Abstract
Fecal bacterial indicator analyses have been widely used for monitoring the water quality. This study was designed to determine the ratio between the density of Escherichia coli and other Thermotolerant Coliforms (TtC) bacteria from freshwater samples collected for a two-year period of monitoring. TtC were enumerated by membrane filtration on mFC agar. E. coli enumeration was done by two methods: TtC colonies identified in mFC were inoculated in EC-MUG or water samples were filtered and inoculated in modified mTEC agar media, and both methods were compared for quantitative recovery of E. coli. The results pointed out a mean percentage of E. coli among other thermotolerant coliforms (E. coli/TtC ratio) of 84.3% in mFC media. Taking these results into account, a mandatory standard of 1000 thermotolerant coliforms would correspond to 800 E. coli and the adoption of these E. coli based standards will represent a major improvement for the monitoring of freshwater quality.
Keywords: Thermotolerant Coliforms (TtC), E. coli, Water quality, microbiological standards
INTRODUCTION
Coliform bacteria are the commonly used bacterial indicator for sanitary quality of water (18, 19). They are defined as members of genera or species within the family Enterobacteriace capable of growth at 37° C (total coliforms) or 44° - 45° C (thermotolerant coliforms) that possess β-galactosidase (9). Coliform bacteria are abundant in the feces of warm-blooded animals but can also be found in soil, aquatic environments and vegetation. Unlike other coliform bacteria, Escherichia coli are almost exclusively of fecal origin and can be detected in elevated densities in human and animal feces, sewage and water subjected to recent fecal pollution. It is therefore considered the best fecal indicator microrganism (9, 24).
Fecal bacteria have been used as an indicator to the possible presence of pathogens in surface waters and the risk of disease based on epidemiological evidence of waterborne diseases. Consequently, because of the difficulties to detect the many possible pathogens (such as Salmonella sp, Shigella sp, diarrheogenic E. coli, Giardia lamblia, Cryptosporidium parvum and enteric viruses) concentrations of fecal bacteria including thermotolerant coliforms, enterococci and E. coli, are used as the primary indicators of fecal contamination (23). Studies suggest that E. coli is a more reliable indicator of fecal pollution and the occurrence of pathogens in water than total and thermotolerant coliforms (9, 16). Therefore, the use of E. coli as the main bacterial indicator instead of other coliform bacteria has been proposed in water quality monitoring programs which tailors the microbiological quality of water.
In fact, the United States Environmental Protection Agency recommends E. coli or enterococci to replace fecal coliform bacteria in state water quality standards based on the studies which showed a statistically significant relationship between E. coli and enterococci concentration in freshwater and rates of swimming-related illness. These studies, carried out from 1979 to 1982 in freshwater beaches of Lake Erie, Pennsylvania and Keystone Lake, Oklahoma, clearly demonstrated a higher risk of gastrointestinal illness at those beaches having the highest degree of fecal contamination. Among the fecal contamination indicators evaluted (fecal coliforms, E. coli and enterococci) E. coli and enterococci showed the best correlation with swimming-associated gastrointestinal symptons, whereas fecal coliform showed little or no correlation (20). The current EPA criteria for recreational water determines that the geometric mean of the indicated bacterial densities should not exceed 126 for E. coli or 33 for enterococci in 100 mL of freshwater, based on a statistically number of samples (generally not less than 5 samples equally spaced over a 30-day period) (21). Considering the previous criteria used by USEPA for TtC enumeration (geometric mean of 200/100 mL of freshwater), the current E. coli density of 126/100 mL corresponds to 63% of total TtC density (23).
Recently, a study performed by Garcia-Armisen et al. (12) showed a mean percentage of 77% of E. coli among other TtC in 166 samples collected from Sena river (France). The correlation found in this study suggests a scientifically based parameter that can be used to convert TtC historical data, since European Union (EU) will adopt the E. coli as the microbiological criteria to state recreational water quality (10).
Apart from that, many countries still use TtC enumeration to provide a legal basis to State water quality. For example, according to Canadian Legislation either E. coli or fecal coliforms can be used if experience shows that greater than 90% of the fecal coliforms are E. coli (15).
The surface water quality in Brazil is mainly regulated by federal laws that define water classification and guidelines based on water uses. The Rule 357/2005 from the National Council of the Environment (7) establishes water microbiological standards for different purposes based on thermotolerant coliforms but allows the State Environmental Agencies to adopt E. coli for such evaluation by setting their own criteria. Regarding recreational activities there is a specific regulation (6) that has already fixed standards for TtC, E coli and enterococci.
The present study was designed to evaluate the ratio between E. coli and TtC in water bodies as an effort to scientifically support a future redefinition on the current standards that define sanitary water quality. The knowledge of E. coli/TtC ratio suitable for freshwater sites will allow the conversion of historical microbiological records expressed in TtC, into E. coli, providing a comparison parameter for present and future water monitoring. It will also be possible to standardize more stringent criteria to monitor the water quality throughout the water system supply.
MATERIALS AND METHODS
Sampling
This study was carried out during two years, from January 2004 to December 2005, in 25 sites from different water bodies across Sao Paulo State (Table 1). Sampling was performed bimonthly in 18 sites in rivers and monthly in seven sites used as recreational areas, located in two reservoirs, amounting to 380 samples. These water bodies were selected based on their level of contamination and sources of pollution.
Table 1.
Geographical location of the 25 collection sites
| Geographical location | |||
|---|---|---|---|
| Water body | Collection site | Latitude | Longitude |
| Paraiba river | 1 | 23 18 48 | 45 58 20 |
| Grande river | 2 | 23 24 42 | 45 06 39 |
| Pardo river | 3 | 21 06 00 | 47 45 44 |
| 4 | 22 57 14 | 49 52 02 | |
| Atibaia river | 5 | 23 06 12 | 46 32 42 |
| Jaguari river | 6 | 22 41 56 | 47 09 07 |
| Corumbataí river | 7 | 22 38 01 | 47 40 58 |
| Capivari river | 8 | 23 00 22 | 47 06 00 |
| Tietê river | 9 | 23 31 11 | 46 44 47 |
| 10 | 23 32 55 | 46 08 09 | |
| 11 | 22 57 25 | 47 49 23 | |
| Baquirivú-Guaçú river | 12 | 23 24 50 | 46 23 05 |
| Jundiaí river | 13 | 23 38 56 | 46 11 48 |
| Mogi river | 14 | 23 51 08 | 46 22 41 |
| Mogi-Guaçú river | 15 | 21 00 44 | 48 10 20 |
| Sorocaba river | 16 | 23 10 21 | 47 47 47 |
| Preto river | 17 | 20 37 40 | 49 21 18 |
| Aguapeí river | 18 | 21 40 35 | 50 35 21 |
| 19 | 23 46 37 | 46 32 01 | |
| Billings reservoir | 20 | 23 46 18 | 46 30 50 |
| 21 | 23 46 37 | 46 37 09 | |
| 22 | 23 40 30 | 46 43 51 | |
| 23 | 23 41 57 | 46 44 41 | |
| Guarapiranga reservoir | 24 | 23 41 48 | 46 43 11 |
| 25 | 23 42 53 | 46 42 58 | |
The water samples were collected in sterile 500 mL, wide mouth, plastic bottles, according to American Public Health Association (2), kept on ice for transportation and processed within 24 h.
Bacteriological Analysis
Throughout the monitoring period 380 water samples were analyzed by the membrane filtration technique. The enumeration of thermotolerant coliforms was performed using a lactose-based agar (mFC: membrane fecal coliform) recommended by the American Public Health Association (2) since 1971. Typical colonies on mFC agar were differentiated to E. coli with EC-MUG (Escherichia coli-methylumbelliferyl-β-D-glucuronide) medium.
The 166 samples collected during 2005 were also analyzed directly for E. coli using modified mTEC (membrane Thermotolerant Escherichia coli) agar (4, 22). This enzymatic based medium can provide reliable confirmed results in 24 h (8) and has been employed by others authors to study the E. coli/thermotolerant coliform ratio (11, 14).
According to the expected degree of fecal contamination different volumes of each sample (70, 25 and 5 mL) or decimal dilutions of 1 mL were filtered in 4.5 cm diameter, 0.45 μm-pore-size, gridded, mixed ester membrane filters, in order to achieve a range of 20 to 60 countable TtC or E. coli Colony Forming Units (CFU). The filters containing different volumes of the samples were transferred to the surface of the mFC or mTEC plates. mFC plates were incubated at 44.5 ± 0.2° C for 20 ± 2 h. Modified mTEC plates were pre-incubated at 35 ± 0.5° C for 2 h and then incubated at 44.5 ± 0.2° C for 22 h. Blue colonies on mFC were counted as TtC. According to the classical procedures stated for verification or differentiation of colonies in the membrane filtration technique (2), ten typical blue coliform colonies on mFC medium were randomly selected from membrane filter for each tested sample. Each selected colony was inoculated in EC-MUG medium and incubated at 44.5 ± 0.2° C for 24 h. Simultaneously, colonies submitted to differentiation on EC-MUG were inoculated in eosine-methylene blue agar (EMB agar). Coliform-typical colonies on EMB agar, originated from EC-MUG-negative colonies, were identified with API 20E (Biomérieux, France). Growth in the EC-MUG was examined for fluorescence under long-wavelength (365 nm) UV light to detect β-glucuronidase activity. Colonies that were positive for β-glucuronidase activity were considered E. coli colonies. With the modified mTEC media, red-magenta colonies were identified as E. coli CFU without further confirmative tests.
Statistical Analysis
Thermotolerant coliform (mFC agar) and E. coli colony counts from both media (mFC + EC-MUG and modified mTEC) were converted to log 10 values to ensure data normality. In order to compare the analysis methods, mFC + EC-MUG vs. modified mTEC, a paired t-test was performed. Linear regressions between TtC counts on mFC agar and E. coli counts with EC-MUG as well as with E. coli counts obtained with the modified mTEC agar were performed in order to determine the proportion E. coli/ TtC obtained by these two methods.
RESULTS AND DISCUSSION
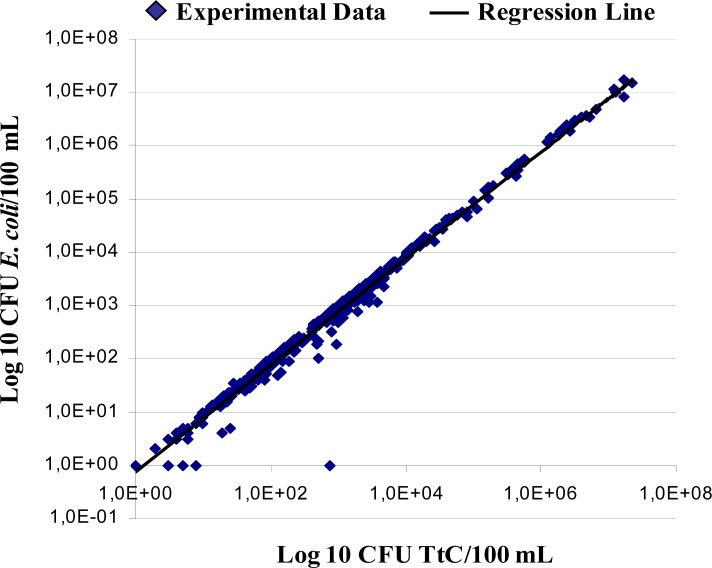
The mean percentage of E. coli isolated from mFC media and further confirmed with the EC-MUG test was 84.3%. Figure 1 shows the regression line depicting a strong positive correlation between TtC counts on mFC agar and E. coli counts with EC-MUG with a correlation coefficient (r) of 0.996 suggesting that near of 80% of TtC colonies isolated from mFC media are E. coli isolates in the analyzed water samples.
Figure 1.
Log regression line between thermotolerant coliform concentrations on mFC agar and E. coli concentrations on EC-MUG
When the results obtained with both methodologies used to quantify E. coli CFU were compared, the paired t-test demonstrated that there were no significant differences between colonies counts obtained with the mFC + EC-MUG technique and the counts obtained with mTEC isolation. Therefore, the membrane filtration method using modified mTEC agar can be used to monitor E. coli for freshwater quality.
Isolated colonies that displayed a negative β-glucuronidase activity with EC-MUG medium were tested for enteric bacteria with the API-20E system (BioMérieux, France).
The distribution of MUG-negative coliforms isolated from mFC media is presented in Table 2. It was observed a predominance of the Klebsiella genre (60.6% of K. pneumoniae). Also, 25.9% of the colonies were identified as MUG-negative E. coli bacteria. These data are in accordance with Bordalo (5) who demonstrated a predominance of Klebsiella genre bacteria among MUG-negative bacteria isolated from aquatic environments. Alonso et al. (1) compared the performance of chromogenic culture media for the recovery of E. coli and TtC and found that Citrobacter freundii was the dominant species in river and marine water samples.
Table 2.
Number, percentage and species of thermotolerant coliforms isolated on mFC agar and negative on EC-MUG
| SPECIES | NUMBER OF ISOLATE/PERCENTAGE (%) |
|---|---|
| Klebsiella pneumoniae pneumoniae | 354/60.6 |
| Escherichia coli (MUG -) | 151/25.9 |
| Enterobacter cloacae | 20/3.4 |
| Klebsiella terrigena | 20/3.4 |
| Enterobacter aerogenes | 15/2.6 |
| Citrobacter freundii | 14/2.4 |
| Pantoea sp | 2/0.3 |
| Klebsiella pneumoniae ozaenae | 2/0.3 |
| Klebsiella ornithinolytica | 1/0.2 |
| Stenotrophomonas maltophila | 1/0.2 |
| Citrobacter youngae | 1/0.2 |
| Kluyvera sp | 1/0.2 |
| Leclercia adecarboxylata | 1/0.2 |
| Serratia liquefaciens | 1/0.2 |
| TOTAL | 584/100 |
Twelve samples displayed a higher concentration of E. coli in mTEC media than the TtC concentration found in mFC media, which means that for these samples the proportion E. coli/TtC ranged from 1.3 to 3.19 (data not shown). Since E. coli is a member of the thermotolerant coliform group, this ratio should not be superior to 1.0. These results can be explained by the greater sensitivity of modified mTEC agar (a chromogenic method based on the detection of β-glucuronidase) over mFC agar (a lactose based method).
In a recent methodology review, Hamilton et al. (14) discussed the E. coli/TtC ratio proportion found in several studies that analyzed water samples from different environments, showing that this proportion could overpass the ideal limit of 1.0 in many cases. In fact, the authors conclude that the differences observed could be due to the recovery range obtained with different methodologies, and also, that media containing chromogenic substrates are usually more efficient in E.coli recovery. The relationship between enzymatic and traditional methods to detect coliforms in freshwaters was studied by George et al. (13). They used the fluorogenic substrates MUGal (4 methylumbelliferyl-β-D-galactoside) and MUGlu (4 methylumbelliferyl-β-D-glucuronide) and verified a significative correlation between both methods. According to their study viable but not culturable E. coli could be detected by the enzymatic method. However, Francy and Darner (11) compared E. coli concentrations in recreational freshwaters using traditional and modified mTEC agar and concluded that the chromogenic medium recovered less bacteria.
Other authors have also studied the proportion of these fecal contamination indicators aiming to convert historic fecal coliform bacteria data to estimate E. coli densities or to establish E. coli values to replace thermotolerant coliforms criteria. Rasmussen and Ziegler (17) observed an E.coli/TtC ratio of 0.77 during a study performed to evaluate the sanitary quality of selected Kansas streams. Garcia Armisen et al. (12) reported values of 77% for E.coli/TtC ratios in differently contaminated freshwater samples.
The linear regression models applied to the results obtained in the present study demonstrated an E. coli/TtC proportion of 80%. As an example, taking this proportion into account, mandatory standards of 1000 thermotolerant coliforms for freshwater would correspond to approximately 800 E. coli. In order to use more stringent criteria, the inferior confidence level of the linear regression model (620 E. coli) could be adopted instead.
As E. coli is recommended by many studies (9, 12, 14, 16) and publications (3, 23, 24) as a better indicator to protect public health than thermotolerant coliforms, the adoption of E. coli based standards will represent a major improvement for the microbiological monitoring of water quality intended to be used as drinking water source, irrigation of crops and aquaculture.
REFERENCES
- 1.Alonso J.L., Soriano A., Carbajo O., Amoros I., Garelick H. Comparison and recovery of Escherichia coli and thermotolerant coliforms in water with a chromogenic medium incubated at 41 and 44.5° C. Appl Environ Microbiol. 1999;65(8):3746–3749. doi: 10.1128/aem.65.8.3746-3749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Public Health Association. Standard Methods for the Examination of Water and Wastewater. 21st ed., 2005. Section 9222. Membrane filter technique for members of the coliform group. Washington, DC: American Public Health Association; [Google Scholar]
- 3.American Public Health Association. Standard Methods for the Examination of Water and Wastewater. Standard Methods online. 2007. Section 9060. Samples. Washington, DC: American Public Health Association; 2007. [Google Scholar]
- 4.American Public Health Association. Standard Methods for the Examination of Water and Wastewater. Standard Methods on line. 2007. Section 9213. Recreational waters. Washington, DC: American Public Health Association; 2007. [Google Scholar]
- 5.Bordalo A.A. Faecal coliform recovery in two standard media along an estuarine gradient. Water Res. 1994;28(11):2331–2334. [Google Scholar]
- 6.Brasil. Ministério do Meio Ambiente. Comissão Nacional do Meio Ambiente. Resolução CONAMA 274, de 29 de dezembro de 2000. Available from [ http//www.mma.gov.br/port/conama/legiabre.cfm?codlegi=272]. Accessed 30 May 2008.
- 7.Brasil. Ministério do Meio Ambiente. Comissão Nacional do Meio Ambiente. Resolução CONAMA 357, de 17 de março de 2005. Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento. Available from [ http//www.mma.gov.br/port/conama/res/res05/35705.pdf]. Accessed 23 February 2006.
- 8.Ciebin B.W., Brodsky M.H., Eddington R., Horsnell G., Choney A., Palmateer G., Ley A., Joshi R., Shears G. Comparative evaluation of modified m-FC and m-TEC media for membrane filter enumeration of Escherichia coli in water. Appl Environ Microbiol. 1995;61(11):3940–3942. doi: 10.1128/aem.61.11.3940-3942.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edberg S.C., Rice E.W., Karlin R.J., Allen M.J. Escherichia coli: the best biological drinking water indicator for public health protection. J Appl Microbiol Symposium Supplement. 2000;88:106S–116S. doi: 10.1111/j.1365-2672.2000.tb05338.x. [DOI] [PubMed] [Google Scholar]
- 10.European Parliament. Directive 2006/EC of the European Parliament and of the council concerning the management of quality of bathing water. Official Journal of the European Union. 2006;I.64(64):37–51. 4/3/2006. [Google Scholar]
- 11.Francy D.S., Darner R.A. Comparison of methods for determining Escherichia coli concentrations in recreational waters. Water Res. 2000;34(10):2770–2778. [Google Scholar]
- 12.Garcia-Armisen T., Prats J., Servais P. Comparison of culturable fecal coliforms and Escherichia coli enumeration in freshwaters. Can J Microbiol. 2007;53(6):798–801. doi: 10.1139/W07-033. [DOI] [PubMed] [Google Scholar]
- 13.George I., Petit M., Servais P. Use of enzymatic methods for rapid enumeration of coliforms in freshwaters. J Appl Microbiol. 2000;88(3):404–13. doi: 10.1046/j.1365-2672.2000.00977.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton W.P., Kim M.K., Thackston E.L. Comparison of commercially available Escherichia coli enumeration tests: implications for attaining water quality standards. Water Res. 2005;39(20):4869–4878. doi: 10.1016/j.watres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Health and Welfare Canada. Guidelines for Canadian Recreational Water: Minister of National Health and Welfare. Otawwa. Canada: Canadian Governement Publishing Centre Supply and Services Canada; 1992. [Google Scholar]
- 16.Leclerc H., Mossel D.A.A., Edberg S.C., Struijk C.B. Advances in the bacteriology of the coliform group: their suitability as markers of microbial water safety. Annu Rev Microbiol. 2001;55:201–234. doi: 10.1146/annurev.micro.55.1.201. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen P.P., Ziegler A.C. Comparison and continuous estimates of fecal coliform and Escherichia coli bacteria in selected Kansas streams, May 1999 through April 2002. Proceedings of 2003. Spring Specialty Conference on Agricultural Hydrology and Water Quality; May 12-14, 2003; Kansas City, Missouri: Middelburg, Virginia. Middelburg^eVirginia Virginia: American Water Resources Association; 2003. AWRA Technical Publication Series No. TPS-03-01. [Google Scholar]
- 18.Rompré A., Servais P., Baudart J., de-Roubin M.R., Laurin P. Detection and enumeration of coliforms in drinking water: current methods and emerging approaches. J Microbiol Methods. 2002;49(1):31–54. doi: 10.1016/s0167-7012(01)00351-7. [DOI] [PubMed] [Google Scholar]
- 19.Tallon P., Magajna B., Lofranco C., Leung K.T. Microbial indicators of faecal contamination in water: a current perspective. Water, Air, Soil Pollut. 2005;166(1-4/ September):139–166. [Google Scholar]
- 20.United States Environmental Protection Agency. Health effects criteria for fresh recreational waters. 1984. EPA 600/1-84-004. August 1984.
- 21.United States Environmental Protection Agency. Ambient Water Quality for Bacteria – 1986. 1986. EPA 440/5-84-002.
- 22.United States Environmental Protection Agency. Method 1603. Escherichia coli (E. coli) in water by membrane filtration using modified membrane thermotolerant Escherichia coli agar (modified mTEC) 2002. EPA 821-R-02-023. September 2002.
- 23.United States Environmental Protection Agency. Implementation guidance for ambient water quality for bacteria. 2004. EPA 823-B-004-02.
- 24.World Health Organization. Guidelines for drinking water quality: Volume 1. Recommendations. 3rd. Genebra: World Health Organization; 2004. [Google Scholar]