Abstract
Solid state fermentation was carried out using various agro- industrial wastes with the best amylase producing strain isolated from soil. Different physicochemical conditions were varied for maximum enzyme production. The strain produced about 5400 units/g of amylase at 1:3 moisture content, 20% inoculum, after 72 h of incubation with Mustard Oil seed cake as the substrate. The optimum temperature and pH of the enzyme activity were found to be 50°C and 6 respectively. The enzyme was found to be thermostable at 70°C for about 2 h without any salt. It showed stability at pH range 5–7. The metal ions as Na+, Ca++, Mg++ and Co++ enhanced the enzyme activity.
Keywords: Solid State Fermentation, Agro-industrial wastes, Amylase, Enzyme optimization, Stability, Bacillus sp.
INTRODUCTION
Amylase is one of the most widely used enzymes in the industry. It hydrolyses starch and is used commercially for the production of sugar syrups from starch which consist of glucose, maltose, and higher oligosaccharides (10). Amylases are of great significance in biotechnological applications ranging from food, fermentation, detergent, pharmaceutical, brewing and textile to paper industries (18, 12). To meet the higher demands of these industries, low cost production of amylase is required.
Amylase is produced in bacteria, fungi, plants and animals. However, due to efficient production strategies, microorganisms have substantial potential to contribute to a number of industrial applications (23). Such industrially important microorganisms are found within the Bacillus species because of their rapid growth rates that lead to short fermentation cycles, their capacity to secrete proteins into extra cellular medium, and general handling safety (19).
Production of these α amylases has been investigated through submerged (SmF) and solid-state fermentation (SSF) (21). However, the contents of a synthetic medium are very expensive and uneconomical, so they need to be replaced with more economically available agricultural and industrial by-products, as they are considered to be good substrates for SSF to produce enzymes (17). In recent years the technique of solid-state fermentation (SSF) process has been developed and used more extensively. It has advantages over SmF like simple technique, low capital investment, cheaper production of enzyme having better physiochemical properties, lower levels of catabolite repression and better product recovery (5). The major factors that affect microbial synthesis of enzymes in a SSF system include selection of a suitable substrate and microorganism, particle size of the substrate, inoculum concentration and moisture level of the substrate. Thus it involves the screening of a number of agro-industrial materials for microbial growth and product formation (23). Temperature and pH are known to be important parameters in the production of enzymes from bacteria; hence, the thermal and the pH stability of the enzyme, which is a function of the exposure time, must also be taken into account.
The present work represents an investigation into amylase production by SSF with Wheat bran, Gram husk, Rice Bran and Mustard Oilseed cake as substrates and the determination of optimized production conditions.
MATERIALS AND METHODS
Isolation of Bacteria
About 50 strains were isolated from soil samples collected from various sites at Delhi and NCR, India. These strains were characterized by colony morphologically, shape and gram character; and maintained in 50% glycerol stock at -20°C till further use.
Screening of bacterial isolates
Primary screening of bacterial isolates for production of alpha amylase was done by the starch agar plate method (2). Out of 50 strains, the 3 strains that showed the biggest zone of clearance in starch hydrolysis were selected for production in Solid State Fermentation.
Inoculum preparation: The selected bacterial strains were inoculated in nutrient broth [consisting of (g L-1): peptone, 5; Beef extract, 3; NaCl, 5] followed by incubation at 37ºC for 24 h to get a standardized inoculum (0.5 OD at 600nm with 3.5 x 105 cfu/ml)
Substrate
Four different types of agro-industrial wastes to be used as substrate viz., Wheat bran, Gram husk, Rice Bran and Mustard Oilseed cake were procured from local market of Delhi and powdered obtain a particle size of 1.0 to 2.0 mm. SSF was performed with all the four substrates and their enzyme production was checked by assay.
SSF Technique
Experiments were conducted in 100ml Erlenmeyer flasks containing 5g of the substrate impregnated with 10ml of sterile liquid nutrient medium containing(%): [KH2PO4 - 0.1, NaCl- 0.25, MgSO4.7H2O- 0.01, CaCl2- 0.01]. The flasks were autoclaved and inoculated with 1ml of the prepared inoculum, thoroughly mixed and followed by incubation at 37°C for 5 days. Samples were aseptically withdrawn periodically and assayed for amylase activity.
Enzyme assay
Estimation of amylase activity was carried out according to the DNSA (3, 5 dinitro salicylic acid) method. One ml of 1% starch was incubated with different dilutions of the enzyme extract and 1ml of citrate- phosphate buffer (pH 6.0). The reaction mixture was incubated at 50°C for 30 min. The reaction was stopped by adding 2ml of DNS and kept in boiling water bath for 10min. The absorbance was read at 540nm using a Spectrophotometre (Shimadzu, Thermoelectric cell holder,S-1700), against glucose as the standard. One unit of enzyme activity is defined as the amount of enzyme, which releases 1μmole of reducing sugar as glucose per minute, under the assay conditions (U/ml/min). The experiments were carried out in triplicates and standard error was calculated.
Optimization studies for enzyme production
In a sequential order, the various physicochemical factors as substrate, moisture content and inoculum size affecting the enzyme production were optimized for maximal enzyme production by using the solid substrate for which best amylase activity was observed.
Substrate
The enzyme production was studied with all the four types of agro-industrial wastes viz., Wheat bran, Gram husk, Rice Bran and Mustard Oilseed cake.
Moisture
Moisture content for the solid state fermentation of the enzyme was evaluated by varying the moisture content from 1:2, 1:3, 1:4 and 1:5.
Inoculum Size
The enzyme production was checked by varying the inoculum size at 1%, 5%, 10% and 20% of bacterial culture with colony count at 3.5x105 cfu/ml.
Partial purification of enzyme
Partially purified enzyme was obtained by addition of solid (NH4)2SO4 to the crude enzyme obtained after solid state fermentation, with constant stirring at room temperature. The precipitate was collected by centrifugation at 12000 x g for 20 min at 4°C. This precipitate was dissolved in 0.1 M phosphate buffer (pH 6.0) and dialyzed overnight against the same buffer. This dialysate was used as the enzyme solution.
Evaluation of factors affecting enzyme activity
Various process parameters as temperature and pH affecting enzyme activity were optimized. The strategy was to optimize each parameter for the best enzyme activity, independently of the others and subsequently optimal conditions were employed in all experiments.
Temperature Optima
Optimum temperature for the enzyme assay was assessed by varying the incubation temperature of the assay from 30°C to 70°C.
pH Optima
Optimum pH for the enzyme assay was assessed by performing the assay with buffers ranging from pH 5 to 9 (citrate phosphate buffer for pH 5–8 & Tris-HCl buffer for pH 9).
Thermostability of Amylase
The thermal stability of the enzyme was assessed by incubating the enzyme without the substrate at various temperatures between 30 to 60ºC for 2h. Enzyme was taken at every 30min intervals, and was assayed for activity, thus assessing the stability of the enzyme at different temperatures.
pH stability
The stability of the enzyme in different pH was assessed by incubating the enzyme for 2 hrs in buffers of different pH (citrate phosphate buffer for pH 5–8 & Tris-HCl buffer for pH 9). The stability of the enzyme was investigated by checking the enzyme activity at every 30 min
Effect of Metal Ions
Enzyme activity was assayed in the presence of 5mM and 10mM concentrations of various metal ions (Na+, Mg++, Ca++ and Co++) in chloride salts. The relative activity of the enzyme was compared with the activity obtained in 0.1M citrate phosphate buffer.
RESULTS
Three strains RSA-27, RSB-75 and RSE-162, were selected on the basis of the zone of clearance they exhibited in the starch test, for the SSF technique. Fig 1 shows the enzyme production by these strains in utilizing Wheat bran as substrate in the solid state fermentation.
Figure 1.
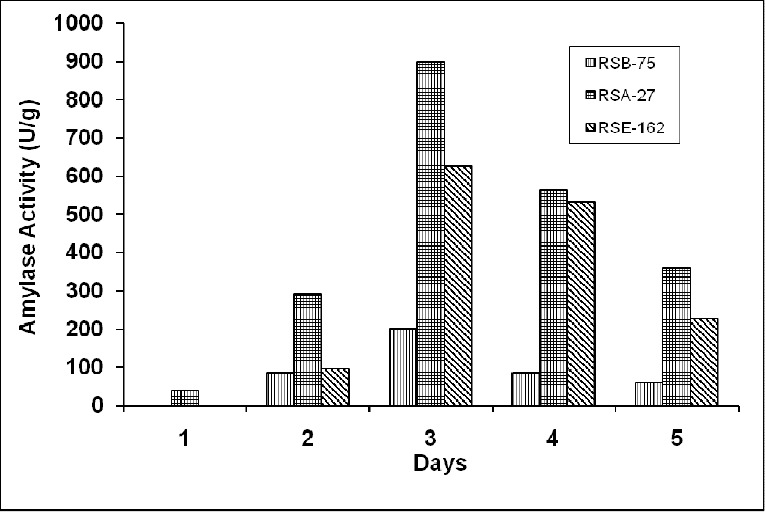
Amylase production by three strains RSB- 75 (▥), RSA- 27 (▦) and RSE 162 (▤) by using Wheat Bran as substrate.
Strain RSA-27 showed the maximum activity of 900 U/g, while Strain RSB-75 and Strain RSE-162 showed the maximum activity of 200 U/g and 626 U/g respectively on the third day. The production in all the three strains increased till day 3, where they showed the maximum enzyme activity, thereafter, decreasing substantially till day 5. Hence, strain RSA-27, a gram positive rod shaped bacterium, was selected for further testing and optimization.
Effect of different Substrate, Moisture content and Inoculum size on enzyme production
Fig. 2 shows the enzyme production of the strain with all the four substrates (Wheat Bran, Gram Husk, Rice Bran and Mustard Oilseed cake), the effect of variation in the moisture content and the inoculum size. The best productivity was observed with Mustard Oilseed Cake with which about 5166 U/g of enzyme was produced which was much high compared to 1233, 900 and 933 U/g of enzyme produced by using Wheat Bran, Gram Husk, Rice Bran respectively. Hence Mustard Oilseed Cake was selected as substrate for further optimization.
Figure 2.
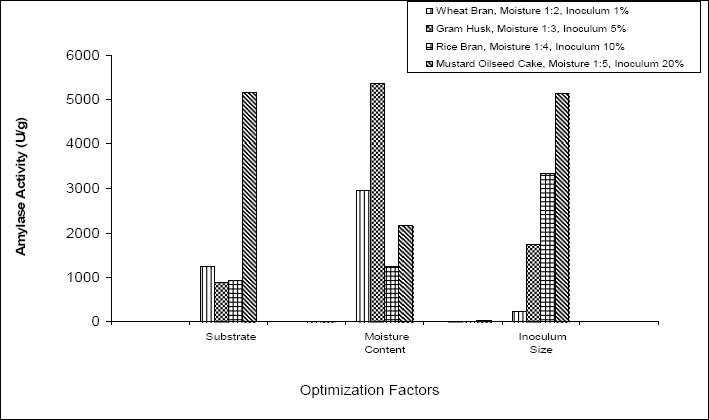
Effect of variation in Substrate, Moisture Content and Inoculum Size in enzyme production (▥)- Substrate Wheat Bran, Moisture Content 1:2, Inoculum Size 1%, (▩) – Substrate Gram Husk, Moisture Content 1:3, Inoculum Size 5%, (▦) - Substrate Rice Bran, Moisture Content 1:4, Inoculum Size 10%, (▧) - Substrate Mustard Oilseed Cake Moisture Content 1:5, Inoculum Size 20%,
The best production was given at moisture content 1:3. At 1:3 moisture content, the strain showed an enzyme activity of 5366 U/g compared to 2966 units, 2166 units and 1233 U/g at 1:5, 1:4 and 1:2 ratios respectively.
Similarly, the best inoculum size was found to be 20% which yielded 5133 U/g of enzyme on the third day. At 1%, 5% and 10% the enzyme production was 226, 1733 and 3333 U/g respectively.
Optimum Temperature and pH
Fig. 3(a) shows the activity of the enzyme at different temperatures ranging from 30°C to 70°C. The Optimum temperature was found to be 50°C. Fig. 3(b) shows the activity of the enzyme at different pH ranging from 5 to 9. The Optimum pH was found to be 6.
Figure 3.
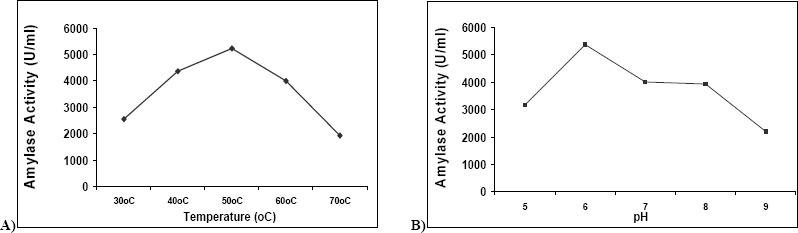
(a) :Temperature optimization for enzyme production [30°C to 70°C]. (b): pH optimization for enzyme production [5 to 9]
Thermostability and pH stability of the enzyme
Fig. 4(a) represents the stability of the enzyme at various temperatures. The enzyme was found to be stable at 30°C for 2h. At 40 and 50°C the enzyme was stable for 1h, thereafter retaining 90% of its activity. At 60 and 70°C the enzyme retained 75- 80% of its activity after 2h.
Figure 4.
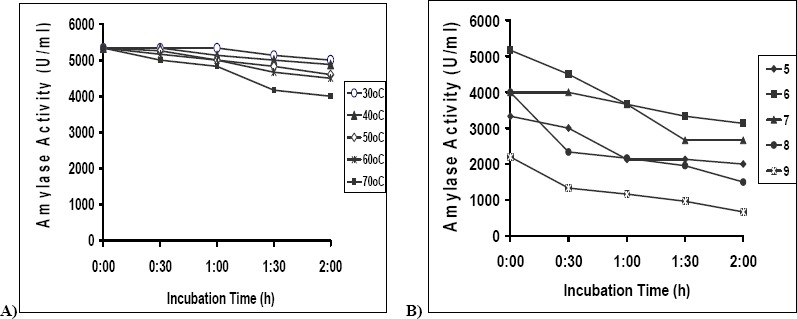
(a): Stability of enzyme at temperatures 30°c to 70°C (◆) 30°C, (●) 40oC, (▲) 50°C, (■) 60°C, (□) 70°C. (b): Stability of enzyme at pH 5 – 9 (◆) 5, (■) 6, (▲) 7, (●) 8, (Ж) 9
Fig. 4(b) represents the stability of the enzyme at different pH. The enzyme was very stable at neutral pH (6-7). At pH 5 the enzyme showed stability for 1 h, thereafter losing only about 20% activity. At pH 8 and 9 it lost about 50% of its activity in 30 min.
Effect of Metal Ions
The presence of salts as Ca2+, Mg2+, Co2+ and Na+ at 5mM concentration enhanced the activity of the enzyme (Table 1). Addition of 10mM CaCl2 increased the activity of the enzyme two fold, and retained 50% of the activity after 11/2 h and decreasing further in 2 h at 70°C. However, at 5mM CaCl2 the activity of the enzyme enhanced by 30% and retained more than 90% of its activity in 2h (Fig. 5). In contrast, CoCl2 and NaCl reduced the amylase activity at 10mM (Table 1).
Table 1.
Effect of various metal ions on enzyme activity
| Salt Concentration | Ca++ | Mg++ | Co++ | Na+ |
|---|---|---|---|---|
| Without Salt | 5166* | 5166* | 5166* | 5166* |
| 5mM | 8500 | 6000 | 6166 | 7500 |
| 10mM | 10666 | 3500 | 5000 | 5666 |
Enzyme activity in U/mL/min
Figure 5.
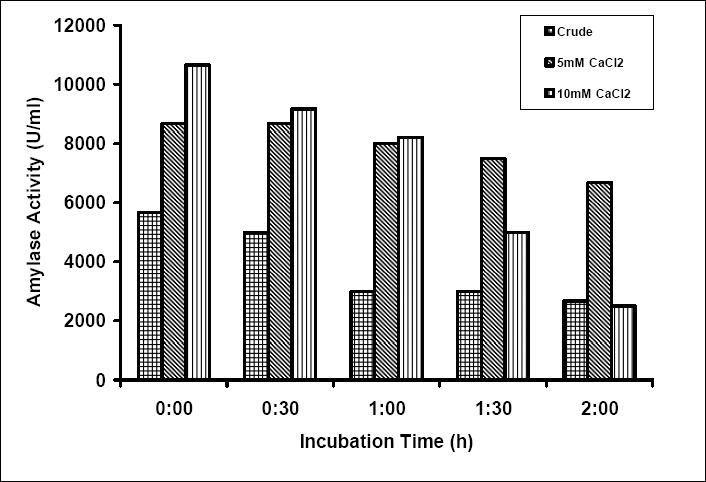
Effect of Ca++ ions on the thermal stability of the enzyme at 70°C (▦) – Crude, (▧) – 5Mm CaCl2, (▥) – 10mM CaCl2
DISCUSSION
The isolated strain exhibited a large zone of clearance in the starch test which showed that it was able to produce amylase in a substantial quantity. From the industrial viewpoint it is necessary to have a strain that can produce large quantity of the enzyme in short fermentation time. Our strain produced very high amount of amylase, about 5400 U/ g in only 72 h which is much higher than that reported by other researchers in SSF (14, 24)
Moisture content is a critical factor for SSF processes because this variable has influence on growth and biosynthesis and secretion of different metabolites. Lower moisture content causes reduction in solubility of the nutrients of the substrate, low degree of swelling and high water tension (4). On the other hand, higher moisture levels can cause a reduction in enzyme yield due to steric hindrance of the growth of strain by reduction in porosity (interparticle spaces) of the solid substrate, thus interfering with oxygen transfer (21). The moisture levels in SSF processes vary between 30 and 85% (4). The optimum moisture content for growth and substrate utilization depends on the organism and the substrate used for cultivation. In this study the optimum moisture content for the enzyme production was found to be 33%.
Inoculum size is an important factor for the production of enzyme. Lower inoculum level results in a lower number of cells in the production medium. This requires a longer time to grow to an optimum number to utilize the substrate and form the desired product (13). The best inoculum size in our study was 20%.Bayasl et al (2008) have also reported 20% inoculum size as optimum in SSF of wheat bran in amylase production, though Anto et al. (2006), have reported that increase in inoculum size adversely affected the enzyme production.
It is evident from this study that oilseed cakes may serve as ideal fermentation bases for obtaining high yields of amylase from Bacillus sp. Mustard oilseed cake contains a lower level of proteinaceous matter and a higher level of carbohydrate than all the other substrates used (16), thus is a suitable nutrient source by itself for amylase production. However, Ikram-ul-Haq et al. (2003) have reported wheat bran as the best substrate for α-amylase production by Bacillus licheniformis using different agricultural by-products.
Amylases are known to be active in a wide range of temperature (40–90°C) and pH (4–11). The optimum temperature of this amylase was 50ºC, which is similar to that described for other Bacillus amylases (15, 7). This identifies the unique characteristic of this Bacillus sp that grows at 37°C as a mesophile, but the enzyme it produces is active and stable at high temperatures (50–70°C). Similar finding has been reported by Hagihara et al. (2006). The stability of the enzyme was found to be independent of divalent calcium ions. The enzyme stability trend, as reported in the present study, agrees with the behavior of amylases from Bacillus sp. investigated by Cordeiro et al. (2002). Thermal stabilization of the enzyme in the presence of calcium has also been reported (8).
The pH optima of the enzyme was found to be 6 with stability in range 5–7. Amylases are active and stable over a wide range of pH (3.5–12), (15, 7), though some are only stable within a narrow pH range (9).
The enzyme activity was found to be enhanced by lower concentrations of calcium, cobalt, magnesium and sodium ions. Similar findings have been reported by Ajayi and Fagade (1), However their reports of NaCl being less effective for enzyme activity contradicts our study where NaCl has been found to enhance the activity, equally.
The unique capability of amylase to retain about 80 % enzyme activity at 70 °C compared to the optimum at 50°C makes it useful for various industrial applications like starch liquefaction at increased temperature as starch liquefaction is carried out at higher temperatures of 70–90 °C (3). Furthermore, the enzyme was active under acidic to neutral condition (pH 6–7), which facilitates its use in dough preparation in the food industry, juice and fruit processing, baking and brewing industry.
REFERENCES
- 1.Ajayi A.O., Fagade O.E. Utilization of corn starch as substrate for β- amylase by Bacillus spp. African Journal of Biomedical Research. 2003;6(1):37–42. [Google Scholar]
- 2.Aneja K.R. Experiments in Microbiology, Plant Pathology, Tissue culture and Mushroom Production Technology. New Delhi, India: New Age International (P). Ltd Publishers; 2002. pp. Pp169–171. [Google Scholar]
- 3.Anto H., Trivedi U., Patel K. Alpha Amylase Production by Bacillus cereus MTCC 1305 Using Solid-State Fermentation. Food Technol. Biotechnol. 2006;44(2):241–245. [Google Scholar]
- 4.Balkan B., Ertan F. Production of α-Amylase from P. chrysogenum, under SSF by using some agricultural by-products. Food Technol. Biotechnol. 2007;45(4):439–442. [Google Scholar]
- 5.Baysal Z., Uyar F., Aytekin C. Solid state fermentation for production of α-amylase by a thermotolerant B. subtilis from hot-spring water. Process Biochem. 2003;38:1665–1668. [Google Scholar]
- 6.Baysal Z., Uyar F., Dogru M., Alkan H. Production of extracellular alkaline α Amylase by SSF with a newly isolated Bacillus sp. Prep. Biochem. Biotechnol. 2008;38:184–190. doi: 10.1080/10826060701885167. [DOI] [PubMed] [Google Scholar]
- 7.Bernhardsdotter E.C.M.J., Ng. J.D., Garriott O.K., Pusey M.L. Enzymic properties of an alkaline chelator-resistant α amylase from an alkaliphilic Bacillus sp. isolate L1711. Process Biochem. 2005;40:2401–2408. [Google Scholar]
- 8.Chung Y.C., Kobayashi T., Kanai H., Akiba T., Kudo T. Purification and properties of extracellular amylase from the hyperthermophilic Archaeon Thermococcus profundus DT 5432. Appl Environ Microbiol. 1995;61(4):1502–1506. doi: 10.1128/aem.61.4.1502-1506.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coronado M., Vargas. C., Hofemeister J., Ventosa A., Nieto J.J. Production and biochemical characterization of an α amylase from the moderate halophile Halomonas meridiana. FEMS Microbiol. Lett. 2000;183:67–71. doi: 10.1111/j.1574-6968.2000.tb08935.x. [DOI] [PubMed] [Google Scholar]
- 10.Hagihara H., Igarashi K., Hayashi Y., Endo K., Ikawa–Kitayama K., Ozaki K., Kawai S., Ho S. Novel α amylase that is highly resistant to chelating reagents and chemical oxidants from the alkaliphilic Bacillus isolate KSM.K.38. Appl. Environ. Microbiol. 2001;67:1744–1750. doi: 10.1128/AEM.67.4.1744-1750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikram-ul-Haq H., Ashraf J., Iqbal M.A., Qadeer. Production of alpha amylase by Bacillus licheniformis using an economical medium. Bioresour. Technol. 2003;87:57–61. doi: 10.1016/s0960-8524(02)00198-0. [DOI] [PubMed] [Google Scholar]
- 12.Kathiresan K., Manivannan S. α Amylase production by Penicillium fellutanum isolated from mangrove rhizosphere soil. African J. Biotech. 2006;5(10):829–832. [Google Scholar]
- 13.Kashyap P., Sabu A., Pandey A., Szakas G., Soccol C.R. Extracellular L-glutaminase production by Zygosaccharomyces rouxii under SSF. Process. Biochem. 2002;38:307–312. [Google Scholar]
- 14.Kokab S., Ashgar M., Rehman K., Asad M.J., Aedeyo O. Bio-Processing of banana peel for α-Amylase production by B. subtilis. Internat. J Agric Biol. 2003:1560–8530. 36–39. [Google Scholar]
- 15.Konsula Z., Liakopoulou-Kyriakides M. Hydrolysis of starches by the action of an α amylase from Bacillus subtilis. Process Biochem. 2004;39:1745–1749. [Google Scholar]
- 16.Krishnan T., Chandra A.K. Effect of Oilseed Cakes on α-Amylase Production by Bacillus licheniformis CUMC 305. Appl. Environ. Microbiol. 1982;44:270–274. doi: 10.1128/aem.44.2.270-274.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunamneni A., Perumal K., Singh S. Amylase production in solid state fermentation by the thermophilic fungus Thermomyces Lanuginosus. J. Biosci. Bioeng. 2005;100(2):168–171. doi: 10.1263/jbb.100.168. [DOI] [PubMed] [Google Scholar]
- 18.Miller G.L. Use of Dinitro salicylic acid reagent for determination of reducing sugar. Analytical Chemistry. 1959;31:426–429. [Google Scholar]
- 19.Pandey A., Nigam P., Soccol C.R., Soccol V.T., Singh D., Mohan R. Advances in microbial amylases. Biotechnol. Appl. Biochem. 2000;31:135–152. doi: 10.1042/ba19990073. [DOI] [PubMed] [Google Scholar]
- 20.Pandey A, Soccol C.R, Rodriguez-Leon J.A., Nigam P. Solid-state fermentation in biotechnology, Fundamentals and Applications. 1st edition. 2001. pp. 8–29. [Google Scholar]
- 21.Perez-Guarre N., Torrado-Agrasar A., Lopez-Macias C., Pastrana L. Main characteristics and application of solid substrate fermentation. Electron. J. Environ. Agric. Food Chem. 2003;2:243–350. [Google Scholar]
- 22.Ramachandran S., Patel A.K., Nampoothiri K.M., Chandran S., Szakacs G., Soccol C.R., Pandey A. Alpha amylase from a fungal culture grown on oil cakes and its properties. Braz. Arch. Biol. Technol. 2004;47:309–317. [Google Scholar]
- 23.Sodhi H.K., Sharma K., Gupta J.K, Soni S.K. Production of a thermostable α amylase from Bacillius sp. PS-7 by solid state fermentation and its synergistic use in the hydrolysis of malt starch for alcohol production. Process Biochem. 2005;40:525–534. [Google Scholar]
- 24.Varalakshmi K.N., Kumudini B.S., Nandini B.N., Solomon J.D., Mahesh B., Suhas R., Kavitha A.P. Characterization of Alpha Amylase from Bacillus sp.1/isolated from paddy seeds. J Appl Biosci. 2008;1(2):46–53. [Google Scholar]


