Abstract
In the present investigation, the basic esters of meta-alkoxyphenylcarbamic acid bearing variously substituted N-phenylpiperazine fragment were screened for their in vitro antimicrobial activity against Staphylococcus aureus, Escherichia coli and Candida albicans, respectively. The most effective against Escherichia coli was found the compound 6d (MIC=195,3 μg/mL) bearing simultaneously para-fluoro substituent at the 4-phenylpiperazin-1-yl core and meta-methoxy side chain in the lipophilic part of the molecule. From whole analyzed set of the molecules the substance 8e with propoxy side chain forming meta-alkoxyphenylcarbamoyl fragment and lipophilic, sterically bulky meta-trifluoromethyl group attached at N-phenylpiperazine moiety was evaluated as the most active against Candida albicans (MIC=97,7 μg/mL). On the contrary, all investigated structures were practically inactive against Staphylococcus aureus (MIC>1000 μg/mL)
Keywords: Phenylcarbamates, substituted N-phenylpiperazines, Candida albicans
INTRODUCTION
To survive in the environment, bacteria and yeasts must respond to several stress factors that lead to non-ideal growth conditions. The emergence of a pathogen community depends on its ability to survive in different enviroments and to interact successfully with the host (18). As an additional stress, they may be exposed to a wide range of antimicrobially active agents, such as antibiotics, that can act as a selective pressure for the development of microorganisms resistance. As a result of antibiotic use and misuse, the prevalence of antimicrobial resistance among bacterial pathogens has increased resulting in more complicated treatment. In general, bacteria have genetic ability to transmit and acquire resistance to the drugs, which are utilized as the therapeutic agents. The problem of microbial resistance is growing and the outlook for the use of antimicrobial drugs in the future is still uncertain. Therefore, actions must be taken to reduce the resistance by controlling the use of antibiotics, by develop of the research for a better understanding the genetic mechanisms of resistance. Projection and synthesis of new antimicrobial agents active against emerging resistant pathogens is thus an essential process (15, 16, 17).
The objective of present study is to investigate in vitro susceptibility of selected clinically significant microbial strains, the opportunist pathogens, to novel lipophilic basic esters of meta-alkoxyphenylcarbamic acid containing variously substituted 4-phenylpiperazin-1-yl fragment in the structure.
MATERIALS AND METHODS
Chemistry
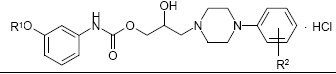
The preparation of evaluated compounds, labelled as 6d– 6g and 8c–8e (Table 1), their spectral characteristics (1H NMR, 13C NMR, IR, MS, UV/VIS) as well as the elemental analyses data were published previously (9, 10). The procedures of physicochemical parameters determination (surface activity γ, dissociation constant pKa, lipophilicity descriptors – log Pexp estimated by the shake-flask method in the octan-1-ol/ buffer medium with pH = 7,3, log k’ from RP-HPLC, RM from RP-TLC) and the corresponding readouts and conclusions were published in paper of Malík et al. (11).
Table 1.
In vitro antimicrobial activity of structures 6d–8e against selected microbial strains (MICs expressed in μg/mL and μmol/L units, respectively).
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | Escherichia coli | Candida albicans | ||||||
| Entry | R1 | R2 | ATCC 6538 | CNCTC 377/79 | CCM 8186 | |||
| μg/mL | μmol/L | μg/mL | μmol/L | μg/mL | μmol/L | |||
| 6d | CH3 | 4’-F | 12500 | 28414 | 195,3 | 444 | 1562,5 | 3552 |
| 6e | C2H5 | 4’-F | 12500 | 27537 | 781,3 | 1721 | 1562,5 | 3442 |
| 6f | C3H7 | 4’-F | 6250 | 13356 | 390,6 | 835 | 781,3 | 1670 |
| 6g | C4H9 | 4’-F | 6250 | 12967 | 390,6 | 810 | 781,3 | 1621 |
| 8c | CH | 3’-CF | 6250 | 12757 | 6250 | 12757 | 195,3 | 399 |
| 8d | C2H5 | 3’-CF3 | 12500 | 24804 | 6250 | 12402 | 195,3 | 388 |
| 8e | C3H7 | 3’-CF3 | 6250 | 12066 | 6250 | 12066 | 97,7 | 189 |
In vitro antimicrobial activity assay
Microorganisms: The antimicrobial activity of concerned compounds was investigated against Gram-positive bacteria Staphylococcus aureus ATCC 6538 (Micrococcaceae), Gram-negative bacteria Escherichia coli CNCTC 377/79 (Enterobacteriaceae) and yeast Candida albicans CCM 8186 as well. The tested bacterial strains were purchased from American Type Culture Collection and Czech Collection of Type Cultures (National Institute of Public Health, Prague, Czech Republic), yeast was obtained from Czech Collection of Microorganisms (Brno, Czech Republic).
Culture media: The blood agar, Endo agar and Sabouraud’s agar were used for cultivation of microorganisms (Imuna, Šarišské Michaľany, Slovak Republic). The blood agar was prepared by adding of 10% of defibrine sheep’s blood to the melted and cooled (50ºC) competent components.
Determination of minimum inhibitory concentration (MIC): The values of MIC of studied structures were estimated according to approach which was detailed in the paper of Mlynarčík et al. (14).
The tested substances were solubilised in dimethyl sulfoxide medium due to their very limited solubility in water. The standard suspension of microorganisms was prepared from 24h bacterial cultures cultivated at a blood agar (Gram-positive bacteria) and Endo agar (Gram-negative bacteria) as well as from the 48h cultures cultivated at the Sabouraud’s agar for yeasts. The suspension exhibited the concentration of 5 × 107 colony forming unit (cfu)/mL of bacteria and 5 × 105 cfu/mL of Candida, respectively. The UV/VIS spectrophotometry was used for the determination of the microorganisms concentration, all the prepared suspensions were adjusted to the absorbance value of 0,35 at the wavelength of 540 nm.
The microorganism suspension prepared in such a way was then particularly added in amount of 5 μL into the solutions consisting of evaluated substances (100 μL) and to the double concentrated peptone broth medium (8%) assigned to bacteria or the Sabouraud’s medium (12%) related to Candida, respectively (14). For the prepared stock solutions of all evaluated substances there was a concentration of 25000 μg/mL.
The solutions were then serially diluted by a half (the final concentrations were 12500; 6250; 3125; 1562,5; 781,3; 390,6; 195,3 and 97,7 μg/mL, respectively). The quantitative screening was performed using 96-well microtiter plates, microorganisms were incubated there at 37°C for 24h. After that, from particular well the amount of 5 μL of evaluated suspension was taken and cultured on a blood agar (bacteria) or on Sabouraud’s agar (yeasts). Then were the Petri dishes incubated for 24h at 37°C.
The values of MIC were read as the lowest concentration of antimicrobially active compound which inhibited the visible microbial growth (14).
RESULTS AND DISCUSSION
The investigated substances, chemically 1-[3-(3-alkoxyphenylcarbamoyloxy)-2-hydroxypropyl]-4-(4-fluoro-/3-trifluoromethylphenyl)piperazinium chlorides (alkoxy = methoxy to butoxy group; labelled as 6d–6g and 8c–8e, respectively), were previously in vitro tested against Mycobacterium tuberculosis CNCTC My 331/88 (19). The estimated values of MIC for 6d–6g were in the range of 55– 241 μg/mL (related to the interval of 125–500 μmol/L), in the series of 8c–8e the evaluated compounds exhibited the MICs in the area of 7,8–8,3 μg/mL which corresponded to 16 μmol/L in all cases. However, they were less active than isoniazide (INH) for which the value of 0,07 μg/mL (0,5 μmol/L) as MIC was assigned. On the other hand, considered molecules performed relatively higher in vitro efficiency against potential pathogenic non-tuberculous Mycobacterium kansasii My 235/80 comparing the standard INH (20). The structures 6d–6g had the MICs against listed mycobacterial strain in the range of 27,5–129,5 μg/mL (62,5–250 μmol/L), the molecules 8c–8e were actually more effective, their MICs were in the area of 8,3–15,7 μg/mL (16–32 μmol/L). Simultaneously estimated MIC related to INH was more than 34 μg/mL (250 μmol/L).
Consequently, there was continuous interest to extend the knowledge about the spectrum of antimicrobial activities of the target structures from both sets 6 and 8 which were then tested against Staphylococcus aureus ATCC 6538 (Gram-positive bacteria, Micrococcaceae), Escherichia coli CNCTC 377/79 (Gram-negative bacteria, Enterobacteriaceae) and Candida albicans CCM 8186 (yeast) as well. The idea for a selection of such structures was based on the knowledge that meta- as well as para-alkoxy substituted phenylcarbamic acid derivatives were previously more antimicrobially active comparing the ortho-substituted ones (13).
The level of a concrete antimicrobial activity of tested structures from both sets 6 and 8 was dependent on the meta-alkoxysubstituent length and type of the substituent attached at the phenyl ring in the basic part of the molecule.
The experimental investigations revealed that all evaluated substances were practically inactive against Staphylococcus aureus ATCC 6538, the most virulent Staphylococcus species, with the MICs higher than 1000 μg/mL. Previously analyzing the influence of 2-piperidinoethyl-4-heptyloxyphenylcarbamate hydrochloride on some metabolic function of mentioned Gram-positive bacterial strain Mlynarcík et al. 1981 (14) suggested that the bacteriostasis could be equated to a loss of the cell’s ability to synthesize ATP, which, in turn, may stem from an uncoupling of oxidative phosphorylation. Čižmárik et al. 1987 (5) comprehensively studied influence of piperidinoethyl esters of ortho-, meta- and ^ara-alkoxyphenylcarbamic acid (alkoxy=metoxy to decyloxy) against given strain. They observed that meta- and ^ara-substituted structures were antibacterially more active than ort/zo-substituted ones because of the steric effect. The proximity of ort/zo-alkoxy string to carbamate bond led to the twist of the benzene ring plain towards the carbamate group. Described process resulted in the planarity violation of molecule imlying subsequent conjugation of 7r-bonds of benzene ring over NH fragment up to carbonyl group. The result was the different electronic density (charge) at carbonyl moiety which could be one of the possible binding sites to the reactive membrane locations. In the case of meta-and para-alkoxy derivatives described secondary steric effect did not manifest, even para-substituted derivatives were practically linear type of the molecule. In response to the paper (5), the lipophilicity of meta- and para-alkoxyphenylcarbamates was found as the factor conducived to the activity of such structures. More lipophilic structures displayed relatively higher efficiency. Carbonyl group also represents the centre at which the split occured because of degradation (5). Čižmárik and Trupl (4) previously studied in very detail an antimicrobial effect of one structure from mentioned alkoxyphenylcarbamic acid esters set, heptacaine. They suggested that Staphylococcus aureus enzymically splitted heptacaine to ortho-heptyloxyaniline, which was then considered the actual antimicrobial agent. The observations from current evaluation of series 6 and 8 could confirmed a hypothesis postulated in a paper (4). There was an assumption that the carbamate bond in evaluated compounds was splitted. After that, the activities of formed products, especially appropriate meta-alkoxyanilines, were much more lower than effectiveness of ortho-heptyloxyaniline due to a lower lipophilicity.
The investigated molecules in which structure was incorporated only a single atom of fluorine, 6d–6g, were more active against Escherichia coli CNCTC 377/79 with MICs in the interval of 195,3–781,3 μg/mL (444–1721 μmol/L) than the substances with trifluoromethyl substitution, 8c–8e, which were against listed bacterial strain inactive (Table 1).
These preliminary findings indicated that for the activity of such tested phenylcarbamates against Escherichia coli CNCTC 377/79 was important the suitable length of alkoxy side string (methoxy substitution was preferred) as well as a presence of a moiety, attached at the N-phenylpiperazine fragment, with a comparable van der Waals radii towards atom of hydrogen but with different electronic configuration. The lowest MIC value against this Gram-negative bacterial strain was noticed for the compound 6d (195,3 μg/mL, 444 μmol/L).
The effect of fluorination of an aromatic system in the basic part of tested molecules could be documented by the hydrophobic Hansch-Leo π value (π = log PC6H5X – log PC6H6 for substituted benzenes) taken from the literature (1, 8). The π readout related to fluorine is 0,14; according to Bondi’s van der Waals radii estimation, fluorine is moderately larger (1,47 Å) than hydrogen (1,20 Å). Its Hammet sigma constant for para-position (σp) is 0,06 and represents only slightly higher value than is a readout for hydrogen (σp=0,00). For fluorine atom is also typical inductive electron-withdrawing as well as resonance electron-donating influencing (1, 8).
Presumably the electronic effects were primarily responsible for a better internalisation of the compounds tested (series 6) through the Gram-negative outer membrane. Present idea is supported by the research of Beveridge 1999 (2) or Hamadi et al. 2008 (7). One of an unusual features of the outer membrane of mentioned bacteria is its asymmetric distribution of lipids over inner and outer faces. The outer face contains (virtually) all of the lipopolysaccharides (LPSs), whereas the inner face has most of the phosholipids. LPSs contain more charge per unit of surface area than any phospholipids, and most of this charge is anionic at neutral pH because of exposed phosphoryl and carboxyl groups which can be readily ionized. The outer face of the outer membrane is highly charged. The resonance electron-donating effect of fluorine atom attached at the para-posititon of aromatic ring performed there relatively electronic density enhancement. The consequent electronic interactions with different membrane components of Escherichia coli generally favoured molecules from the series 6 comparing the members from the set 8, in which was evident strongly deactivating influence of meta-trifluoromethyl fragment towards phenyl ring. Except of mentioned, for fluorine atom is typical relatively smaller van der Waals radii (and possible better internalisation into the membrane’s structures) comparing trifluoromethyl group.
On the contrary, the significant enhancement of an activity against Candida albicans CCM 8186 was observed for the compounds 8c–8e, their estimated MICs were in the interval of 97,7–195,3 μg/mL (189–399 μmol/L). The substances 6d–6g were against this yeast practically inactive (781,3–1562,5 μg/mL, 1621–3552 μmol/L). In general, the evaluated series 8c–8e was even more effective against the yeast than previously tested dimethylaminoethyl, piperidinoethyl as well as perhydroazepinoethyl esters of meta--alkoxyphenylcarbamic acids with an identical meta-alkoxy substituent attached at the lipophilic aromatic ring (12, 13).
The experimental findings revealed that the level of an activity against Candida albicans of tested structures was dependent on the length and type of the substituent attached at the N-phenylpiperazine moiety (Table 1). From both analyzed sets 6 and 8 as the most active was considered compound 8e (with the lowest value of MIC) containing meta-propoxy side chain and lipophilic, sterically bulky trifluoromethyl fragment with its strong inductive electron-withdrawing effect. For an illustration, Hansch-Leo π value for trifluoromethyl group is 0,88; its electron-withdrawing influencing, expressed by the value of σm=0,43, is evident (1, 8). According to Tafts steric effects constants Es, given substituent is also much larger in steric bulk than hydrogen or methyl moiety; for the comparison the corresponding Es data are present: Es CF3=1,16 (trifluoromethyl); Es CH3=0,00 (methyl); Es H=-1,24 (hydrogen).
Following present experimental findings, the lipophilicity of tested structures played a key role in their ability to act against Candida albicans CCM 8186. Approximately 80 to 90% of the cell wall of this yeast consists of the carbohydrate, three basic constituents represent the major polysaccharides of its cell wall (3) – β-glucans, chitin and glyco[manno]proteins. In addition, cell wall of mentioned yeast contains proteins (6 to 25%) and minor amounts of lipids (1 to 7%). The inner layer, enriched for chitin and polysaccharide matrix, is more electron translucent than outer layers which are enriched for glyco[manno]proteins. Relatively higher lipophilicity allowed easier internalisation of the compounds tested into the eukaryotic pathogens, as compared with the prokaryotic ones, causing a pertubation of the membranes especially by the substances from the series 8 resulting in the anticandidacidal effect at relatively lower MIC values. Present suggestion of a possible acting of evaluated structures is consistent with another published conclusions of Limban et al. 2011 (6).
In the future, we intend to extend our research within this class of alkoxyphenylcarbamic acid derivatives containing N-phenylpiperazine ring by the testing of new compounds with meta- and para-alkoxy substitution and with various substituents attached at the basic moiety for which are typical with electron-donating or electron-withdrawing effects.
CONCLUSION
In order to find new structures in the group of meta-alkoxyphenylcarbamic acid derivatives with improved antimicrobial activity, nine molecules were tested. Their chemical structures were previously confirmed by spectral (1H NMR, 13C NMR, IR, MS, UV/VIS) data and by elemental analysis. The tested compounds exhibited a different level of antimicrobial activity against some selected Gram-positive (Staphylococcus aureus ATCC 6538), Gram-negative (Eschericha coli CNCTC 377/79) bacterial strains and against a yeast (Candida albicans CCM 8186). The activity was dependent on the length of meta-alkoxy side string co-creating the lipophilic part, electronic and lipophilic properties of substituent attached at N-phenylpiperazine ring which formed a basic part of the molecule. The presence of relatively shorter alkoxy side chain and a single atom of fluorine in para-position at aromatic system favoured the antimicrobial activity against Escherichia coli CNCTC 377/79, while the highest inhibitory effect against Candida albicans CCM 8186 was exhibited by the most lipophilic structure from whole tested series containing propoxy fragment and meta-trifluoromethyl group attached at N-phenylpiperazine fragment. On the other hand, the substances tested were inactive against Staphylococcus aureus ATCC 6538.
ACKNOWLEDGEMENTS
This study was supported by the Grant of Faculty of Pharmacy, Comenius University, Grant No. FaF UK/10/2011 and by the Slovak Grant Agency for Science, Grants No. 1/0055/11 and 1/0039/12.
REFERENCES
- 1.Bégué J.-P., Bonnet-Delpon D. Bioorganic and medicinal chemistry of fluorine. Hoboken: John Wiley & Sons; 2008. [Google Scholar]
- 2.Beveridge T.J. Structures of Gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999;181(16):4725–4733. doi: 10.1128/jb.181.16.4725-4733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaffin W.L., López-Ribot J.L., Casanova M., Gozalbo D., Martinez J.P. Cell wall and secreted proteins of Candida albicans: Identification, function, and expression. Microbiol. Mol. Biol. Rev. 1998;62(1):130–180. doi: 10.1128/mmbr.62.1.130-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Čižmárik J., Trupl J. Antimicrobial effects of heptacaine and some intermediary products obtained during its synthesis. Folia Microbiol. 1978;23:80–81. doi: 10.1007/BF02876602. [DOI] [PubMed] [Google Scholar]
- 5.Čižmárik J., Trupl J., Pešák M. A correlation between the antimicrobial activity to Staphylococcus aureus and selected physico-chemical parameters in the series of hydrochlorides of piperidinoethylesters of alkoxy-substituted phenyl-carbamic acids. Českoslov. Farm. 1987;36(8):345–348. [Google Scholar]
- 6.Limban C., Missir A.V., Chirita I.C., Nitulescu G.M., Caproiu M.T., Chifiriuc M.C., Israil A.M. Synthesis and antimicrobial properties of new 2-((4-ethylphenoxy)methyl)benzoylthioureas. Chem. Pap. 2011;65(1):60–69. [Google Scholar]
- 7.Hamadi F., Latrache H., Zahir H., Elghmari A., Timinouni M., Ellouali M. The relationship between Escherichia coli surface functional groups’ composition and their physicochemical properties. Braz. J. Microbiol. 2008;39(1):10–15. doi: 10.1590/S1517-83822008000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiyama T., Yamamoto H., Kanie K., Kusumoto T., Morizawa Y., Shimizu M. Organofluoric compounds: Chemistry and applications. Berlin: Springer-Verlag; 2000. [Google Scholar]
- 9.Malík I., Sedlárová E., Csöllei J., Andriamainty F., Kurfürst P., Vančo J. Synthesis, spectral description, and lipophilicity parameters determination of phenylcarbamic acid derivatives with integrated N-phenylpiperazine moiety in the structure. Chem. Pap. 2006;60(1):62–67. [Google Scholar]
- 10.Malík I., Sedlárová E., Csöllei J., Račanská E., Čižmárik J., Kurfürst P. Synthesis, physico-chemical properties and biological activity of 1-(4-fluorophenyl)-4-{3-(2-, 3- and 4-alkyloxyphenylcarbamoyloxy)-2-hydroxypropyl}piperazinium chlorides. Sci. Pharm. 2004;72(4):283–291. [Google Scholar]
- 11.Malík I., Sedlárová E., Čižmárik J., Andriamainty F., Csöllei J. Study of physicochemical properties of 4-alkoxyphenylcarbamic acid derivatives with various substituted N-phenylpiperazin-1-yl moiety in the basic part of the molecule. Farm. Obzor. 2005;74(8):211–215. [PubMed] [Google Scholar]
- 12.Mlynarčík D., Čižmárik J. Antimicrobial efficiency of ω-piperidinoethyl esters of n-alkoxy-phenylcarbamic acids. FoliaMicrobiol. 1976;21(1):75–76. doi: 10.1007/BF02879011. [DOI] [PubMed] [Google Scholar]
- 13.Mlynarčík D., Čižmárik J. Antimikrobielle Eigenschafteneiniger basischer Ethylester von Alkoxyphenylcarbaminsäuren. Pharmazie. 1979;34(9):575. [PubMed] [Google Scholar]
- 14.Mlynarčík D., Denyer S.P., Hugo W.B. A study of the action of a bisquaternary ammonium salt, an amine oxide and an alkoxy phenylcarbamic acid ester on some metabolic functions in Staphylococcus aureus. Microbios. 1981;30(119):27–35. [PubMed] [Google Scholar]
- 15.Nascimento G.F., Locatelli J., Freitas P.C., Silva G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000;31(4):247–256. [Google Scholar]
- 16.Nørskov-Lauritsen N., Marchandin H., Dowzicky M.J. Antimicrobial susceptibility of tigecycline and comparators against bacterial isolates collected as part of the TEST study in Europe (2004—2007) Int. J. Antimicrob. Ag. 2009;34(2):121–130. doi: 10.1016/j.ijantimicag.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Simões M., Rocha S., Coimbra M.A., Vieira M.J. Enhancement of Escherichia coli and Staphylococcus aureus antibiotic susceptibility using sesquiterpenoids. Med. Chem. 2008;4(6):616–623. doi: 10.2174/157340608786242016. [DOI] [PubMed] [Google Scholar]
- 18.Talia J.M., Debattista N.B., Pappano N.B. New antimicrobial combinations: substituted chalcones-oxacillin against methicillin resistant Staphylococcus aureus. Braz. J. Microbiol. 2011;42(2):470–475. doi: 10.1590/S1517-838220110002000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waisser K., Doležal R., Čižmárik J., Malík I., Kaustová J. The potential antituberculotics of the series of 2-hydroxy-3-(4-phenylpiperazin-1-yl)-propylphenylcar-bamates. Folia Pharm. Univ. Carol. 2007:35–36. 45–48. [Google Scholar]
- 20.Waisser K., Doležal R., Čižmárik J., Malík I., Kaustová J. The antimycobacterial derivatives against potential pathogenic strains: 2-Hydroxy-3-(4-phenylpiperazin-l-yl)-propylphenylcarbamates. Sci. Pharm. 2007;75(2):55–61. [Google Scholar]


