Abstract
Endophytic microorganisms, defined as fungi or bacteria that colonize the interior of plants without causing any immediate negative effects or damages, have reciprocal relationships with host plants. In some cases their presence is beneficial to the host due to the synthesis of bioactive compounds, among which several alcohols, esters, ketones and others that may react with other compounds and may be lethal to pathogenic microorganisms. Diaporthe helianthi (Phomopsis helianthi in its anamorphic phase) is available worldwide, especially in Europe, Asia and America. Isolated in Europe as an agent of the sunflower stem cancer, it has also been endophytically isolated from tropical and temperate plants. A D. helianthi strain isolated from Luehea divaricata has been employed in current research. An investigation of the secondary metabolite from D. helianthi by CC and NMR of 1H and 13C yielded the separation of 10 fractions and the identification of the phenolic compound 2(-4 hydroxyphenyl)-ethanol (Tyrosol). Its antimicrobial reaction was tested and the ensuing antagonistic effects on the human pathogenic bacteria Enterococcus hirae, Escherichia coli, Micrococcus luteus, Salmonella typhi, Staphylococcus aureus, phytopathogenic Xanthomonas asc. phaseoli and phytopathogenic fungi were demonstrated. Results show that bioactive compounds and Tyrosol produced by D. helianthi have a biotechnological potential.
Keywords: endophytic fungi, Diaporthe helianthi, secondary metabolite, phenolic compound, Tyrosol
INTRODUCTION
An endophytic microorganism or endophyte is a term for fungi and bacteria that live in plant tissues (leaves, fruits, seeds, stems and roots) without causing any apparent damage to plants (14). Although studies on these organisms started in the 1980s, scanty information is available. It is known that all plants under analysis have endophytes (3) and that studies on community organization have shown that endophyte communities are usually specific to the hosts at species level (13). It has been estimated that worldwide fungus distribution reaches 1.5 million species. Whereas there is a ratio of 6:1 for vascular plants in the U.K., the proportion reaches 33:1 for endophyte and saprophyte fungi in tropical plants (3).
Endophytic microorganisms that live the interior of plants may transform the latter chemical components from which chemical compounds are produced (2). Substances produced by endophytic fungi may be used in agroindustries for the biological control of pests and diseases.
Endophytes may directly produce chemical defense in plants through the production of secondary compounds which inhibit insects and pathogenic organisms. The in vitro secretion of substances by endophytes that limit the growth of other microbial species, including pathogens, is currently interesting in cases of bioprospection and biological control by endophytic fungi (3, 4, 16).
Research has shown that the symbiosis of many fungi with plants produce bioactive metabolites in chemical defenses against herbivores. For instance, Neotyphodium uncinatum produces an insecticide alkaloid called Loline (20).
Endophytes are not required to produce a great quantity of chemical products to defend the host’s tissues. A genetically uniform plant will thus become non-palatable, quality-less and lacking pathogen infectivity for herbivores.
Although an increasing number of researches have produced solid bases for endophyte studies, the degrees of abundance, composition, taxonomic diversity and acceptance affinity within the endophyte-plant relationships are still hazy when geographical areas are concerned. Actually the determination of place is a relevant aspect for the formation of host’s identity and for the symbiosis established with the microorganism (10).
Owing to the importance of endophytes in the biological control of pests, current research establishes the chemical characteristics of the bioactive compounds of Diaporthe helianthi isolated from Luehea divaricata.
MATERIALS AND METHODS
Endophytic fungi
Endophytic fungus D. helianthi in current experiment was isolated from L. divaricata Mart. (Tiliaceae) by Bernardi-Wenzel et al. (6) and stored in the Microbial Biotechnological Laboratory of the Department of Cell Biology (DBC) of the State University of Maringá (UEM), Maringá PR Brazil.
Obtaining secondary metabolite
Secondary metabolite of endophyte D. helianthi was obtained following methodology by Li et al. (11), with modifications, this information is confidential in patent PI, 1005011–6
Column chromatography
Column Chromatography (CC) was undertaken in a glass column, Sephadex LH-20, diameter 1.5cm and height 30cm, as stationary phase. Movable phase consisted of pure solvents or combined solvents in polarity gradients.
CC was undertaken in thin layer chromatography (TLC) in 5.0 x 20.0 cm glass plates, with silica gel 60 G and 60 GF254(Merck), suspended in distilled water at 1:2, and distributed at an approximate thickness of 0.25mm and 1.0mm.
The visualization of compounds was undertaken by UV irradiation at 254 and 366 nm; pulverization with H2SO4/MeOH (1:1) occurred, followed by heating in a iodine atmosphere.
Whereas fractions were analyzed in TLC, re-sublimated iodine and H2SO4/MeOH (1:1) were the revealers, followed by heating.
Resulting 28 fractions were redistributed into 10 new fractions according to similarity shown in TLC (Table 1).
Table 1.
Data from fractions from D. helianthi metabolic extract.
| Identification | Joint Fractions | Mass (mg) | Methanol (mL) | Concentration tested (μg) |
| 1 | Vl-2 | 43.5 | 2.5 | 17.4 |
| 2 | V 3 | 17.6 | 2.5 | 7.04 |
| 3 | V 4 | 11.7 | 2.5 | 4.68 |
| 4 | V 5 | 1.8 | 2.0 | 0.9 |
| 5 | V 6–9 | 4.6 | 2.0 | 2.3 |
| 6 | V 10–13 | 2.1 | 2.0 | 1.05 |
| 7 | V 14–18 | 3.6 | 2.0 | 1.8 |
| 8 | V 19–20 | 0.5 | 2.0 | 0.25 |
| 9 | V 21–24 | 0.5 | 2.0 | 0.25 |
| 10 | V 25–28 | 1.5 | 2.0 | 0.75 |
Nuclear Magnetic Resonance of 1H and 13C (NMR of 1H and 13C)
NMR spectra of 1H and 13C were obtained by spectrometer MERCURY plus BB, at 300 MHz in the case of *H and at 75.5 MHz in the case of 13C. Chemical displacements were given in ppm, with tetramethylsilane TMS (δ = O.Oppm) or solvent as internal reference. Solvents were D20 and DMSO-d6.
Evaluation of antibacterial and antifungal activity of secondary metabolite
Qualitative biological assays in triplicate were employed to evaluate antimicrobial activity. The following human pathogenic bacteria were used: Enterococcus hirae (ATCC 1227), Escherichia coli (ATCC 25922), Micrococcus luteus (ATCC 9341), Salmonella typhi (ATCC 19430), Staphylococcus aureus (ATCC 25923), (collection of microorganisms of the Microbiological Laboratory - State University of Maringâ), phytopathogenic bacterium Xanthomonas asc. Phaseoli (collection of pathogenic microorganisms of Cenargen - Embrapa Genetic and Biotechnological Resources) and phytopathogenic fungi Alternaria alternata, Colletotrichum sp., Guignardia citricarpa and Moniliophthora perniciosa (donated by Joäo Lúcio de Azevedo Lab - ESALQ-USP), Colletotrichum gloesporioides, Dydimella bryoniae, Fusarium solani f. sp. glycines, Sclerotinia sclerotiorum (donated by Phytopathology Lab -Department of Agronomy - UEM).
Cup plate diffusion technique was used for antimicrobial analysis from the metabolite extraction of endophyte isolate. Bacteria under analysis were grown for 24h in LB liquid medium (pH 7.0) (18), adjusted to a concentration of 106cell/mL. Antibiotic Tetracycline (Sigma) (50 μg.mL−1 absolute ethanol) and water methanol were respectively employed as positive and negative controls. Bacteria were inoculated (100 HL) on petri plates with LB medium and spread with a Drigalsky spatula. Four Whatman sterile paper dises n. 4 (Ø 6 mm) were then placed at equal distance and inoculated by 10 μL metabolite extract. Plates were kept incubated at 37°C during 24h. Antimicrobial activity was evaluated by inhibition halo formation, according to Souza et al. (21).
Phytopathogen inhibition test was undertaken according to Li et al. (11), with modifications. Fungi were grown in medium BDA at 27°C for 7 days; solution of spores in tween was prepared; ΙΟΟμΙ suspension was inoculated in petri plates and spread with a Drigalsky spatula. Four Whatman sterile paper disc n. 4 (Ø 6 mm) were then placed at equal distance and inoculated by 10 μL metabolite extract. Fungicide Derosal plus® with a 10−1 dilution of Methyl benzimidazol-2-ylcabamato (Carbendazim) 150g/L (15,0% m/v); Tetramethylthiuram dissulfide (TIRAM) 350g/L (35,0% m/v). Inert ingredient 667g/L (66,7% m/v), water and methanol were respectively employed as positive and negative controls. Plates were kept incubated at 27°C for 7 days. Antimicrobial activity was evaluated by inhibition halo formation.
Results were statistically evaluated by variance analysis (p<0.05) for the comparison of averages using SAS (19).
RESULTS AND DISCUSSION
Analysis of Nuclear Magnetic Resonance (NMR) of 1H and 13C
The chemical analysis of the crude extract (Figures IA, 1B, IC, and 1D) and of the fractions separated by CC from the bioactive compound produced by endophytic fungus D. helianthi yielded the isolation of carbinolic hydrogens, namely, glucosides and aromatic hydrogens, and identified the compound 2-(4 hydroxyphenyl) ethanol, known as Tyrosol.
The structure of the isolated substance was identified by uni- and bi-dimensional NMR spectroscope data analysis and by their comparison with data from the literature (12, 25).
Fraction 1, representing 49.8% of total weight in mg of metabolite extracted from D. helianthi, is constituted by carbinolic hydrogens (glucosides) H-C-OH, fraction 2, weight 17.6 mg, is similar to fraction 1 and features glucosides in its composition. Fraction 3 shows hydrogens of the methyl group (CH3); aliphatic hydrogens of CH2 , carbinolic hydrogens, hydrogens of the aromatic system. Similar to fraction 3, fraction 4 has an aromatic region.
The compound 2-(4 hydroxyphenyl)-ethanol is identified in fraction 5 (Figure 1E), with 4.6 mg, by means of aromatic hydrogens with defined doubles with the same coupling constant δH 7.23 (2H, d, J=8.4 Hz) and δH 6.91 (2H, d, J=8.4 Hz). It may be affirmed that these hydrogens are orthoposition-coupled owing to J rate. Triplets corresponding to aliphatic hydrogens δH 3.84 (2H, t, J=6.7 Hz), δH 2.83 (2H, t, J=6.7 Hz) have also been reported. Hydrogens are coupled owing to J rate, whereas the integration of two hydrogens occurs in all peaks. Above data have been confirmed by the literature (12, 17, 22). A bi-dimensional spectrum of correlation 1H x 1H (COSY) (Figure 1F) was undertaken due to the purity degree of the fraction, to the obtained peaks and to the good results in the microbiological assay. Correlation of hydrogen δH 7.23 with δH6.91 and hydrogen δ 3.84 with δ 2.83 was reported. Since a small amount of mass was involved, NMR spectrum of 13C was not undertaken. However, when chemical displacement of carbon (δc) of 13C of fraction 5 by the HSQC spectrum of the crude fraction was analyzed (Figure 1C), correlations of 1H x 13C between 1H for δH 7,23 ppm with 13C δc 130 ppm (δc129.85 ppm, carbons 4’ and 8’) and between 1H δH 6.91 ppm with 13C δc 115 ppm (δc 115.19 ppm, carbons 5’ and 7’) were reported.
Figure 1.

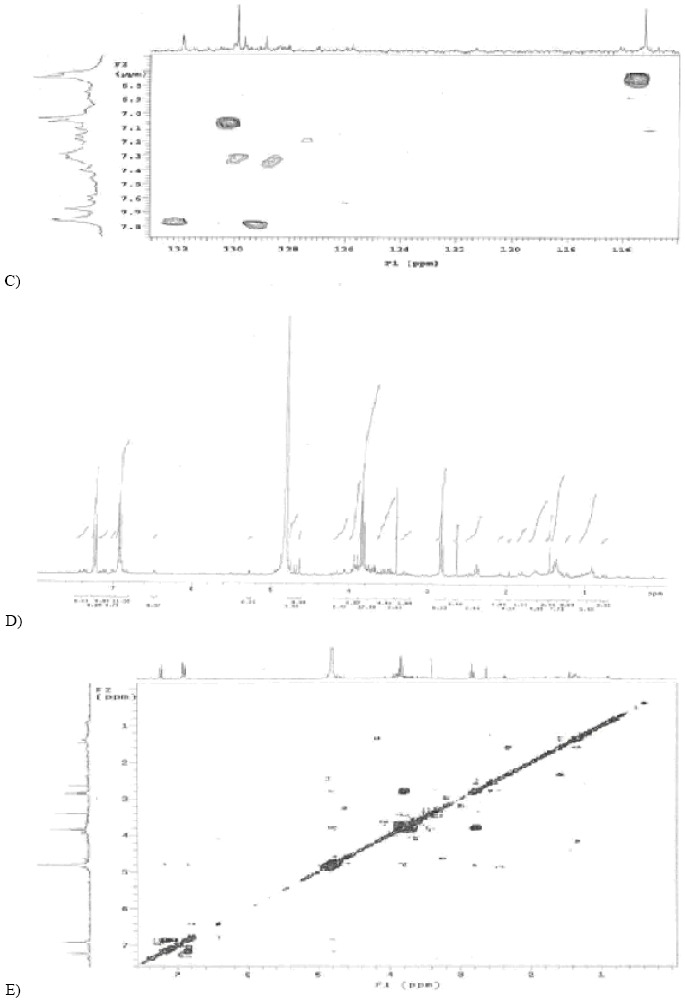
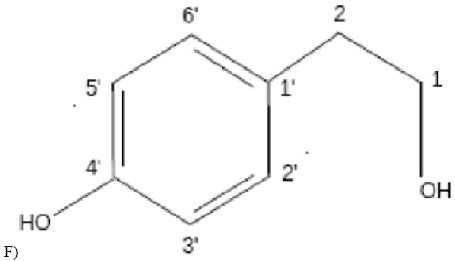
RMN spectruns of ¹H and ¹³C. (A) RMN spectrum of 1H (DMSO, 300 MHz) from crude extract. (B) RMN spectrum of 13C (DMSO, 75 MHz) from crude extract. (C) Espectrum de HSQC (1H x 13C) (DMSO from crude extract. (D) RMN spectrum of 1H (D2O, 300 MHz) from fraction 5. (E) Spectrum of 1H x 1H – COSY (D2O, 300 MHz) from fraction 5. (F) 2-(4-hidroxifenil)-etanol (Tirosol).
Conferring the literature (Table 2), data indicate Tyrosol (12, 17, 25), a well-known phenolic compound with antioxidant qualities found in wine and olive oil. It is produced by soil fungi and is beneficial when human pathologies, such as cardiovascular diseases and thromboses, occur. It also presents antifungal activity against Lagenidium callinectes and Gibberella pulicaris (9).
Table 2.
1H e 13C data for compound Tyrosol (300 MHz).
| Fraction 5 | Owen et al. (2000) | Ravirosa et al. (2006) | |||
| N° | 1 NMR | 13 C NMR | 1NMR | 1NMR | 13C NMR |
| 1’ | 3.84 t (6.7) | 3.67 (t) | 3.83 t (6.5) | 63.4 t | |
| 2’ | 2.83 t (6.7) | 2.70 (tt) | 2.80 t (6.5) | 38.2 t | |
| 3’ | |||||
| 4’ | 7.23 d (8.4) | 129.85 d | 7.01 (dt) | 7.10 d (8.4) | 130.1 d |
| 5’ | 6.91 d (8.4) | 115.19 d | 6.69 (d) | 6.78 d (8.4) | 115.4 d |
| 6’ | |||||
| 7’ | 6.91 d (8.4) | 115.19 d | 6.68 (d) | 6.78 d (8.4) | 115.4 d |
| 8’ | 7.23 d (8.4) | 129.85 d | 7.01 (dt) | 7.10 d (8.4) | 130.1 d |
Tyrosol has been identified as a quorum-sensing molecule, a signaler in the growth and morphogenesis of Candida albicans. Tyrosol is self-stimulated and increases its concentration according to population increase. In fact, it induces response as a bioluminescence, antibiotics production, biofilm formation and virulence (7).
In their assays with the endophytic fungus Glomerella cingulata isolated from Viguiera arenaria, Guimarães et al. (8) identified Tyrosol in one of the analyzed fractions and tested its activities on human T leukemia cells, although no cytotoxic activity was reported. Its anti-microbial activity against E. coli was tested, with minimum inhibitory concentration at 187µg/mL.
Fractions 7, 8 and 9 yielded a highly similar spectrum to that of Fraction 3 above. However, a better analysis of these fractions, especially 8 and 9, failed due to their small mass. Fraction 10 show that the main component is an aromatic compound. However, due to its small quantity, a better purification of the fraction was unavailable and new tests for its complete identification were impossible.
Evaluation of antibacterial activity
The metabolic extract from the fermented medium of endophytic fungus D. helianthi used for the evaluation of antimicrobial activity. When compared with the control, the metabolite had a significant statistically antibacterial activity on all pathogenic bacteria under analysis. Results were more pronounced for E. coli and S. typhi (Table 3). Results for E. coli corroborated those by Bernadi-Wenzel (5).
Table 3.
Antagonistic activity (halo size) of secondary metabolite (crude extract) of D. helianthi with pathogenic bacteria
| Bacteria | Halo size (cm) | Control 1 | Control 2 | Control 3 |
| E. hirae | 0.75±0.07 b | 4.00±0.00 a | 0.00±0.00 c | 0.00±0.00 c |
| S. typhi | 0.98±0.11 b | 3.79±0.07 a | 0.00±0.00 c | 0.00±0.00 c |
| S. aureus | 0.83±0.14 b | 3.63±0.13 a | 0.00±0.00 c | 0.00±0.00 c |
| E. coli | 1.07±0.05 b | 3.17±0.14 a | 0.00±0.00 c | 0.00±0.00 c |
| M. luteus | 0.72±0.02 b | 3.92±0.07 a | 0.00±0.00 c | 0.00±0.00 c |
| Xanthomonas sp | 0.77±0.03 b | 4.00±0.00 a | 0.00±0.00 c | 0.00±0.00 c |
| Control: 1 – Antibiotic; 2 - Methanol; 3 – Water. |
Means followed by same letter on the line do not differ among themselves by Tukey’s test (p>0.05)
Fraction test on the same bacteria was undertaken so that the activities of each separate compound in the crude extract may be evaluated. With its composition identified, fraction 5 showed inhibitory activity on S. aureus, E. coli and M. luteus (Table 4).
Table 4.
Antagonistic activity (halo size in cm) of D. helianthi fractions with pathogenic bacteria
| Fractions | E. hirae | S. typhi | S. aureus | E. coli | M. luteus | Xanthomonas sp |
| 1 | 0.0±0.00 d | 0.75±0.07 bc | 0.75±0.02 b | 0.75±0.02 c | 0.90±0.14 b | 0.0±0.00 f |
| 2 | 0.0±0.00 d | 0.73±0.04 bc | 0.72±0.04 b | 0.74±0.01 c | 0.0±0.00 f | 0.83±0.04 c |
| 3 | 0.0±0.00 d | 0.77±0.00 bc | 0.74±0.03 b | 0.77±0.00 c | 0.0±0.00 f | 0.0±0.00 f |
| 4 | 0.0±0.00 d | 0.0±0.00 d | 0.72±0.02 b | 0.80±0.00 c | 0.78±0.11 cd | 0.0±0.00 f |
| 5 | 0.0±0.00 d | 0.0±0.00 d | 0.79±0.08 b | 0.78±0.00 c | 0.70±0.00 e | 0.0±0.00 f |
| 6 | 0.87±0.06 bc | 0.73±0.03 c | 0.76±0.05 b | 0.73±0.06 c | 0.0±0.00 f | 0.0±0.00 f |
| 7 | 0.87±0.03 bc | 0.85±0.18 bc | 0.77±0.03 b | 1.00±0.09 b | 0.0±0.00 f | 0.95±0.35 b |
| 8 | 1.06±0.20 b | 0.74±0.05 c | 0.91±0.10 b | 0.76±0.05 c | 0.0±0.00 f | 0.0±0.00 f |
| 9 | 0.96±0.15 bc | 0.91±0.02 b | 0.86±0.13 b | 0.80±0.12 c | 0.80±0.14 c | 0.70±0.00 e |
| 10 | 0.77±0.09 c | 0.70±0.00 c | 0.73±0.03 b | 0.79±0.09 c | 0.72±0.02 de | 0.80±0.00 d |
| Control 1 | 4.0±0.00 a | 3.0±0.00 a | 3.42±0.19 a | 2.58±0.07 a | 3.00±0.00 a | 4.00±0.00 a |
| Control 2 | 0.0±0.00 d | 0.0±0.00 d | 0.0±0.00 c | 0.0±0.00 d | 0.0±0.00 f | 0.0±0.00 f |
| Control 3 | 0.0±0.00 d | 0.0±0.00 d | 0.0±0.00 c | 0.0±0.00 d | 0.0±0.00 f | 0.0±0.00 f |
Control: 1 – Antibiotic; 2 - Methanol; 3 – Water.
* Means followed by the same letter in the column do not differ among themselves by Tukey’s test (p>0.05)
Wang et al. (23) tested the bacterial and fungicide potential of isolated endophytic fungi of Quercus variabilis. Tests were undertaken for E. coli, Bacillus subtilis and Pseudomonas fluorescens and for five fungi Trichophyton rubrum, Candida albicans, Aspergilus niger, Epidermphyton floccosum and Microsporum canis. Moreover, 53.7% of the isolated 67 endophytes inhibited the tested pathogenic microorganisms. Endophytic fungi have an important bactericide potential since good results against 40.3% of the pathogenic bacteria were obtained.
Abdou et al. (1) tested the antibacterial activity of the endophyte Botryosphaeria rhodina isolated from Bidens pilosa. Results showed that two out of the four metabolic compounds tested were efficient against Bacillus subtilis. The authors concluded that the production of bioactive compounds by endophyte fungi may be involved within the endophyte-plant relationship owing to their ability in protecting the plant against pathogens.
Phongpaichit et al. (15) tested antimicrobial activity in an endophyte fungi culture which had been isolated from five species of Garcinia sp all a total of 377 fungi. Results showed that 18.6% had an antimicrobial activity at least against one pathogenic microorganism, 53% were efficient against S. aureus and Cryptococcus neoformans strains and 4.3% inhibited C. albicans. However, no endophyte inhibited gramnegative bacteria such as E. coli, Pseudomonas aeruginosas and Microsporum gypseum.
Xu et al. (24) tested the antibacterial activities of endophytic fungi isolated from the rhizomes of the Chinese medicinal herb Dioscorea zingiberensis. Seven out of the nine endophytes isolated showed antibacterial activity at least against three of the four tested pathogenic bacteria (E. coli, Xanthomonas vesicatoria, B. subtilis, Staphylococcus haemolyticus). Results show that endophytes are a source of bioactive compounds.
Evaluation of antifungal activity
D. helianthi metabolite, produced by fermentation and extracted by ethyl acetate, was tested for seven phytopathogenic fungi. Fraction 5 was then tested against pathogen M. perniciosa.
However, when compared to control, there was no statistically significant anti-fungus activity in the crude extract or in any of its fractions. This is probably due to the manner the experiment was undertaken. If certain modifications in the protocol had been undertaken, results would have been probably positive with regard to the inhibition of certain phytopathogens as the literature shows.
The chemical investigation of the secondary metabolite of D. helianthi identified the phenolic compound 2(-4 hydroxyphenyl)-ethanol known as Tyrosol, already found in wine and olive oil. Other plant-associated fungi produce Tyrosol with its antimicrobial characteristics and its activity in human pathogens such as cardiovascular diseases and thrombosis.
Assays in current research using the crude extract and fractions of secondary metabolites and D. helianthi-produced compound showed an antibacterial activity. Since there is evidence of the bioactive potential of endophyte fungi, the importance of the chemical and biological importance of these microorganisms is thus enhanced.
REFERENCES
- 1.Abdou R., Scherlach K., Dahse H.M., Sattler I., Hertweck C. Botryorhodines A-D, antifungal and cytotoxic depsidones from Btryosphaeria rhodina, an endophyte of the medicinal plant Bidens pilosa. Phytochemistry. 2009;71:110–116. doi: 10.1016/j.phytochem.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Agusta A., Maehara S., Ohashi K., Simanjuntak P., Shibuya H. Stereo-selective oxidation at C-4 of flavans by the endophytic fungus Diaporthe sp. isolated from a tea plant. Chem. Pham. Bull. 2005;53:1565–1569. doi: 10.1248/cpb.53.1565. [DOI] [PubMed] [Google Scholar]
- 3.Arnold A.E. Endophytic fungi: hidden components of tropical community ecology. In: Carson W.P., Schnitzer S.A., editors. Tropical forest community ecology. Blackwellp: Wiley; 2008. pp. 254–271. [Google Scholar]
- 4.Azevedo J.L., Macchroni W.J., Pereira J.O., Araújo W. L. Endophytic microorganisms: a review on insect control and recent advances on tropical plants. EJB: Electronic Journal of Biotechnology. 2000;3:40–65. [Google Scholar]
- 5.Bernardi-Wenzel J. Bioprospecção e caracterização citológica e molecular de fungos endofíticos isolados de Luehea divaricata (Martius et Zuccarini): Estudo da interação endófito-planta hospedeira. Brasil: Maringá; 2008. p. 103. M.Sc. Dissertation. Biologia Comparada. UEM) [Google Scholar]
- 6.Bernardi-Wenzel J., Garcia A., Rubim Filho C. J., Prioli A. J., Pamphile J. A. Evaluation of foliar fungal endophyte diversity and colonization of medicinal plant Luehea divaricata (Martius et Zuccarini) Bio. Res. 2010;43:375–384. [PubMed] [Google Scholar]
- 7.Chen H., Fujita M., Feng Q., Clardy J., Fink G. R. Tyrosol is a quorum-sensing molecule in Candida albicans. Proc. Natl. Sci. U. S. A. 2004;101:5048–5052. doi: 10.1073/pnas.0401416101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guimarães D. O., Borges W.S., Kawano C.Y., Ribeiro P.H., Goldman G.H., Nomizo A., Thiemann O.H., Lopes N.P., Pupo M.T. Biological activities from extracts of endophytic fungi isolated from Viguiera arenaria and Tithonia diversifolia. FEMS Immunol Med Microbiol. 2008;52:134–144. doi: 10.1111/j.1574-695X.2007.00354.x. [DOI] [PubMed] [Google Scholar]
- 9.Guimarães D.O., Borges K.B., Bonato P.S., Pupo M.T. A simple method for the quantitative analysis of Tyrosol by HPLC in liquid Czapek cultures from endophytic fungi. J. Braz. Chem. Soc. 2009;20:188–194. [Google Scholar]
- 10.Hoffman M.T., Arnold A.E. Geographic locality and host identity shape fungal endophyte communities in cupressaceous trees. Mycol. Res. 2008;112:331–344. doi: 10.1016/j.mycres.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Li H., Qing C., Zhang Y., Zaho Z. Screening for endophytic fungi with antitumour and antifungal activities from Chinese medicinal plants. World J. Microb. Biot. 2005;21:1515–1519. [Google Scholar]
- 12.Owen R.W., Mier W., Giacosa A., Hull W.E., Spiegelhalder B., Bartsch H. Identification of lignans as major components in the phenolic fraction of olive oil. Clin. Chem. 2000;46:976–988. [PubMed] [Google Scholar]
- 13.Pamphile J.A., Azevedo J.L. Molecular characterization of endophytic strains of Fusarium verticillioides (Fusarium moniliforme) from maize (Zea mays.L) World Journal of Microbiology & Biotechnology. Holanda. 2002;18(5):391–396. [Google Scholar]
- 14.Petrini O. Fungal endophyte of tree leaves. In: Andrews J., Hirano S.S., editors. Microbial Ecology of Leaves. New York: Springer Verlag; 1991. pp. 179–197. [Google Scholar]
- 15.Phongpaichit S., Rungjindamai N., Rukachaisirikul V., Sakayaroj J. Antimicrobial activity in cultures of endophytic fungi isolated from Garcinia species. FEMS Immunol. Med. Microbiol. 2006;48:367–372. doi: 10.1111/j.1574-695X.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez R.J., White-Jr. J. F., Arnold A.E., Redman R.S. Fungal endophytes: diversity and functional roles. New Phytologist. 2009;182:314–330. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- 17.Rovirosa J., Diaz-Marrero A., Darias J., Painemal K., Martin A.S. Secondary metabolites from marine Penicillium brevicompactum. J. Chil. Chem. Soc. 2006;51:775–778. [Google Scholar]
- 18.Sambrook J., Russel D.W. Molecular cloning: a laboratory manual. 3. Cold Spring Harbor, New York, N.Y. USA.: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 19.SAS. Statistical Analysis System. Cary, NC, USA.: Sas Institute Inc.; 2001. [Google Scholar]
- 20.Schneider P., Misiek M., Hoffmeister D. In vivo and in vitro production options for fungal secondary metabolites. Mol. Pharmaceutics. 2008;5:234–242. doi: 10.1021/mp7001544. [DOI] [PubMed] [Google Scholar]
- 21.Souza A.Q.L., Souza A.D.L., Filho S.A., Pinheiro M.L.B., Sarquis M.I.M., Pereira J.O. Atividade antimicrobiana de fungos endofíticos isolados de plantas tóxicas da Amazônia: Palicourea longiflora (aubl.) rich e Strychnos cogens bentham. Acta Amazônica. 2004;34(2):185–195. [Google Scholar]
- 22.Suárez L. E. C., Cendales D. R. M., Orozco P. C. I. Compuesto fenólicos aislados de la especie Solanum validinervium (Solanaceae) sección geminata. Rev. Colomb. Quim. 2006;35:59–65. [Google Scholar]
- 23.Wang F. W., Jiao R.H., Cheng A.B., Tan S.H., Song Y.C. Antimicrobial potentials of endophytic fungi residing in Quercus variabilis and brefeldin A obtained from Cladosporium sp. World J. Microb. Biot. 2007;23:79–83. [Google Scholar]
- 24.Xu L., Zhou L., Zhao J., Li J., Li X., Wang J. Fungal endophytes from Dioscorea zingiberensis rhizomes and their antibacterial activity. Appl. Microbiol. 2008;46:68–72. doi: 10.1111/j.1472-765X.2007.02264.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Yi, Zhang G.L., Li B.G., Chen Y.Z. Two new compounds from Melanosciadum pimpinelloideum H. Boiss. Chin. Chem. Lett. 2001;12:333–334. [Google Scholar]


