Abstract
In order to find out optimum culture condition for algal growth, the effect of light irradiance and temperature on growth rate, biomass composition and pigment production of Spirulina platensis were studied in axenic batch cultures. Growth kinetics of cultures showed a wide range of temperature tolerance from 20 °C to 40 °C. Maximum growth rate, cell production with maximum accumulation of chlorophyll and phycobilliproteins were found at temperature 35 °C and 2,000 lux light intensity. But with further increase in temperature and light intensity, reduction in growth rate was observed. Carotenoid content was found maximum at 3,500 lux. Improvement in the carotenoid content with increase in light intensity is an adaptive mechanism of cyanobacterium S.platensis for photoprotection, could be a good basis for the exploitation of microalgae as a source of biopigments.
Keywords: Spirulina platensis, Growth kinetics, Chlorophyll a, Carotenoid, and Phycobiliproteins
INTRODUCTION
Microalgae are utilized for the production of several fine chemicals which are either unique to the algae or found at relatively high concentration and command a high market value. In this respect Spirulina is one of the most promising micro-alga. Spirulina is one of the economically important cyanobacterial genus due to its industrial production of proteins, P-carotene, vitamins (especially B12), phycocyanin and y-1inolenic acid (6), and has now become the most well-known and the most broadly cultivated species of both cyanobacteria and other algae in the world (24).
Spirulina is attracting commercial conglomerates as a source of various nutraceuticals, biomass and pigments (5, 16). Among various nutraceuticals, pigments attain growing commercial importance due to their high end applications and easy extraction procedures. Algal cultures are influenced by a variety of environmental factors and they play a significant role in the production and composition of the photosynthetic pigments.
In order to produce high biopigments containing biomass, much attention must be paid to culture status. Large-scale production of Spirulina biomass depends on many factors, the most important of which are nutrient availability, temperature and light (3). There have been several reports relating to the effect of various environmental conditions which influence the growth of Spirulina and the composition of the biomass produced by causing changes in metabolism (14, 23).
The objective of the present investigation is to evaluate the influence of different environmental factors i.e. temperature and illumination on the growth and biopigments of Spirulina platensis to optimize the culture condition for its large scale production.
MATERIALS AND METHODS
Microorganism and Culture condition
The unialgal culture of cyanobacterium S.platensis (Jal mahal, Jaipur isolate) was grown axenically in batch culture of Zarrouk’s media (26). In order to find out the best culture condition for growth and biopigment accumulation in S.platensis, in vitro experiments were carried out. The culture was grown with photoperiod of 12 hours light/dark provided by white fluorescent lamps at a light intensity of 2,500 lux and temperature of 25 ± 2°C. Influence of the environmental conditions was studied by varying temperature and light intensity in stepwise individual experiments. Varying light intensities i.e. 500 lux to 4,000 lux were provided by adjusting the light with the help of Lux meter (Lutron LX-101A).
Cultivation
For the cultivation of S.platensis four days old prepared inoculum of unialgal culture was added to three sets of 500 ml conical flasks containing 250 ml sterilized Zarrouk’s medium. To observe the influence of temperature on growth and pigment accumulation, the cultures of S.platensis were grown at different temperatures i.e. 20°C to 45°C (at 5°C interval) at 2,500 lux light intensity. Similarly, the growth of S.platensis was also tested under different light intensities i.e. 500 lux to 4,000 lux (at an interval of 500 lux) at temperature of 25 ± 2°C. During the process of growth, the cultures were shaken thrice a day to avoid clumping and accelerate the growth process.
Analytical methods
Growth was followed through dry weight, optical density and growth rate. Optical density (OD) was recorded with the help of photo-colorimeter at 670 nm and Spectrophotometer was used for pigment estimation. The chlorophyll a content was estimated by Parson and Strickland (15) method, Carotenoids by Jensen (9), and Bennett and Bogorad’s (2) method was used for phycobiliprotein quantitation. Cultures were analyzed for their growth and pigment contents every 5th day.
Statistical analysis
Results of the analyses were compared by one way analysis of variance (ANOVA). The significance between pairs of variable means were analysed using least significant difference (LSD) test at 5 % level of significance (7).
RESULTS
Biomass productivity
Biomass productivity of S.platensis was evaluated at various temperatures and light intensities for a period of 25 days. Growth analysis of cultures grown at different temperatures showed significant difference (P < 0.05) in growth pattern. Maximum biomass concentration (as dry weight) i.e. 0.73 g.L-1 was observed at temperature 35°C and least i.e. 0.26 g.L-1 was found at temperature 20°C (Fig. 1). During the growth of cultures, a wide range of temperature tolerance from 20°C to 40°C was observed. But at 45°C, the growth was almost negligible (data not recorded). The maximum growth rate i.e. 0.091 doubling day-1 was observed at 35°C, but with further increase in temperature reduction in growth rate was observed. At 40°C culture showed 0.041 doubling day-1 which is almost half as compared to growth rate at 35°C (Fig. 2).
Figure 1.
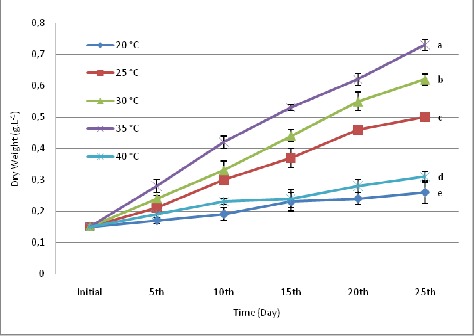
Effect of various temperatures on biomass concentration of S.platensis. Values are means ± SD (n = 3). Variable means with the same letter are not significantly different (p > 0.05).
Figure 2.
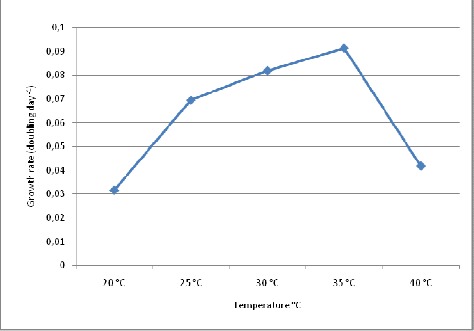
Effect of different temperatures on growth rate (doubling day-1) of S.platensis.
At different experimental irradiances, 2,000 lux was found to be the optimum light intensity for biomass production. Highest biomass (0.71 g.L-1), optical density (0.42) and growth rate (0.094 doubling day-1) was found at 2,000 lux light intensity but with further increase in light intensity, reduction in growth rate was observed (Fig. 3, 4, 5).
Figure 3.
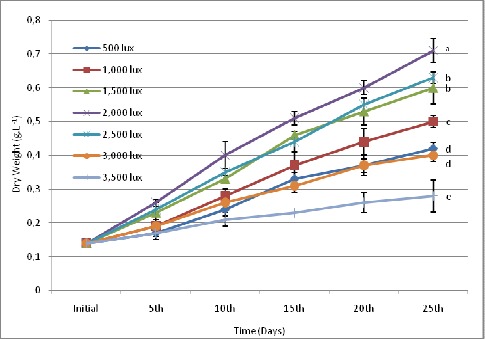
Effect of different light intensities on biomass concentration (dry weight g.L-1) of S.platensis. Values are means ± SD (n = 3). Variable means with the same letter are not significantly different (p > 0.05).
Figure 4.
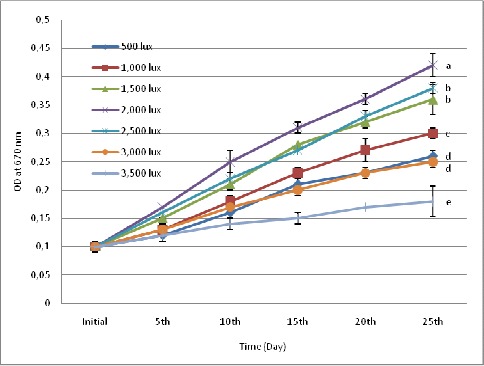
Effect of different light intensities on growth (OD670) of S.platensis. Values are means ± SD (n = 3). Variable means with the same letter are not significantly different (p > 0.05).
Figure 5.
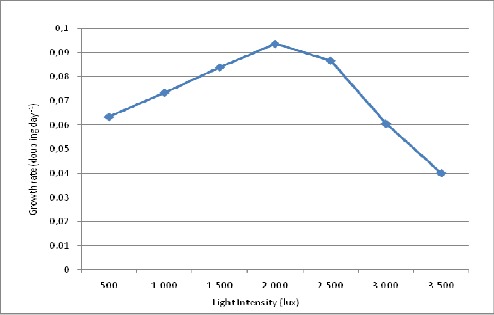
Effect of different lightintensities on growth rate (doublingday -1) of S.platensis.
Effect of temperature and light intensity on biopigments of S.platensis
Biopigment accumulation at different temperatures: After every 5th day, known amount of cell mass was harvested and analyzed for its pigment contents. At different experimental temperatures up to 35 °C pigment content gradually increased but with further increase in temperature reduction in pigments was observed. Cultures grown at 35 °C showed the highest chlorophyll a and carotenoid accumulation i.e. 1.54 % and 0.27 % (of dry weight) respectively (Fig.6). Phycobiliproteins (PBP) were also found maximum in cultures grown at 35 °C i.e. 7.73 % phycocyanin (PC), 3.46 % allophycocyanin (APC) and 1.798 % phycoerythrin (PE) and minimum was observed at 20 °C (5.39 % PC, 2.59 % APC and 0.639 % PE). But the PBP accumulation (except PE) did not show any significant difference (P > 0.05) at temperatures 30 °C and 35 °C (Table 1).
Table 1.
Effect of different temperatures and light Intensities on pigment composition of S.platensis. Values are means ± Standard deviation (n = 3). For each individual experiment variable means with the same letter are not significantly different (p > 0.05). PC – Phycocyanin, APC – Allophycocyanin, PE – Phycoerythrin, Chl. a – Chlorophyll a, Cart – Carotenoid.
| Temperature | PC | APC | PE | Cart/Chl. a | PC/ Chl. a |
|---|---|---|---|---|---|
| 20 C | 5.39 ± 0.26a | 2.59 ± 0.11a | 0.639 ± 0.014a | 0.152 | 4.991 |
| 25 °C | 6.61 ± 0.36b | 3.07 ± 0.11b | 1.193 ± 0.010b | 0.166 | 5.008 |
| 30 °C | 7.34 ± 0.21c | 3.32 ± 0.13c | 1.582 ± 0.013c | 0.171 | 5.027 |
| 35 °C | 7.73 ± 0.52c | 3.46 ± 0.15c | 1.798 ± 0.017d | 0.175 | 5.019 |
| 40 °C | 5.59 ± 0.26a | 2.7 ± 0.14a | 0.86 ± 0.015e | 0.149 | 4.904 |
| Light Intensity | |||||
| 500 lux | 6.125 ± 0.32a | 2.88 ± 0.15a | 0.972 ± 0.006a | 0.158 | 4.940 |
| 1,000 lux | 6.53 ± 0.35a | 3.02 ± 0.12a | 1.126 ± 0.015b | 0.163 | 5.023 |
| 1,500 lux | 7.05 ± 0.3b | 3.22 ± 0.09b | 1.436 ± 0.012c | 0.169 | 5.000 |
| 2,000 lux | 7.65 ± 0.35c | 3.4 ± 0.15b | 1.7 ± 0.035d | 0.174 | 5.100 |
| 2,500 lux | 7.255 ± 0.32bc | 3.29 ± 0.13b | 1.52 ± 0.051e | 0.177 | 5.003 |
| 3,000 lux | 6.1 ± 0.35a | 2.83 ± 0.11a | 0.959 ± 0.017a | 0.178 | 4.959 |
| 3,500 lux | 5.38 ± 0.42d | 2.58 ± 0.19c | 0.63 ± 0.016f | 0.181 | 4.981 |
Biopigment accumulation at different light intensities
Among various light irradiances tested, 2,000 lux light intensity was found optimum for pigment accumulation. The yield of chlorophyll a was maximum i.e. 1.5 % at 2,000 lux light intensity (Fig. 6). But carotenoid/chlorophyll value (0.181) was found maximum at 3,500 lux. There was an improvement in total carotenoid by increasing light irradiance level (Table 1). Maximum PBP accumulation was found at 2,000 lux light intensity, but increase in light irradiance showed reduction in PBP production. Cultures receiving 2,000 lux had 7.65 % PC, 3.4 % APC and 1.7 % PE and significantly (P < 0.05) least PBP (i.e. 5.38 % PC, 2.58 % APC and 0.63 % PE) was found in cultures irradiated with 3,500 lux light intensity. At 2,000 and 2,500 lux light intensity no significant difference observed for PC and APC accumulation, but 2,500 lux showed the significant reduction in PE, so 2,000 lux was found optimum light intensity for PBP accumulation (Table 1).
Figure 6.
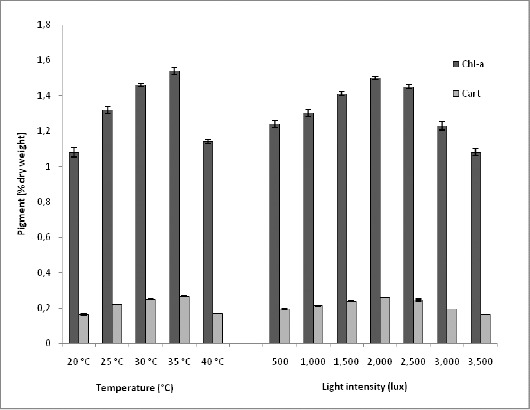
Effect of different temperatures and light intensities on pigments of S.platensis. Values are means ± SD (n = 3). Chl. a – Chlorophyll a, Cart – Carotenoid.
DISCUSSION
Growth analysis of cultures grown at different temperatures showed different growth patterns. Maximum growth rate and biomass concentration were observed in cultures receiving 35°C temperature. Growth curve of cultures at various temperatures revealed that S.platensis has a wide range of temperature tolerance from 20°C to 40°C. Cultures at 20°C and 40°C did not show exponential growth, which proved that extreme temperatures did not support the growth of S.platensis (Fig. 1). But at 45°C temperature, the absence of growth was observed with the disappearance of pigmentation. Growth rate of S.platensis increased with increase in temperature and was found maximum at temperature 35°C. Increased growth rate of S.platensis was due to decrease in doubling time. Later it drops with further increase in temperature due to decrease in chlorophyll and other pigments. Similar observations were also reported by many other workers, Ranjitha and Kaushik (17) reported that the highest biomass in Nostoc muscorum was obtained at 30°C and 35°C. According to Richmond (18), from observations of different strains of Spirulina, the optimal growth temperature was between 35°C and 37°C with 40°C being definitely injurious. In addition, Ronda and Lele (19) and Ogawa et al. (13) reported similar observations for optimum temperature of S.platensis. Earlier reports of Srivastava (22), Bajaj (1) and Singh and Srivastava (21) confirm these observations. Temperature is the most fundamental factor for all living organisms as it affects metabolic processes and biochemical composition of cells. The optimal growth temperature and tolerance to the extreme values usually vary from strain to strain.
Chlorophyll a, carotenoid and phycobiliprotein were also found maximum in cultures growing at temperature 35 °C. PC/Chl. a value indicates that depending on extent of temperature there was decrease in PC accumulation. This decrease in the PC could be due to the bleaching of PBP. In the present study, optimum temperature for phycobiliprotein was 35°C (Table 1). Other workers have reported 37 °C as optimum for Arthronema africanum (4), 36°C for Synechococcus (20) and 25°C for red algae Audoinella pygmaea, Batrachospermum delicatulum and Comsopogon coureuleus (27).
The growth rate, biomass production and biopigment accumulation of S.platensis at different experimental irradiances showed 2,000 lux as the optimum light intensity. Further increase in light intensity shows reduction in growth due to drop in chlorophyll a synthesis. But the carotenoid/chlorophyll value shows the maximum accumulation of carotenoid at 3,500 lux light intensity. Improvement in the carotenoid content by increasing the light intensity is an adaptive mechanism by the organism for photoprotection. S.platensis was also reported to display increased carotenoid levels under strong illumination by Liu (11) and in astaxanthin-accumulating algae Haematococcus pluvialis by Kobayashi et al. (10). PBP also show decreased value with increase in light irradiance. It has been suggested that cyanobacteria prefer low light intensities and stimulate phycobiliprotein synthesis (8) because of their low specific maintenance energy rate and their pigment composition (12). Not only this, low irradiances actually broadens the overall light absorption band in such a way that the balance of light energy distribution between the two photo systems is maintained this optimizes the rate of light energy conversion (25).
On the basis of our findings we concluded that the maximum biomass production with high pigment contents in S.platensis were shown by cultures receiving 35°C temperature and 2,000 lux light intensity. S.platensis has a wide range of temperature tolerance from 20°C to 40°C with different growth rates. High light intensity also helps in improvement of carotenoid level in S.platensis for photoprotection which could be a good basis for the exploitation of S.platensis as a source of biopigments.
REFERENCES
- 1.Bajaj V. India: Jaipur; 1986. The effect of antibiotics and mutagenic chemicals on certain members of green Algae- Scendesmus spp. (PhD Thesis, University of Rajasthan, Jaipur) [Google Scholar]
- 2.Bennett A., Bogorad L. Properties of subunits and aggregates of blue-green algae biliproteins. Biochem. 1971;10:3625–3634. doi: 10.1021/bi00795a022. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho A.P., Malcata F.X. Kinetic modeling of the autotrophic growth of Pavlova lutheri: study of the combined influence of light and temperature. Biotechnol. Prog. 2003;19:1128–1135. doi: 10.1021/bp034083+. [DOI] [PubMed] [Google Scholar]
- 4.Chaneva G., Furnadzhieva S., Minkova K., Lukavsky J. Effect of light and temperature on the cyanobacterium Arthronema africanum a prospective phycobiliprotein producing strain. J. Appl. Phycol. 2007;19:537–544. [Google Scholar]
- 5.Ciferri O., Tiboni O. The biochemistry and industrial potential of Spirulina. Annu. Rev. Microbiol. 1985;39:503–526. doi: 10.1146/annurev.mi.39.100185.002443. [DOI] [PubMed] [Google Scholar]
- 6.Cohen Z. The chemicals of Spirulina. In: Vonshak A, editor. Spirulina platensis (Arthrospira): physiology, cell-biology and biotechnology. USA: Taylor and Francis; 1997. pp. 175–204. [Google Scholar]
- 7.Gomez K.A., Gomez A.A. New York: John Wiley and Sons, Inc; 1984. Statistical procedures for agricultural research; p. 680. [Google Scholar]
- 8.Grossman A.R., Schaer M., Chiang G., Collier J. Environmental effects on the light harvesting complex of cyanobacteria. J. Bacteriol. 1993;175:575–582. doi: 10.1128/jb.175.3.575-582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen A. Chlorophylls and carotenoids. In: Hellebust JA, Craigie JS, editors. Handbook of phycological methods, physiological and biochemical methods. Cambridge: Cambridge University Press; 1978. pp. 59–70. [Google Scholar]
- 10.Kobayashi M.K., Toshihide N.M., Nagai S. Effects of light intensity, light quality and illumination cycle on astaxanthin formation in a green alga, Haematococcus pluvialis. J. Ferment. Bioeng. 1992;74:61–63. [Google Scholar]
- 11.Liu H.I. Effects of temperature and light intensity on growth rate, physiological and biochemical characteristics of Spirulina platensis. Zhonghua Nongye Yanjiu. 1984;33:276–291. [Google Scholar]
- 12.Mur L.R., Elema R.P. Niewe Achtergracht 127, 1018 WS, Amsterdam, The Netherlands: Laboratory of Microbiology, University of Amsterdam; 1983. The influence of light quality on the growth of some phytoplankton species. [Google Scholar]
- 13.Ogawa T., Kozasu H., Terui G. Studies on the growth of Spirulina platensis II. Growth kinetic of an autotrophic culture. J. Ferm. Tech. 1971;50:143–149. [Google Scholar]
- 14.Olguin E.J., Galicia S., Angulo-Guerrero O., Hernandez E. The effect of low light flux and nitrogen deficiency on the chemical composition of Spirulina sp. (Arthrospira) grown on digested pig waste. Bioresour. Technol. 2001;77:19–24. doi: 10.1016/s0960-8524(00)00142-5. [DOI] [PubMed] [Google Scholar]
- 15.Parson T.R., Strickland J.D.H. Particulate organic matter III. I. pigment analysis III, I.I. Determination of Phytoplankton pigments. J. Fish. Res. Bd. Canada. 1965;18:117–127. [Google Scholar]
- 16.Pulz O., Gross W. Valuable products from biotechnology of microalgae Appl. Microbiol. Biotechnol. 2004;65:635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- 17.Ranjitha K., Kaushik B.D. Influence of environmental factors on accessory pigments of Nostoc muscorum. Indian J. Microbiol. 2005;45(1):67–69. [Google Scholar]
- 18.Richmond A. Spirulina. Cambridge: Micro-algal Biotechnology, Cambridge University Press; 1988. pp. 85–121. [Google Scholar]
- 19.Ronda S.R., Lele S.S. Culture conditions stimulating high y-Linolenic acid accumulation by Spirulina platensis. Braz. J. Microbiol. 2008;39:693–697. doi: 10.1590/S1517-838220080004000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakamoto T., Bryant D.A. Growth at low temperature causes nitrogen limitation in the cyanobacterium Synechococcus sp. PCC 7002. Arch. Microbiol. 1998;169:10–19. doi: 10.1007/s002030050535. [DOI] [PubMed] [Google Scholar]
- 21.Singh G.P., Srivastava P. Impact of varied culture conditions on growth and morphology of Ankistrodesmus fusiformis. J. Indian Bot. Soc. 1991;71:341–345. [Google Scholar]
- 22.Srivastava P. India: PhD Thesis, Osmania Univerisity Hyderabad; 1967. Studies on the experimental cultures of certain Chlorococcales. [Google Scholar]
- 23.Volkmann H., Imianovsky U., Oliveira J.L.B., Sant’Anna E.S. Cultivation of Arthrospira (Spirulina) platensis in desalinator wastewater and salinated synthetic medium: protein content and amino-acid profile. Braz. J. Microbiol. 2008;39:98–101. doi: 10.1590/S1517-838220080001000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vonshak A. Use of Spirulina biomass. In: Vonshak A, editor. Spirulina platensis (Arthrospira): physiology, cell-biology and biotechnology. USA: Taylor and Francis; 1997. pp. 205–212. [Google Scholar]
- 25.Wyman M., Fay P. Underlight water climate and the growth and pigmentation of planktonic blue green algae (cyanobacteria). I. The influence of light quantity. Proc. R. Soc. Lond. B. 1986;227:367–380. [Google Scholar]
- 26.Zarrouk C. Paris, France: PhD Thesis, University of Paris; 1966. Contribution a l’etude d’une cyanophycee. Influence de divers facteurs physiques et chimiques sur la croissance et photosynthese de Spirulina maxima (Setch et Gardner) Geitler; pp. 4–5. [Google Scholar]
- 27.Zucchi M.R., Neechi O. Effects of temperature, irradiance and photoperiod on growth and pigment content in some fresh water red algae in culture. Phycol. Res. 2001;49:103–114. [Google Scholar]


