Abstract
About 40 different types of ginsenoside (ginseng saponin), a major pharmacological component of ginseng, have been identified along with their physiological activities. Among these, compound K has been reported to prevent the development of and the metastasis of cancer by blocking the formation of tumors and suppressing the invasion of cancerous cells. In this study, ginsenoside Rb1 was converted into compound K via interaction with the enzyme secreted by β-glucosidase active bacteria, Leuconostoc citreum LH1, extracted from kimchi. The optimum time for the conversion of Rb1 to compound K was about 72 hrs at a constant pH of 6.0 and an optimum temperature of about 30°C. Under optimal conditions, ginsenoside Rb1 was decomposed and converted into compound K by 72 hrs post-reaction (99%). Both TLC and HPLC were used to analyze the enzymatic reaction. Ginsenoside Rb1 was consecutively converted to ginsenoside Rd, F2, and compound K via the hydrolyses of 20-C β-(1 → 6)-glucoside, 3-C β-(1 → 2)-glucoside, and 3-C β-glucose of ginsenoside Rb1.
Keywords: bioconversion, compound K, ginsenoside Rb1, Leuconostoc citreum LH1
INTRODUCTION
Ginsenosides or ginseng saponins are the most important components of ginseng. Essentially, saponin is a glycoside formed through the bonding of carbohydrates, such as glucose, arabinose, xylose, and rhamnose, to structurally steroidal triterpenoids. These ginseng saponins can be either classified into tetracyclic saponins, including protopanaxadiol (PPD), protopanaxatriol (PPT), and/or pentacyclic saponins such as oleanane. Furthermore, it has been reported that ginsenoside has various effects on the central nervous system (4) and brain function (21). It also exhibits effects on carcinogenesis, cancer treatment (13), immune function regulation (26), diabetes (10), hepatic function (19), blood pressure regulation (11), cardiovascular disorder amelioration (14), menopausal disorder regulation (18), fatigue (5), antioxidant function (17), and anti-stress function (22). Meanwhile, compound K (20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol) is a metabolic product of ginseng protopanaxadiol saponins, such as Rb1, Rb2, Rc, and Rd. This compound prevents the development of cancer malignancy and metastasis by preventing the tumorigenesis of chromosomes and suppressing the invasion of cancer cells. Moreover, it exhibits excellent cancer treatment effects similar to those of BI6-BL6, HepG2, K562, and 95-D (25, 35).
The oral absorption of major saponins is poor; extremely small amounts of Rb1 and Rb2 are oxygenated by gastric juices, with only limited components being soluble (12). In this regard, the biological usage rates of Rb1, Rb2, and Rg1 are 0.1–44%, 3.7%, and 1.9%-18.4%, respectively, which indicates that the majority of these saponins are excreted in the urine (28, 29, 32). To enhance its pharmaceutical efficacy, the major saponin forms must be converted into the minor forms that more easily absorbed. Several studies have shown that the transformation of ginsenosides into deglycosylated ginsenosides is required for increased in vivo physiological activities (30). Various transformation methods have been used, including mild acid hydrolysis (9), enzymatic conversion (16), and microbial conversion (1), although the chemical methods produce side reactions such as epimerization, hydration, and hydroxylation. Most of the microorganisms that have been used for ginsenoside transformation are not food-grade standard.
In the present study, we characterized the transformation of the major protopanaxadiol ginsenoside Rb1 using cell-free extracts of the Leuconostoc citreum LH1 strain isolated from kimchi, a traditional Korean fermented food.
MATERIALS AND METHODS
Materials
The Leuconostoc citreum LH1 strain was isolated from kimchi. MRS broth was purchased from Difco (Miller, Becton Dickinson and Co., MD, U.S.A.). Ginsenoside Rb1 was obtained from Panax quinquefolius, and standard ginsenosides including 20(S)-Rb1, 20(S)-Rd, 20(S)-Rg3, 20(S)-Rh2, and compound-K were obtained from KT&G (Daejeon, Korea). A 60 F-254 silica gel plate (Merck) was used for thin-layer chromatography (TLC), and silica gel 60 column (70–230 mesh, Merck) was used for column chromatography. A high performance liquid chromatography system (HPLC) (NS 3000i system, FUTECS Co., Ltd., Korea) equipped with a UV/Vis detector and gradient pump was also used for analysis.
Screening of β-glucosidase -producing lactic acid bacteria
Esculin-MRS agar (31) was used to isolate β-glucosidase-producing lactic acid bacteria. This growth medium contains (per 1 L) 3 g esculin and 0.2 g ferric citrate in MRS agar (Becton Dickinson and Co.). The lactic acid bacteria which produced the β-glucosidase that hydrolyzes esculin appeared on the esculin-MRS agar as colonies surrounded by a reddish-brown to dark brown zone. Subsequently, single colonies from those plates were subjected to an additional two-day incubation at 37°C.
Phylogenetic analysis
The complete sequence of the LH1 strain’s 16S rRNA gene was compiled through SeqMan software and edited using the BioEdit program. The 16S rRNA gene sequences of the related taxa were obtained from GenBank, and the phylogenetic tree was constructed via the neighbor-joining method using the MEGA 3.1 program. A bootstrap analysis with 1,000 replicates was likewise conducted to obtain confidence levels for the branches. Finally, the closest type strains were included in the phylogenetic tree.
Preparation of crude microbial enzymes
The LH1 strain was grown in the MRS broth at a temperature of 37°C until absorbance at 600 nm reached 1.0. After culture broth centrifugation (15,000 × g for 10 min at 4°C) using a MICRO 17R microcentrifuge (Hanic Science Industrial, Korea), four volumes of ethanol (EtOH) were added to the supernatant. This solution was mixed sufficiently and placed in an ice chamber for 20 min to foster further reactions. Meanwhile, the protein pellet was collected via centrifugation (10,000 × g for 40 min at 4°C) and was dissolved in a 20 mM sodium phosphate buffer (pH 6.0).
Parameter optimization for the bioconversion of ginsenoside Rb1
The effects of temperature on the transformation of ginsenoside Rb1 were investigated at temperatures in the range of 25–60°C. Reactions were performed in sodium phosphate buffer (pH 7.0) containing ginsenoside Rb1 (1 μmol) and the crude enzyme (250 μL) for 48 hrs.
The optimum reaction pH for the conversion of ginsenoside Rb1 varied between 3.0 and 13.0. Reactions were performed in various buffers (pH 3.0–13.0) containing ginsenoside Rb1 (1 μmol) and the crude enzyme (250 μL) at 30°C for 48 hrs, followed by extraction with water-saturated n-butanol. Subsequently, the n-butanol fraction was evaporated to dryness, and the methanolic extract was analyzed using HPLC.
Conversion of ginsenoside Rb1 with crude enzymes
The Leuconostoc citreum LH1strain was grown in MRS broth at 37°C until its absorbance at 600 nm reached 1.0. The crude enzymes from each culture broth were dissolved in 20 mM sodium phosphate buffer (pH 6.0) and then mixed with 1 mM ginsenoside Rb1 dissolved in distilled water at a 1:4 ratio (v/v). Subsequently, the mixture was incubated at 30°C and stirred at 190 rpm for 72 hrs. During the reaction period, a 1.25-mL aliquot was collected every 12 hrs, extracted in water-saturated n-butanol and then analyzed using both TLC and HPLC.
Analysis of ginsenosides using TLC
TLC was performed with silica gel plates (60F254, Merck, Darmstadt, Germany), and CHCl3-CH3OH-H2O (65:35:10, V/V, lower phase) was used as its developing solvent. The spots on the TLC plates were detected through spraying with 10% H2SO4, followed by heating at 110°C for 10 min (24).
Analysis of ginsenosides using HPLC
The HPLC-grade acetonitrile and water were purchased from SK Chemicals (Ulsan, Korea). The reaction mixture was extracted in n-butanol saturated with H2O and evaporated in vacuo. Additionally, the residue was dissolved in CH3OH and injected for HPLC analysis. This experiment also employed a C18 (250 × 4.6 mm, ID 5 μm) column using acetonitrile (solvent A) and distilled water (solvent B) mobile phases at A/B ratios of 15:85, 21:79, 58:42, 90:10, 90:10, 15:85, and 15:85; with run times of 0–5, 5–25, 25–70, 70–72, 72–82, 82–84, and 84–100 min, respectively. The flow rate was 1.6 ml/min and the sample was detected at UV 203 nm.
Analysis of the nuclear magnetic resonance spectrum
The reaction products 1, 2, and 3 from the Rb1 bacterial bioconversion of strain LH1 were separated on a silica gel column using chloroform/methanol/water (8:3:1, by vol., lower phase). Their structures were assessed via the proton and carbon nuclear magnetic resonance (1H-NMR, 13C-NMR) spectrum methods, using an FT-NMR spectrometer (Varian Inova AS 400, Varian, USA; 400 MHz), with pyridine-d5 solvent. The ratio of solvent to CHCl3/MeOH/H2O was 65:35:10 by volume (lower phase), and saponin standards were used as references. The results of the NMR analyses were as follows.
Metabolite 1: White powder; mp.: 204~206°C ; 1H-NMR (pyridine-d5, 400 MHz): d0.73 ppm (3H, s, H-19), d0.88 ppm (3H, s, H-30), d0.89 ppm (3H, s, H-18), d1.05 ppm (3H, s, H-29), d1.22 ppm (3H, s, H-28), d1.56 ppm (6H, s, H-26, H-27), d1.58 ppm (3H, s, H-21), d4.87 ppm [1H, d, J = 7.6Hz, H-3-glc (inner)-1H], d5.14 ppm [1H, d, J = 7.6Hz, H-20-glc-1H], d5.34 ppm [1H, d, J = 7.6Hz, H-3-glc (outer)-1H]; for 13C-NMR (pyridine-d5, 100 MHz) data see Table 1.
Table 1.
13C-NMR chemical shifts of material Rb1 and metabolites 1, 2, 3 (100 MHz, solvent: pyridine-d5). Ginsenoside Rb1 and metabolite 1 (7), metabolite 2 (8), and metabolite 3 (27).
| Carbon site | Material Rb1 | Rd (ppm) Ref. Exp. | F2 (ppm) Ref. Exp. | C-K (ppm) Ref. Exp. | |||
|---|---|---|---|---|---|---|---|
| Aglycone moiety | |||||||
| C-1 | |||||||
| C-2 | 39.2 | 39.1 | 39.1 | 39.2 | 39.2 | 39.5 | 39.6 |
| C-3 | 26.6 | 26.7 | 26.6 | 26.8 | 26.8 | 28.3 | 28.4 |
| C-4 | 89.0 | 88.5 | 88.9 | 88.8 | 88.8 | 78.1 | 78.2 |
| C-5 | 39.7 | 39.6 | 39.7 | 39.7 | 39.9 | 39.6 | 39.7 |
| C-6 | 56.4 | 56.4 | 56.3 | 56.4 | 56.4 | 56.5 | 56.5 |
| C-7 | 18.5 | 18.5 | 18.4 | 18.4 | 18.5 | 18.8 | 18.9 |
| C-8 | 35.1 | 35.2 | 35.1 | 35.1 | 35.2 | 35.3 | 35.3 |
| C-9 | 40.0 | 40.0 | 40.0 | 40.1 | 40.1 | 40.2 | 40.2 |
| C-10 | 50.2 | 50.2 | 50.1 | 50.2 | 50.2 | 50.4 | 50.4 |
| C-11 | 36.9 | 36.9 | 36.8 | 36.9 | 37.0 | 37.5 | 37.5 |
| C-12 | 30.8 | 30.8 | 30.8 | 30.8 | 30.8 | 30.9 | 30.9 |
| C-13 | 70.2 | 70.2 | 70.2 | 70.2 | 70.1 | 70.2 | 70.3 |
| C-14 | 49.5 | 49.4 | 49.3 | 49.5 | 49.5 | 49.6 | 49.7 |
| C-15 | 51.4 | 51.4 | 51.4 | 51.4 | 51.5 | 51.5 | 51.6 |
| C-16 | 30.7 | 30.8 | 30.8 | 30.9 | 31.0 | 31.1 | 31.2 |
| C-17 | 26.8 | 26.7 | 26.7 | 26.6 | 26.7 | 26.7 | 26.8 |
| C-18 | 51.6 | 51.7 | 51.7 | 51.7 | 51.7 | 51.7 | 51.8 |
| C-19 | 16.3 | 16.3 | 16.3 | 16.3 | 16.4 | 16.3 | 16.4 |
| C-20 | 16.0 | 15.9 | 15.9 | 16.0 | 16.0 | 16.1 | 16.2 |
| C-21 | 83.5 | 83.8 | 83.3 | 83.3 | 83.3 | 83.4 | 83.4 |
| C-22 | 22.4 | 22.4 | 22.5 | 22.4 | 22.5 | 22.4 | 22.3 |
| C-23 | 36.2 | 36.0 | 36.0 | 36.1 | 36.2 | 36.3 | 36.4 |
| C-24 | 23.1 | 23.2 | 23.3 | 23.2 | 23.3 | 23.3 | 23.6 |
| C-25 | 126.0 | 125.9 | 125.8 | 126.0 | 126.6 | 126.0 | 126.0 |
| C-26 | 131.1 | 130.9 | 130.8 | 130.9 | 131.5 | 131.0 | 130.9 |
| C-27 | 25.8 | 25.8 | 25.8 | 25.8 | 25.8 | 25.8 | 25.9 |
| C-28 | 17.9 | 17.8 | 17.8 | 17.8 | 17.8 | 17.8 | 17.6 |
| C-29 | 28.1 | 28.0 | 28.1 | 28.2 | 28.2 | 28.7 | 28.8 |
| C-30 | 16.6 | 16.6 | 16.6 | 16.8 | 16.9 | 16.4 | 16.5 |
| sugar moiety 3-O-inner-Glc | 17.4 | 17.3 | 17.3 | 17.4 | 17.4 | 17.5 | 17.3 |
| C-1 | |||||||
| C-2 | 105.1 | 105.0 | 105.0 | 106.9 | 106.8 | ||
| C-3 | 83.5 | 83.3 | 83.1 | 75.8 | 75.9 | ||
| C-4 | 77.2 | 78.1 | 77.9 | 79.3 | 78.7 | ||
| C-5 | 71.6 | 71.6 | 71.6 | 71.7 | 71.6 | ||
| C-6 | 78.1 | 78.1 | 78.2 | 78.3 | 78.3 | ||
| 3-O-outer-Glc | 62.7 | 62.7 | 62.6 | 62.9 | 62.8 | ||
| C-1 | |||||||
| C-2 | 105.4 | 105.9 | 105.8 | ||||
| C-3 | 77.1 | 77.0 | 77.0 | ||||
| C-4 | 79.3 | 79.1 | 79.1 | ||||
| C-5 | 71.6 | 71.6 | 71.4 | ||||
| C-6 | 78.0 | 78.1 | 78.0 | ||||
| 62.9 | 62.7 | 62.6 | |||||
| 20-O-inner-Glc | |||||||
| C-1 | |||||||
| C-2 | 98.1 | 98.2 | 98.2 | 98.3 | 98.2 | 98.3 | 98.3 |
| C-3 | 74.9 | 75.0 | 75.1 | 75.1 | 75.1 | 75.2 | 75.2 |
| C-4 | 78.1 | 78.1 | 78.2 | 78.8 | 79.2 | 79.3 | 79.3 |
| C-5 | 71.6 | 71.6 | 71.6 | 71.9 | 71.8 | 71.9 | 71.9 |
| C-6 | 77.1 | 78.1 | 78.2 | 78.3 | 78.4 | 78.1 | 78.2 |
| 20-O-outer-Glc | 71.6 | 62.7 | 62.8 | 63.1 | 63.0 | 63.1 | 63.1 |
| C-1 | |||||||
| C-2 | 105.4 | ||||||
| C-3 | 74.9 | ||||||
| C-4 | 78.4 | ||||||
| C-5 | 71.6 | ||||||
| C-6 | 78.0 | ||||||
| 62.8 | |||||||
Metabolite 2: White powder; mp.: 184~186° C; 1H-NMR (pyridine-d5, 400 MHz): δ0.82 ppm (3H, s, H-19), δ0.96 ppm (3H, s, H-30), δ0.9 ppm (3H, s, H-18), δ1.00 ppm (3H, s, H-29), δ1.31 ppm (3H, s, H-28), δ1.60 ppm (3H, s, H-26, H-27), δ1.63 ppm(3H, s, H-21), δ4.95 ppm (1H, d, J = 7.2Hz, H-20-glc-1), δ5.21 ppm (1H, d, J = 7.2Hz, H-3-glc-1); for 13C-NMR (pyridine-d5, 100 MHz) data see Table 1.
Metabolite 3: White powder; mp.: 220~222°C ; H-NMR (pyridine-d5, 400 MHz): δ0.83 ppm (3H, s, H-19), δ0.90 ppm (3H, s, H-18), δ0.94 ppm (3H, s, H-30), δ1.00 ppm (3H, s, H-29), δ1.19 ppm (3H, s, H-28), δ1.57 ppm (6H, s, H-26, H-27), δ1.59 ppm (3H, s, H-21), δ5.16 ppm (IH, d, J = 7.2Hz, H-20-glc-1); for 13C-NMR (pyridined5, 100 MHz) data see Table 1.
RESULTS AND DISCUSSION
Phylogenetic study
The 16S rRNA gene sequence of the LH1 strain was aligned with other neighboring type strains, and the taxonomic relationships were confirmed. As shown in Fig. 1, the phylogenetic tree revealed that the LH1 strain was actually grouped with the Leuconostoc species. Moreover, the highest degree of 16S rRNA gene sequence identity was found with Leuconostoc citreum (99.8%). Hence, based on the phylogenetic tree and homology analysis, the LH1 strain was concluded to be Leuconostoc citreum LH1.
Figure 1.
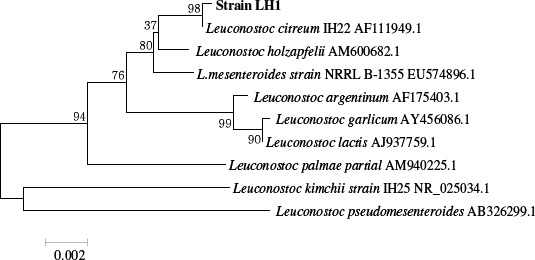
Phylogenetic tree based on the 16S rRNA gene sequence showing the phylogenetic relationships between the Leuconostoc citreum LH1 strain and related Leuconostoc species.
Bioconversion of ginsenoside Rb1
After Leuconostoc citreum LH1 was cultured to an absorbance 600 of 1.0, the crude enzyme solution of the strain was used to transform ginsenoside Rb1 to metabolite 1, metabolite 2 and metabolite 3 as shown in Fig. 2. Evidently, this indicated that metabolite 1 and metabolite 2 were intermediate metabolites, and that metabolite 3 was the final product. The TLC Rf values of metabolite products 1, 2, and 3 were similar to those of ginsenoside Rd, ginsenoside F2 and compound K, respectively, suggesting that Rb1 was converted by the crude enzyme from Leuconostoc citreum LH1. The reaction products 1, 2, and 3, were separated on a silica gel column to confirm their chemical structures.
Figure 2.
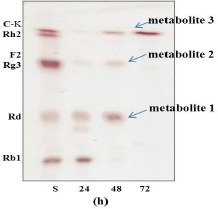
Time course thin-layer chromatography (TLC) analysis of metabolites of ginsenoside Rb1 bioconverted by Leuconostoc citreum LH1. Ginsenoside Rb1 was used as the substrate to yield ginsenoside Rd (1), ginsenoside F2 (2) and compound K (3). Developing solvent: CHCl3/MeOH/H2O (65:35:10, by vol., lower phase). S: saponin standards
Structures of metabolites 1, 2, 3
In the 1H-NMR spectrum of metabolite 1, the proton signals for the H-1s of the 3-O-inner-glucopyranosyl moiety, 3-O-outer-glucopyranosyl moiety, and 20-glucopyranosyl moiety appeared, respectively, at d4.87 ppm [1H, d, J = 7.6Hz, H-3-glc (inner)-1H], d5.34 ppm [1H, d, J = 7.6Hz, H-3-glc (outer) -1H], and d5.14 ppm [1H, d, J = 7.6Hz, H-20-glc-1H]. Metabolite 1 was shown to harbor three β-D-glucoses.
According to the results of comparisons of the 13C-NMR spectrum of metabolite 1 with the material Rb1 (7), the signal for the C-6 of the 20-innerglucopyranosyl moiety was shifted upfield, from d71.6 ppm to d62.8 ppm, and the signal for the C-5 of the 20-inner-glucopyranosyl moiety was shifted downfield, from d77.1 ppm to d78.2 ppm. However, the other signals were similar to those of ginsenoside Rb1. It is believed that the terminal glucose connected at C-20 of aglycon of Rb1 was hydrolyzed by the LH1 strain. Therefore, metabolite 1 was identified as 3-O-[P-D-glucopyranosyl-(1, 2)-P -D-glucopyranosyl]-20-O-[P-D-glucopyranosyl]-20(S)-protopanaxadiol, also known as ginsenoside Rd.
In the 1H-NMR spectrum of metabolite 2, the proton signals for the H-1s of the 3-glucopyranosyl moiety and the 20-glucopyranosyl moiety appeared, respectively, at δ5.21 ppm (1H, d, J = 7.2Hz, H-3-glc-1) and δ4.95 ppm (1H, d, J = 7.2Hz, H-20-glc-1). Metabolite 2 was shown to harbor two β-D-glucoses. According to the comparison of the 13C-NMR spectrum of metabolite 2 with that of metabolite 1, the signal for the C-2 of the 3-inner-glucopyranosyl moiety was shifted upfield, from δ83.1 ppm to δ75.9 ppm, and the other signals were similar to those of metabolite 1. Additionally, based on the fact that the anomeric carbon signals at δ106.8 and δ98.3 ppm were similar to signals for C-1s of the 3-O-inner-glucopyranosyl moiety and 20-O-innerglucopyranosyl moiety of metabolite 1, it was confirmed that the two glucoses were connected at the C-3 and C-20 sites of the aglycon moiety. Therefore, metabolite 2 was identified as 3-O-[P-D-glucopyranosyl]-20-O-[β-D-glucopyranosyl]-20(S)-protopanaxadiol, also known as ginsenoside F2.
The 1 H-NMR spectrum of metabolite 3 showed that only one proton signal for H-1 of the 20-glucopyranosyl moiety appeared at δ5.16 ppm (1H, d, J = 7.2Hz, H-20-glc-1), and metabolite 3 was shown to harbor only one β-D-glucose. According to the comparison of the 13C-NMR spectrum of metabolite 3 with the decomposition of metabolite 2, the signal for C-3 of aglycon was shifted upfield, from δ88.8 ppm to δ78.2 ppm, and the other signals were similar to those of metabolite 1. Therefore, the glucose connected at the C-3 site of aglycon of metabolite 2 was hydrolyzed. The signal for the C-1 of glucopyranosyl moiety appeared at δ98.3 ppm, further indicating that the glucopyranosyl moiety of metabolite 3 was connected to the C-20 site of aglycon. Therefore, metabolite 3 was 20-O-[β-D-glucopyranosyl]- 20(S)-protopanaxadiol, also known as compound K.
Bioconversion pathway
The process through which ginsenoside Rb1 was decomposed by strain LH1 was analyzed using TLC and HPLC, as were any changes in reaction time. As is shown in Fig. 2, the concentrations of Rb1 and those of the decomposition products Rd, F2 and C-K exhibited regular changes with reaction time. The majority of ginsenoside Rb1 had decomposed after 48 hrs of reaction, and ginsenosides Rd (metabolite 1) and F2 (metabolite 2) levels reached a maximum after 24~48 hrs and then gradually decreased. However, the concentration of C-K (metabolite 3) increased continuously from 48 hrs to 72 hrs. These findings indicated that ginsenosides Rd and F2 were intermediate metabolites, and that C-K was the final product after 72 hrs of reaction time. As shown in Fig. 3, HPLC analysis produced results similar to the TLC findings. Ginsenoside Rb1 was 98% degraded after 48 hrs, and ginsenoside Rd levels had decreased, with disappearance of the peak observed at 72 hrs, while the compound K peak increased continuously. This result suggested that strain LH1 exerts potent β-glucosidase activity, and that ginsenoside Rb1 was converted in the following sequence: Rb1 —> Rd —> F2 —> compound K (see Fig. 4) by the enzymes produced from strain LH1, consecutively hydrolyzing 20-C β -(1 —> 6) glucoside, 3-C β -(1 —> 2) glucoside and 3-C β -glucose of ginsenoside Rb1.
Figure 3.
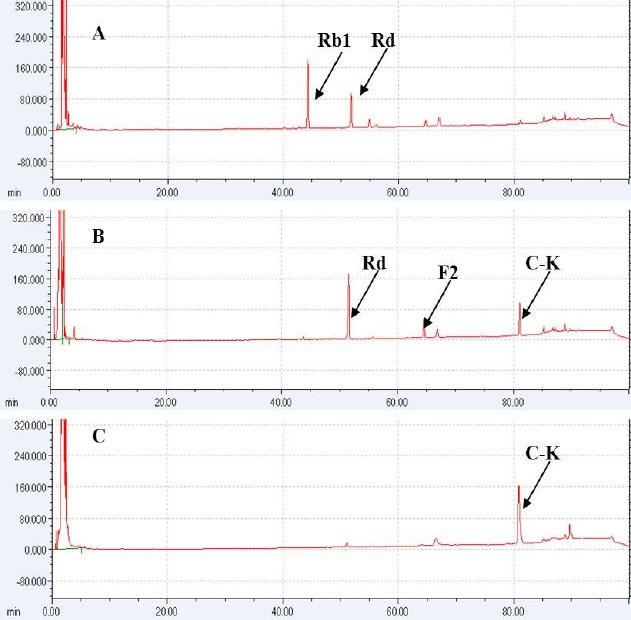
High-performance liquid chromatography (HPLC) analysis of the bioconversions of ginsenosides. The reaction time-course transformation of ginsenoside Rb1 by the crude enzyme Leuconostoc citreum LH1. Incubation time: A, 48 h; B, 60 h; C, 72 h.
Figure 4.
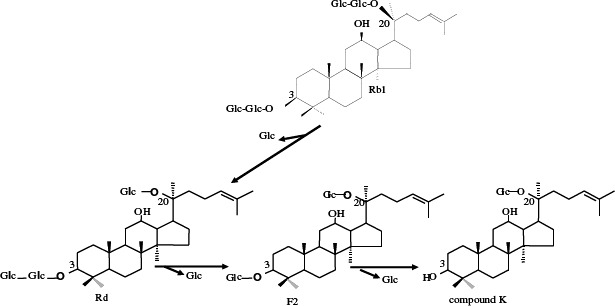
Proposed bioconversion pathway of ginsenoside Rb1 to compound K by Leuconostoc citreum LH1.
The effect of temperature on enzyme activity
Generally, this study focused on determination of the crude enzyme’s optimum temperature through performance at various temperatures (25°C, 30°C, 37°C, 50°C, 60°C). The results shown in Fig. 5 revealed that the reactions of both the LH1 and Rb1 strains’ crude enzymes were greatly influenced by temperature. In addition, the TLC analysis showed that the activity of the LH1 strain’s crude enzyme was greatest at 30°C. The optimum temperature was further confirmed through quantitative HPLC analysis. It was also observed that the concentration of ginsenoside Rb1 began degrading at 25°C, peaked at 30°C and suddenly decreased after 37°C. Hence, the enzyme could not catalyze reactions at temperatures lower than the optimum due to insufficient energy release. At high temperatures, enzymes lose their activities due to denaturation.
Figure 5.
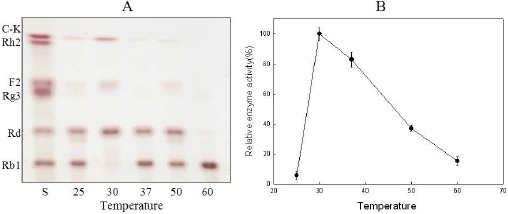
Effects of temperature on the enzymatic conversion of ginsenoside Rb1 according to TLC analysis (A) and quantitative HPLC analysis (B).
The effect of pH on enzyme activity
The ginsenoside conversion activity of the crude enzyme was tested at various pH (4.0–10.0) levels, and TLC analysis revealed that the optimum pH range was pH 5.0- 9.0. Similarly, the quantitative HPLC analysis showed that ginsenoside Rb1 began degrading at pH 4.0 and gradually increased up to pH 5.0. Moreover, maximum enzyme activity was observed at pH 6.0, with a sharp decline at pH 9.0 (Fig. 6).
Figure 6.
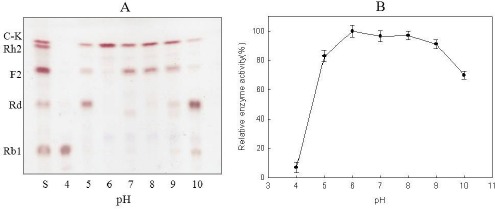
Effects of pH on the enzymatic conversion of ginsenoside Rb1 according to TLC analysis (A) and quantitative HPLC analysis (B).
Ginsenoside Rb1 can be converted to the minor compound K through hydrolysis and loss of a glucose moiety from the 20-C β -(1 → 6) glucoside, 3-C β -(1 → 2) glucoside and 3-C β -glucose of ginsenoside aglycone. Many enzymes are known to hydrolyze the above glycosidic bonds, with β-glucosidase considered the most useful (3, 20). In the present study, attempts were made to screen active β-glucosidase-producing bacteria.
The esculin-MRS agar was specially designed to screen β-glucosidase-producing bacteria. Esculin is needed to detect the β-glucosidase activity, and the MRS agar is optimal for the cultivation of the lactobacillus dominant in kimchi due to its low concentration of nutrients.
In this study, ginsenoside Rb1 was converted into compound K using the enzyme secreted by the β-glucosidase-producing bacteria Leuconostoc citreum LH1, which is isolated from kimchi. The bioconversion pathway to produce compound K followed the sequence: Rb1 → Rd — > F2 → compound K. As such, enzymes secreted by microbes such as human intestinal bacteria (2) and soil bacteria (6) have been used conventionally to transform ginsenoside Rb1 into compound K.
The Leuconostoc citreum LH1 strain is an aerobic and edible lactic acid bacteria. The enzyme activity of this strain was found to be greatest at 30°C and decreased at temperatures higher than 37°C. According to these results, the optimum temperature determined in this study was slightly lower than those reported for other species, such as 45°C for Rhizopus japonicas derivative β-glucosidase (15), 40°C for Aspergillus niger 48g and A. niger 848g derivative β-glucosidase (34), and 40~50°C for general β-glucosidase (23, 33). In addition, the crude enzyme showed a high activity rate at pH 5.0–9.0, while the maximum hydrolysis activity was found at pH 6.0. These pH values were slightly higher than those reported for other species, such as pH 5.0 for A. niger 48g and A. niger 848g derivative β-glucosidase (34), pH 4.8–5.0 for R. japonicas derivative jö-glucosidase (15), pH 6.0 for Fusobacterium K-60 derivative jö-glucosidase (20), and pH 5.0 for ginseng derivative β-glucosidase (25).
Ginsenoside compound K is a promising natural product that could be used for the treatment of numerous human pathologies. Unfortunately, the methods currently available for the commercial production of ginsenoside compound K are difficult. This difficulty has limited the availability and development of this compound. In this study, it was concluded that Leuconostoc citreum LH1 can efficiently transform Rb1 for ginsenoside compound K production.
ACKNOWLEDGEMENTS
This study was supported by KGCMVP for the Technology Development Program of the Ministry of Food, Agriculture, Forestry and Fisheries, Republic of Korea.
REFERENCES
- 1.Bae E.A., Han M.J., Choo M.K., Park S.Y., Kim D.H. Biol. Pharm. Bull.25. 2002. Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities; pp. 58–63. [DOI] [PubMed] [Google Scholar]
- 2.Bae E.A., Kim N.Y., Han M.J., Choo M.K., Kim D.H. Transformation of ginsenoside to compound k (IH-901) by Lactic acid bacteria of human intestine. J. Mìcrobiol. Biotechnol. 2003;13(1):9–14. [Google Scholar]
- 3.Bae E.A., Park S.Y., Kim D.H. Constitutive β-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol. Pharm. Bull. 2000;23:1481–1485. doi: 10.1248/bpb.23.1481. [DOI] [PubMed] [Google Scholar]
- 4.Benishin C.G. Action of ginsenoside Rb1 on choline uptake in central cholinergic nerve endings. Neurochem. Int. 1992;21(1):1–5. doi: 10.1016/0197-0186(92)90061-u. [DOI] [PubMed] [Google Scholar]
- 5.Brekhman I.I. In: Proc. Symp. Gerontology, Lugano, Switzerland. 1976. Ancient ginseng and pharmacology; p. 6. [Google Scholar]
- 6.Cheng L.Q., Kim M.K., Lee J.W., Yang D.C. Conversion of major ginsenoside Rb1 to ginsenoside F2 by Caulobacter leidyia. Biotechnol. Lett. 2006;28:1121–1127. doi: 10.1007/s10529-006-9059-x. [DOI] [PubMed] [Google Scholar]
- 7.Dong A., Ye M., Guo H., Zheng J., Guo D. Microbial transformation of ginsenoside Rb1 by Rhizopus stolonifer and Curvularia lunata. Biotechnol Lett. 2003;25:339–344. doi: 10.1023/a:1022320824000. [DOI] [PubMed] [Google Scholar]
- 8.Dou d., Wen Y., Pei Y., Chen Y., Ma Z. Active constituents reducing side-effects of prednisone acetate in leaves of Panax ginseng C.A.Mey. zhongguo. zhong yao. za zi. 1997;22(3):174–176. [PubMed] [Google Scholar]
- 9.Han B.H., Park M.H., Han Y.N., Woo L.K., Sankawa U., Yahara S., Tanaka O. Degradation of ginseng saponins under mild acidic conditions. Planta. Med. 1982;44:146–149. doi: 10.1055/s-2007-971425. [DOI] [PubMed] [Google Scholar]
- 10.Huo Y., Chen Y. The effect of Panax ginseng extract(GS) on insulin and corticosteroid receptors. J. Traditional Chinese Medicine. 1998;8(4):293–295. [PubMed] [Google Scholar]
- 11.Kang S.Y., Kim N.D. The antihypertensive effect of red ginseng saponin and the endothelium-derived vascular relaxtion. Korean. J. Ginseng Sci. 1992;18:175–182. [Google Scholar]
- 12.Karikura M., Miyase T., Tanizawa H., Taniyama T., Takino Y. Studies on absorption, distribution, excretion and metabolism of ginseng sa1ponins. VI. The decomposition products of ginsenoside Rb2 in the stomach of rats. Chem. Pharm. Bull. 1991;39(2):400–404. doi: 10.1248/cpb.39.400. [DOI] [PubMed] [Google Scholar]
- 13.Kikuchi Y., Sasa H., Kita T., Hirata J., Tode T. Inhibition of human ovarian cancer cell proliferation in vitro by ginsenoside-Rh2 and adjuvant effects of cisplatin in vivo. Anticancer. Drugs. 1991;2(1):63–67. doi: 10.1097/00001813-199102000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Kim H.Y., Chen X., Gillis C.N. Ginsenosides protect pulmonary vascular endothelium against free radical induced injury. Biochem. Biophys. Res. Comm. 1992;189(2):670–676. doi: 10.1016/0006-291x(92)92253-t. [DOI] [PubMed] [Google Scholar]
- 15.Kim S.D., Seu J.H. Enzymatic properties of the convertible enzyme of ginseng saponin produced from Rhizopus japonicus. Kor. J. App. Mìcrobiol. Bioeng. 1989;17(2):126–130. [Google Scholar]
- 16.Ko S.R., Choi K.J., Uchida K., Suzuki Y. Enzymatic preparation of ginsenosides Rg2, Rh1, and F1 from protopanaxatriol-type ginseng saponin mixture. Planta. Med. 2003;69:285–286. doi: 10.1055/s-2003-38476. [DOI] [PubMed] [Google Scholar]
- 17.Mei B., Wang Y.E., Wu JX., Chen W.Z. Protective effect of ginsenosides on oxygen free radical induced damages of cultured vascular endothelial cells in vitro. Yao. Hsueh Hsuuh Pao. 1994;29(11):801–808. [PubMed] [Google Scholar]
- 18.Ogita S., Samugawa K. Clinical effectiveness of Korea ginseng on patients with climacteric disturbances. The Ginseng Review. 1994;18:95–97. [Google Scholar]
- 19.Oura H., Hiai S. Physiological chemistry of ginseng. Metabolism. Disease. 1973;10:564–569. [Google Scholar]
- 20.Park S.Y., Bae E.A., Sung J.H., Lee S.K., Kim D.H. Purification and characterization of ginsenoside Rb1-metabolizing β-glucosidase from Fusobacterium K-60, a huma intestinal anaerobic bacterium. Biosci. Biotechnol. Biochem. 2001;65(5):1163–1169. doi: 10.1271/bbb.65.1163. [DOI] [PubMed] [Google Scholar]
- 21.Saito H., Bao T.T. Effect of red ginseng on mice exposed to various stress. In: Proc. 4th Int’l. Ginseng Symp. Seoul, Korea. 1984:97–105. [Google Scholar]
- 22.Saito H., Nishiyama N. Effect of ginseng and its saponins on experimental amnesis in mice and on cell cultures of neurons. In: Proc. 5th Int’l. Ginseng Symp. Seoul, Korea. 1988:92–98. [Google Scholar]
- 23.Sano K., Amemura A., Harada T. Purification and properties of a beta-1,6-clucosidase from Flavobacterium. Biochim. Biophys. Acta. 1975;377(2):410–420. doi: 10.1016/0005-2744(75)90321-6. [DOI] [PubMed] [Google Scholar]
- 24.Shibata S., Tanaka O., Soma K., Iita Y., Ando T., Nakamura H. Studis on saponins and sapogenens of ginseng: the structure of panaxatriol. Tetrahedron. Letters. 1965;3:207–213. doi: 10.1016/s0040-4039(01)99595-4. [DOI] [PubMed] [Google Scholar]
- 25.Shin J.E., Park E.K., Kim E.J., Hong Y.H., Lee K.T., Kim D.H. Cytotoxicity of compound K (IH-901) and ginsenoside Rh2, main biotransformants of ginseng saponins by Bifidobacteria, against Some Tumor Cells. Ginseng. Res. 2003;27:129–134. [Google Scholar]
- 26.Singh V.K., Agarwal S.S., Gupta B.M. Immunomodulatory activity of panax ginseng extract. In: Proc. 4th Int’l. Ginseng Symp Seoul, Korea. 1984:225–232. doi: 10.1055/s-2007-969773. [DOI] [PubMed] [Google Scholar]
- 27.Sung J.H., Hideo H, Satoshi M., Masamori U. Metabolism of ginseng saponins by intestinal Bacteria. Kor. J. Pharmacogn. 1995;26:360–367. [Google Scholar]
- 28.Takino Y. Studies on the pharmacodynamics of ginsenoside-Rg1, -Rb1 and -Rb2 in rats. Yakugaku. Zasshi. 1994;114(8):550–564. [PubMed] [Google Scholar]
- 29.Tanizawa H, Karikuma M., Miyase T., Takono Y. Studies on the metabolism and/or decomposition and distribution of ginsenoside Rb2 in rats. In: Proc. 6th Int. Ginseng Symp. Seoul. 1993:187–194. [Google Scholar]
- 30.Tawab M.A., Bahr U., Karas M., Wurglics M., Schubert Z.M. Degradation of ginsenosides in humans after oral administration. Drug. Metab. Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 31.Wang B.X., Cui J.C., Liu A.J., Wu S.K. Studies on the anti-fatigue effect of the saponins of stems and leaves of Panax ginseng (SSLG) J. Tradit. Chin. Med. 1983;3(2):89–94. [PubMed] [Google Scholar]
- 32.Xu Q.F., Fang X.L., Chen D.F. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J. Ethnopharmacol. 2003;84(2–3):187–192. doi: 10.1016/s0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 33.Yoshioka H., Hayashida S. Purification and properties of β-glucosidase from Humicola insolens YH-8. Agr. Biol. Chem. 1980;44:1729–1735. [Google Scholar]
- 34.Zhang D., Liu Y.P., Yu H.S., Jin F.X., Chen G.X. Purification of ginsenoside β-glucosidase hydrolase and its characteristics. Ying Yong Yu Huan Jing Sheng Wu Xue Bao. 2003;9(3):259–262. [Google Scholar]
- 35.Zhou W., Feng M.Q., Li J.Y., Zhou P. Studies on the preparation, crystal structure and bioactivity of ginsenoside compound K. Journal of Asian Natural Products Research. 2006;8(6):519–527. doi: 10.1080/10286020500208600. [DOI] [PubMed] [Google Scholar]


