=13(8.4) | |
Abbreviation: SXT; co-trimoxazole, CN cephalxin, CIP; ciprofloxacin, CD; clindamycin, TE; tetracycline, E; erythromycin, GM; gentamicin, RF; rifampin, S sensitive, R; resistant, IR; intermediate resistant.
Table 3.
Cross-resistance of MRSA to the tested antibiotics.
| n | Number of isolates and percent (values in parenthesis) resistant to | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | E | CIP | TE | CN | CD | SXT | RF | ||||
| GM | 155 | 139 (87) | 131 (85) | 129 (83) | 123(79) | 119 (77) | 108 (70) | 17 (11) | |||
| E | 139 | 138 (99) | 124 (89) | 119 (86) | 120 (86) | 113 (81) | 106 (76) | 17 (12) | |||
| CIP | 131 | 130 (99) | 125 (95) | 115 (88) | 115 (88) | 113 (86) | 107(82) | 17 (13) | |||
| TE | 129 | 129 (100) | 119 (93) | 114 (88) | 113 (88) | 106 (82) | 101 (78) | 16 (12) | |||
| CN | 123 | 123 (100) | 120 (98) | 115 (93) | 113 (92) | 107 (87) | 102 (83) | 12 (14) | |||
| CD | 119 | 119 (100) | 113 (95) | 113 (95) | 113 (95) | 106 (89) | 100 (84) | 15 (13) | |||
| SXT | 108 | 108 (100) | 106 (98) | 107 (99) | 107 (99) | 101 (94) | 100 (93) | 11 (10) | |||
| RF | 17 | 17 (100) | 17 (100) | 17 (100) | 16 (94) | 17 (100) | 15 (88) | 11 (65) | |||
Abbreviation: GM; gentamicin, E; erythromycin, CIP; ciprofloxacin, TE; tetracycline, CN cephalxin, CD; clindamycin, SXT; co-trimoxazole, RF; rifampin
Table 4.
Range of MIC50 & MIC90 values for MRSA isolates to the tested antibiotics.
| Antibiotics | OX | VA | TEC | LZD | SYR | MUP | FUS | RF | SXT | CD | CN | CIP | E | GM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC 50µg/ml | 256 | 2 | 0.75 | 0.75 | 0.5 | 0.094 | 0.125 | 0.012 | 32 | 256 | 265 | 32 | 256 | 256 |
| MIC 90µg/ml | >256 | 4 | 1.5 | 1 | 0.75 | 0.125 | 0.19 | 4 | 32 | 256 | >256 | 32 | >256 | 256 |
Abbreviations: OX:oxacillin,VA:vancomycin,TEC:tecoplanin,LZD:linezolid,SYR:quinuprstin/dalfopristin,MUP mupirocin,FUS: fusidic acid, rifampin, SXT:co-trimoxazole, CD:clindamycin, CN: cephalxin, CIP: ciprofloxacin,E: erythromycin and GM:gentamicin.
Figure 1.
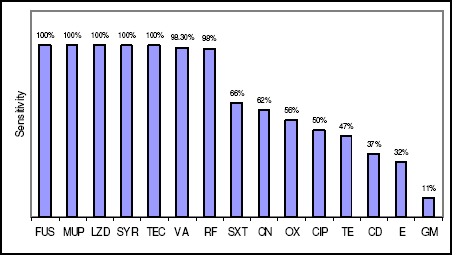
Sensitivity patterns of 356 isolates of MSSA & MRSA to the 15 tested antibiotics Abbreviations: FUS: fusidic acid, MUP: mupirocin, LZD: linezolid, SYR: quinupristin/dalfopristin, TEC: tecoplanin, VA: vancomycin, R: rifampin, SXT: cotrimoxazole, CN: cephalxin, OX: oxacillin, CIP: ciprofloxacin, TE: tetracycline, CD: clindamycin, E: erythromycin and GM: gentamicin.
DISCUSSION
Methicillin-resistant strains of S. aureus are important nosocomial infections worldwide. Our data indicate that S. aureus with high frequency was isolated from the blood samples (30.6 %), of which 50% were MRSA, followed by sputum and deep wound samples with 14 % and 13.5 %, respectively. The high incidence of S.aureus bacteremia (SAB), mostly driven by community acquired (CA) infections was also previously reported (7, 10, 14, 15). Studies from various countries revealed that the rates of SAB are rising and the proportion due to methicillin (β-lactam) resistant MRSA is also increasing (7, 15). Wyllie et al. (29) reviewed the rates of nosocomial SAB in two Oxfordshire hospitals in England between 1997 and 2004. They showed an increase in the rates of nosocomial SAB, but the incidence of methicillin susceptible S. aureus (MSSA) bacteremia was unchanged over the period. However, in the UK, Johnson et al. (13) observed a marked rise both in the number of SAB cases and in the proportion due to MRSA reported by hospital laboratories. According to this report, the number of isolated MRSA from 1990 to 2004 ranged between 2 % to 40 %, where the latter value stands at plateau level in recent years. Kaech et al. (15) viewed SAB in a Swiss hospital with a low incidence of MRSA. The rate of SAB increased by 23%.This change was related to a 140% increase in community acquired bacteremia, which was in turn attributed largely to intravenous drug use (15). The SCOPE project (6), a prospective surveillance project in the USA, found that 20% of nosocomial bacteremia between 1995 and 2002 were due to S. aureus. The proportion of the strains resistant to methicillin rose from 22% in 1995 to 57% in 2001. Collectively, these data lend support to the idea that the increased ratio of MRSA/MSSA especially in patients suffering from bacteremia due to MRSA could be a global issue which demands international cooperation to prevent the potential worldwide crisis.
Relatively high incidence of skin and wound infections (19.5%) was noticed in the present study. The results are consistent with the previous reports on the prevalence of cutaneous MRSA infections in US emergency room patients (18).
Evaluation of the antibiotic susceptibility patterns of MSSA and MRSA indicates the sensitivity of MRSA isolates were markedly reduced as compared with that of MSSA isolates. Nevertheless, complete sensitivity of MSSA and MRSA to the six tested antibiotics including vancomycin, tecoplanin, linezolid, quinupristin/dalfopristin, mupirocin and fusidic acid could provide relatively more alternative choice of the antibiotics available for treatment. Given the low activities of co-trimoxazole, ciprofloxacin, clindamycin, tetracycline, erythromycin and gentamicin against all the S. aureus isolates, we may suggest that they are not suitable for use in clinical practices. Low activities of these antibiotics against S.aureus could be due to the selection of resistant isolates resulting from mutations at drug target sites, or from the disturbance of drug accumulation in cytoplasm due to cell wall or cell membrane rearrangement (14, 16, 17, 30). Alternatively, resistance genes could transfer due to mobile genetic elements such as plasmid, transposon and integrons (1, 11, 23, 26). Nevertheless, comparison of MICs values in particular MIC50 and MIC90 may suggest that more likely the latter phenomenon could be responsible for the emergence of the majority of isolates with high MIC values. To determine the mechanisms of MRSA resistance in our isolates, precise molecular methods such as staphylococcal cassette chromosome typing (SCC typing) and sequencing of resistant determinate should be initiated. The findings indicate that the minority of resistant isolates could emerge due to the mutation of those with low MIC values. Apparently, continuous antibiotics pressure on sensitive isolates could facilitate the domination of any emerging possible resistant isolates. Unfortunately, most of the tested antibiotics in this study are extensively used in our clinics for prophylactic and treatment purposes. Therefore, comprehensive control measures and prudent application of effective antibiotics should be adopted to overcome this critical situation (9). It is worth mentioning that two points should be considered for clinical use of antibiotics; first of all the cost-effectiveness of the antibiotics and the second is the pharmacological availability of different types of antibiotics for various clinical purposes. For example, linezolid is an expensive antibiotic as compared to tecoplanin (19). Mupirocin is likely available in the form of topical cream and ointment preparations (4) which limit its systemic application.
It is well known that MRSA characteristically are multi-drug resistant strains (16). High incidence of cross-resistance of MRSA noticed in this survey may indicate that different resistance mechanisms are involved in the resistance of MRSA to different classes of antibiotics. Therefore, every isolate of MRSA should be considered potentially multi-drug resistant with respect to its clonal selection and transferring of the isolates to the other hospitalized patients (9).
In summary, decreased susceptibility of MRSA to the daily consuming antibiotics indicates that special controlling protocol should be adopted in our clinics and hospitals. This program should include direct or indirect promotion of control measures in hospital wards and clinics to prevent the transferring of MRSA from patient to patient. Moreover, rational prescription of sensitive antibiotics and ceasing of the over-the-counter presenting of them are also recommended. The application of this protocol along with periodical surveillance of antibacterial susceptibility patterns of MRSA and MSSA could preserve their sensitivity levels to the antibiotics and alleviate the situation, accordingly.
ACKNOWLEDGEMENT
Our special thanks are due to H. Khajehei for his great help with linguistic copy editing.
REFERENCES
- 1.Barker, K.F. (1999).Antibiotic resistance: a current perspective Br J Clin Pharmacol, 48: 109-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber, M. (1963). Methicillin-resistant Staphylococci. J Clin Pathol, 1:308-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boerlin, P.; Reid-Smith, R. J.(2008). Antimicrobial resistance: its emergence and transmission.Anim Health Res Rev., 9:115-26. [DOI] [PubMed] [Google Scholar]
- 4.Bork, K.; Brauers, J.; Kresken, M.(1989;). Efficacy and safety of 2% mupirocin ointment in the treatment of primary and secondary skin infections--an open multicentre trial. Br J Clin Pract,. 43:284-248. [PubMed] [Google Scholar]
- 5.Bratu, S.; Eramo, A.; Kopec, R.; Coughlin, E.; Ghitan, M.; Yost, R.; Chapnick, E.K.; Landman, D.; Quale, J. (2005).Community-associated methicillin-resistant Staphylococcus aureus in hospital nursery and maternity units. Emerg Infect Dis., 11: 08-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention [CDC]. (2005). ommunity-associated MRSA information for clinicians [online]].CDC; Available at: http://www.cdc.gov/ncidod/dhqp/ar_mrsa_ca_clinicians. html Accessed 17 Apr 2006.
- 7.Das, I.; O’Connell, N.; Lambert, P. (2007). Epidemiology, clinical and laboratory characteristics of Staphylococcus aureus bacteremia in a university hospital in UK. J Hosp Infect, 65: 117–123. [DOI] [PubMed] [Google Scholar]
- 8.Folahzadeh, B.; Emaneini, M.; Aligholi, M.; Gilbert, G.; Taherikalani, M.; Jonaidi, N.; Eslampour, M.A.; Feizabadi. M.M. (2009). Molecular characterization of methicillin-resistant Staphylococcus aureus clones from a teaching hospital in Tehran. Jpn J Infect Dis, 62:309-301. [PubMed] [Google Scholar]
- 9.Hacek, D. M.; Suriano, T.; Noskin, G.A.; Kruszynski, J.; Reisberg, B.; Peterson, L.R. (1999). Medical and economic benefit of a comprehensive infection control program that includes routine determination of microbial clonality. Am J Clin Pathol, 111:647-654. [DOI] [PubMed] [Google Scholar]
- 10.Hill, P.C.; Birch, M.; Chambers, S.; Drinkovic, D.; Ellis-Pegler, R.B.; Everts, R.; Murdoch, D.; Pottumarthy, S.; Roberts, S.A.; Swager, C.; Taylor, S.L.; Thomas, M.G.; Wong, C.G.; Morris, A.J. (2001). Prospective study of 424 cases of Staphylococcus aureus bacteremia: determination of factors affecting incidence and mortality. Intern Med J, 31: 97–103 [PubMed] [Google Scholar]
- 11.Gold, H.S.; Pillai, S.K. (2009). Antistaphylococcal agents. Infect Dis Clin North Am, 23:99-131. [DOI] [PubMed] [Google Scholar]
- 12.Japoni, A.; Alborzi, A.; Orafa, F.; Rasouli, M.; Farshad, S. (2004). Distribution patterns of methicillin resistance genes (mecA) in Staphylococcus aureus isolated from clinical specimens. Iran. Biomed. J., 8: 173-178. [Google Scholar]
- 13.Johnson, A.P.; Pearson, A.; Duckworth, G. (2005). Surveillance and epidemiology of MRSA bacteraemia in the UK. J Antimicrob Chemother., 56:455–62. [DOI] [PubMed] [Google Scholar]
- 14.Kawai, M.; Yamada, S.; Ishidoshiro, A.; Oyamada, Y.; Ito, H.; Yamagishi, J. ( 2009). Cell-wall thickness: possible mechanism of acriflavine resistance in meticillin-resistant Staphylococcus aureus J Med Microbiol., 58:331-336. [DOI] [PubMed] [Google Scholar]
- 15.Kaech, C.; Elzi, L.; Sendi, P.; Frei, R.; Laifer, G.; Bassetti, S.; Fluckiger, U. (2006). Course and outcome of Staphylococcus aureus bacteremia: a retrospective analysis of 308 episodes in a Swiss tertiary centre. Clin Microbiol Infect, 12: 345–352. [DOI] [PubMed] [Google Scholar]
- 16.Kwong, S.M.; Lim, R.; Lebard, R.J.; Skurray, R.A.; Firth, N. (2008). Analysis of the pSK1 replicon, a prototype from the staphylococcal multi-resistance plasmid family. Microbiology, 154:3084-3094. [DOI] [PubMed] [Google Scholar]
- 17.Memmi, G.; Filipe, S.R.; Pinho, M.G.; Fu, Z.; Cheung, A. (2008). Staphylococcus aureus PBP4 is essential for beta-lactam resistance in community-acquired methicillin-resistant strains. Antimicrob Agents Chemother, 52: 3955-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moran, G.J.; Krishnadasan. A.; Gorwitz, RJ.; Fosheim, G.E.; McDougal, K.; Carey, R.B.; Talan, D,A.; emergency ID Net Study Group. (2006). Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med, 355:666-674. [DOI] [PubMed] [Google Scholar]
- 19.Nathwani, D.; Barlow, G.D.; Ajdukiewicz, K.; Gray, K.; Morrison, J.; Clift, B.; France, A.J. (2003). Cost-minimization analysis and audit of antibiotic management of bone and joint infections with ambulatory teicoplanin, in-patient care or outpatient oral linezolid therapy. J Antimicrob Chemother, 51:391-6. [DOI] [PubMed] [Google Scholar]
- 20.Nikbakht, M.; Nahaei, M.R.; Akhi, M.T.; Asgharzadeh, M.; Nikvash, S.( 2008) Molecular fingerprinting of meticillin-resistant Staphylococcus aureus strains isolated from patients and staff of two Iranian hospitals.J Hosp Infect., 69:46-55. [DOI] [PubMed] [Google Scholar]
- 21.Ogston, A. (1984). “On Abscesses. Classics in Infectious Diseases”, Rev Infect Dis, 6: 122–28. [PubMed] [Google Scholar]
- 22.Performance standards for antimicrobial susceptibility testing (2007). CLSI approved standard M100-S17. Clinical and Laboratory Standards Institute, CLSI. Wayne, PA. [Google Scholar]
- 23.Pinho, M.G.; de Lencastre, H.; Tomasz, A. (2001). An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci. Proc Natl Acad Sci USA, 98:10886-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell, J.P.; Wenzel, R.P. (2008). Antibiotic options for treating community acquired MRSA. Expert Rev Anti Infect Ther, 6: 299–307. [DOI] [PubMed] [Google Scholar]
- 25.Rubin, R.J.; Harrington, C.A.; Poon, A.; Dietrich, K.; Greene, J.; Moiduddin, A. (1999). The economic impact of Staphylococcus aureus infection in New York City Hospitals. Emerg Infect Dis, 5:9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speer, B.S.; Shoemaker, N.B.; Salyers, A. A. (1992). Bacterial resistance to tetracycline mechanisms, transfer, and clinical significance. Clin Microbiol Rev., 5:387-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart, G.T.; Holt, R. J. (1963). Evolution of natural resistance to the newer penicillins. Br. Med. J., 1: 308-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallin, T.R.; Hern, H.G.; Frazee, B.W. (2008). Community-associated methicillin resistant Staphylococcus aureus. Emerg Med Clin North Am, 26: 431–455. [DOI] [PubMed] [Google Scholar]
- 29.Wyllie, D.H.; Crook, D.W.; Peto, T.E.A. (2006). Mortality after Staphylococcus aureus bacteraemia in two acute hospitals in Oxfordshire, 1997–2003: cohort study. Br. Med. J, 333: (7562)-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu, H.; Sullivan, T.J.; Sekiguchi, J.; Kirikae, T.; Ojima, I.; Stratton, C.F.; Mao, W.; Rock, F.L.; Alley, M.R.; Johnson, F.; Walker, S.G.; Tonge, P.J. (2008). Mechanism and inhibition of saFabI, the enoyl reductase from Staphylococcus aureus. Biochemistry., 47:4228-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]


