Abstract
Baffled shake flask cultivation of Aurantiochytrium sp. B-072 was carried out at in a glucose-monosodium glutamate mineral medium at different C/N-ratios (30–165) with glucose fixed at 90 g/L. With increasing C/N-ratio, a modest increase in lipid content (60 to 73 % w/w) was observed whereas fat-free biomass decreased but overall biomass showed little variation. FA-profiles were not affected to a large extent by C/N-ratio and absolute docosahexaenoic (DHA)-levels fell in narrow range (5–6 g/L). However at C/N > 64 a rapid decrease in lipid synthetic rate and/or incomplete glucose utilization occurred. Glucose and FA-fluxes based on fat-free biomass peaked at a C/N ratio of 56. This condition was chosen for calculation of the redox balance (NAD(P)H) and energy (ATP) requirement and to estimate the in vivo P/O ratio during the main period of fatty acid biosynthesis. Several models with different routes for NADPH, acetyl-CoA formation and re-oxidation of OAA formed via ATP-citrate lyase were considered as these influence the redox- and energy balance. As an example, using a commonly shown scheme whereby NADPH is supplied by a cytosolic “transhydrogenase cycle” (pyruvate-OAA-malate-pyruvate) and OAA formed by ATP-citrate lyase is recycled via import into the mitochondria as malate, the calculated NADPH-requirement amounted to 5.5 with an ATP-demand of 10.5 mmol/(g fat-free biomass x h) and an in vivo P/O-ratio (not including non-growth associated maintenance) of 1.6. The lowest ATP requirement is found when acetyl-CoA would be transported directly from the mitochondria to the cytosol by carnitine acetyltransferase. Assay of some enzymes critical for NADPH supply indicates that activity of glucose-6-phosphate dehydrogenase, the first enzyme in the HMP pathway, is far insufficient for the required NADPH-flux and malic enzyme must be a major source. Activity of the latter (ca. 300 mU/mg protein) far exceeds that in oleaginous fungi and yeast.
Keywords: Aurantiochytrium, C/N-ratio, Docosahexaenoic acid, Fatty acid flux, P/O ratio
INTRODUCTION
Docosahexaenoic acid (DHA, 22:6 n-3) is required for maintenance of normal brain function and photoreceptor function in humans (11, 21). DHA is largely derived from fish oils, but declining fish stocks, environmental pollution and season-dependent compositional variation in fatty acid (FA) composition have led to a search for alternative sources, such as various marine microbes.
Various auto- and heterotrophic microbes produce DHA and other polyunsaturated fatty acids (PUFAs), but commercially bulk production of DHA-rich oil or biomass has focused on heterotrophic cultivation of the dinoflagellate Crypthecodinium cohnii and the non-related thraustochytrids which include the genus Aurantiochytrium (29). Physiological knowledge of these organisms remains limited however, but one unusual characteristic of the thraustochytrids and possibly C. cohnii is the possession of a polyketide system (PKS) for synthesis of PUFAs. In this system, introduction of double bonds occurs by isomerization/dehydration rather than by oxygen-dependent desaturases (20).
Surprisingly, especially in view of current interest in specialty FAs as well as biodiesel derived from microbial lipids, flux analysis of lipid metabolism has been largely ignored. As basically no growth occurs in the main lipogenic (N-limited phase), in silico flux analysis can be significantly simplified as carbon is only consumed in lipid formation as well as ATP generation (dissimilation) for the latter process as well as maintenance. Hence by assaying the concentrations of glucose as well as cellular FAs in time, sufficient formation is obtained to calculate the redox- and energy balance.
The aim of this work was firstly to study the effect of C/N-ratio in a mineral medium by varying nitrogen levels at a fixed concentration of glucose on the specific flux of glucose and fatty acids. Secondly, for one cultivation condition the redox balance and bioenergetics formation during lipogenesis were calculated for various metabolic networks. Assay of a limited number of enzymes was used to confirm the feasibility of the various networks. Finally the results are discussed in view of more efficient DHA production, both in terms of absolute DHA-level as well as productivity.
MATERIALS AND METHODS
Strain and maintenance
Aurantiochytrium sp. B-072 was kindly supplied by dr. Somtawin Jaritkhuan, Burapha University, Thailand. The strain was preserved in 20% (v/v) glycerol at –80 ºC and maintained by monthly subculture on YPG agar. The YPG agar contained (g/L): artificial sea salts, 15; glucose, 1; yeast extract, 1; peptone, 1; MnCl2, 1; and agar, 15.
Identification of strain
The strain of Aurantiochytrium sp. B-072 was cultured for 3 days in an agar plate. A single colony that grown on an agar plate was inoculated to a 50 mL tube with 10 mL of liquid medium prepared with artificial seawater containing 4 g/L glucose, 2 g/L yeast extract and 1 g/L peptone. The cell was grown in the medium at 25 ˚C for 4 days with continuous shaking (150 rpm). The cells were collected by centrifugation (9,000×g, 10 min), washed twice with equal volume of sterile deionized water and dried by lyophilizer before DNA extraction and sequencing.
Dried biomass was ground with glass beads by vortexing. Total genomic DNA of cells was extracted by using DNeasy Plant Mini Kit purchased from Qiagen (Germantown, MD, USA) according to the manufacturer’s guideline. The resulting of extracted DNA was determined the quality before amplify by using agarose gel electrophoresis (0.8% agarose). According to Chodchoey et al. (6), Grzebyk et al. (12) and Yang et al. (32), two primers, forward primer: 5′-TCCTGCCAGTAGTCATATGC-3′ and reverse primer: 5′-TGATCCTCTCGCAGGTTCAC-3′ were used to amplify 18S rRNA gene in the genomic DNA. A genomic DNA template of 100 ng was mixed with the polymerase chain reaction (PCR) mixture containing 50 μL of 1× Taq PCR buffer (New England Biolabs, UK), 0.2 mM dNTP, 0.2 μM of 16S1N, and 16S2N primers respectively, and 2.5 U Taq polymerase (New England Biolabs, UK) and denatured at 94°C for 5 min. The PCR program was run for 30 s at 94°C, 30 s at 60.8°C (annealing temperature), 2 min at 72°C for 40 cycles, and 7 min for final extension at 72°C. Then the PCR product was sent for DNA sequencing. The resulting 18S rRNA gene sequences were aligned and compared to the nucleotide sequences of microorganisms in GenBank database of the National Center for Biotechnology Information by using Basic Local Alignment Search Tool (BLAST). The neighbor-jointing (NJ) tree was constructed by the multiple alignment program CLUSTAL W using MEGA5 software (23). The values of bootstrap were analyzed from 1,000 replications (32).
Shake-flask cultivation to study effect of C/N-ratio
Preparation of a standardized inoculum was as described by Unagul et al. (25). A 5% v/v inoculum (adjusted to OD660 of 2.0) was transferred to 500 mL baffled flasks (Bellco, USA) containing 100 mL of the test media and shaken at 25˚C and 200 rpm until glucose was exhausted. Mineral medium consisted of glucose (fixed at 90 g/L unless otherwise noted); monosodium glutamate monohydrate (MSG) 3, 6, 8, 9, 12, 15 and 18 g/L; KH2PO4, 0.6 g/L; artificial sea salts (Sigma, USA, refer to manufacturers product sheet for detailed composition), 15 g/L; trace elements and vitamins (28), both 1 mL/L. The media were sterilized at 120˚C for 15 min in 80% of the final volume, followed by addition of glucose (sterilized at 110˚C as a stock solution of 50% w/v) and vitamins (sterilized by 0.2 micron filter). They were then made up to the final volume (100 mL) with sterilized distilled water. Experiments were performed in duplicate.
Analysis of culture supernatant
Glucose was assayed with a commercial enzymatic kit (Glucose liquicolor, Human, Germany). Glutamate was assayed via an amino nitrogen assay by the TNBS (trinitrobenzo sulfonic acid) procedure (1).
Assay of biomass dry weight
The culture samples (2 mL) were harvested by centrifugation (9,000×g, 10 min, 4 ˚C), and biomass was washed twice with distilled water. After freeze-drying the pellet was placed in a desiccator before gravimetrical determination.
Analysis of fatty acids
Briefly, 15–20 mg of freeze-dried biomass was accurately weighed and esterified by 4% sulphuric acid in methanol and antioxidant, butylated hydroxytoluene for 1 h. at 90 ˚C. Heptadecanoic acid (Sigma, St. Louis, USA) was used as internal standard. Samples were subjected to gas chromatography on a GC-17A instrument (Shimadzu, Japan) equipped with a Supelco OmegawaxTM 250 fused silica capillary column. The procedure and instrumentation has been described in detail by Unagul et al. (25).
Preparation of cell-free extract (cfe) and enzyme assays
Biomass (ca. 300 mg dry weight) was harvested at the late lipogenic phase, washed once with distilled water and extraction buffer (0.1 M Tris-HCl pH 7.2, 10% w/v glycerol, 1 mM dithiothreitol and 1 mM EDTA) and then suspended in 4 mL ice-cold extraction buffer in a glass tube containing 1g of glass beads (diameter 0.6 mM) and sonicated in 4 x 2.5 min intervals with intermediate cooling on ice. The cell homogenate was then centrifuged at 50,000 x g for 10 min at 4˚C. The clear supernatant was collected, kept on ice and used immediately for enzyme assays as detailed below.
Enzyme assays were performed with a Jasco B-530 spectrophotometer at 25˚C. All assays were carried out with two different amounts of cfe and corrected for endogenous activity.
ATP:citrate lyase (ACL) was assayed according to Srere (22). Carnitine acetyltransferase (CAT) was assayed according to Kohlhaw and Tan-Wilson (18). Glucose-6-P-dehydrogenase (G-6-P-DH) and 6-P-gluconate dehydrogenase were assayed according to Harris et al. (15) and Bruinenberg et al. (3), respectively. Isocitrate dehydrogenase was assayed according to Harris et al. (15). Malic enzyme was also assayed according to Harris et al. (15) but malate concentration was decreased from 100 to 25 mM as a concentration of 100 mM resulted in severe substrate inhibition (data not shown). Protein content of cfe was assayed by the Lowry method using fat-free BSA as standard. Enzyme activity was then expressed as nmol substrate/product converted per min per mg protein (or mU/mg protein) using a molar absorption coefficient (340 nm) of 6.22 mM-1.cm-1 for NAD(P)H.
The measured specific activity of enzymes was converted into maximal cellular fluxes assuming the enzymes operate at Vmax in vivo according to Postma et al. (19).
Definitions and calculations
As MSG contains a significant amount of carbon, C/N-ratio was calculated from (Cglucose + CMSG)/NMSG , or in g/L: ([glucose] x 0.4 + [MSG] x 0.32) / [MSG] x 0.074. Fat-free biomass (FFB, g/L) was calculated by subtracting total fatty acid content (g/L) from biomass (g/L). Flux is the formation or consumption rate of a metabolite/product along a metabolic pathway or transport of a substrate across a membrane and is expressed in mmol of component per gram of FFB per hour. It is obtained from the plotting the concentration of the component of interest vs time followed by linear regression after conversion into mmoles.
Stoichiometric model and bioenergetic calculations
Basic set-up and principles of the stoichiometric network are as previously described by e.g. Henriksen et al. (16) and van Gulik et al. (26, 27) for penicillin production by Penicillium chrysogenum, a process with shares a common factor with lipid production, i.e. a high NADPH-demand. Briefly, as no major changes in absolute levels of protein and carbohydrate occurred after N-exhaustion and no extracellular products could be detected by HPLC and GC (tested for C/N-ratio 56, data not shown), it was assumed that storage lipid was the only product and consisted solely of triacylglycerols. The latter make up the bulk of the lipid if significant formation of storage lipids occurs (2). Compartmentation of relevant metabolites and reducing equivalents (NAD(P)H) was taken into account. The PKS system is believed to be soluble and presumably located in the cytosol, hence NADPH need to be available there, as in the case for the synthesis of saturated FAs by the fatty acid synthase. Glucose was assumed to be transported by facilitated diffusion. For intracellular transport it was assumed that movement of four protons across the inner mitochondrial membrane equals 1 ATP, hence for instance pyruvate transport in symport with one proton (27) requires ¼ ATP. Citrate is assumed to have a charge of -2 under physiological conditions and transport is assumed to require ½ ATP. Various schemes for provision of acetyl-CoA and NADPH are shown in Fig. 4a-b and are further discussed in the text.
Figure 4.
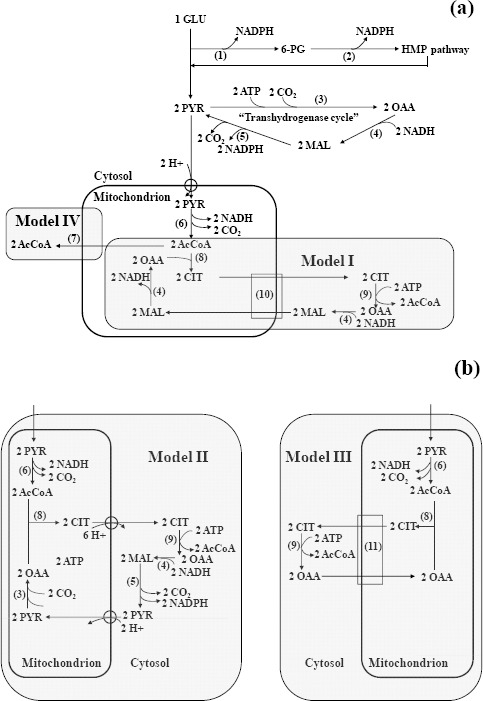
Metabolic models for acetyl-CoA and NADPH provision during lipogenesis. NADPH-producing pathways i.e. HMP and cytosolic “transhydrogenase cycle” as well as general route to pyruvate are shown in (a). Models I to III all use ATP-citrate lyase for generation of acetyl-CoA. Subsequently, recycling of oxaloacetate (OAA) occurs by citrate-malate translocation in model I (a); pyruvate formation in model II (b); or direct transport of OAA in model III (b). Model IV assumes direct acetyl-CoA supply via carnitine acetyltransferase (a). The enzymes involved are: 1, glucose-6-phosphate dehydrogenase; 2, 6-phosphogluconate dehydrogenase; 3, pyruvate carboxylase; 4, malate dehydrogenase; 5, NADP+-linked malic enzyme; 6, pyruvate dehydrogenase; 7, carnitine acetyltransferase; 8, citrate synthase; 9, ATP:citrate lyase; 10, malate-citrate translocase; 11, citrate-oxaloacetate translocase.
Statistics
Each data point represents an average of duplicate experiments and assays. The data were subjected to statistical analysis using SPSS (SPSS Inc., 1998, Chicago, IL, USA) version 15 for windows (Duncan’s test). Significant differences were reported for P < 0.05.
RESULTS AND DISCUSSION
Identification of strain
According to 18S rRNA analysis (sequence deposited at the GenBank database under accession number JF266572), strain B-072 is classified as an Aurantiochytrium sp. (formerly Schizochytrium). Highest similarity to other strains in the database was found with Aurantiochytrium sp. LK4 (98% identity with 100% coverage), which was isolated in a Hong Kong mangrove forest.
Growth and fatty acid content at different C/N-ratios
A typical substrate consumption and product formation profile for a cultivation with at a C/N-ratio of 56 (90 g/L glucose and 9.4 g/L MSG.H2O) is depicted in Fig. 1a. Following exponential growth under non-N limiting conditions with low lipid formation, a linear phase of lipid formation was observed in the N-limited region until glucose was exhausted.
Figure 1.
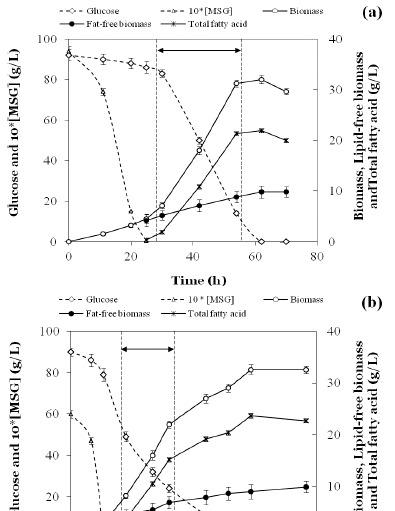
Levels of glucose, glutamate, biomass and TFA during baffled shake flask cultivation of Aurantiochytrium sp. B-072with C/N ratio of 56 (a) and 84 (b). The cultures were grown at 25 °C and agitated at 200 rpm. Arrow indicates the time span for which the lipogenic volumetric flux was calculated.
This pattern was typical for all C/N-ratio’s not exceeding 64 (data not shown). From microscopic observation, cell size of Aurantiochytrium sp. B-072 was 8–15 micron at a C/N-ratio of 56. However, at a C/N-ratio of 84 a clear decrease in the rate of glucose uptake with a concomitant reduction in lipid synthesis rate was observed approximately halfway the lipogenic phase, but eventually all glucose was consumed and a lipid content of 73% w/w was reached (Fig. 1b). At a C/N-ratio of 165, this decrease in glucose uptake/lipid formation rates became even more pronounced and glucose consumption terminated completely at a residual concentration of ca. 30 g/L. At this point, the biomass level and TFA (Total Fatty Acid) contents were only 29 g/L and 65.7% w/w, respectively (data not shown). Data pertaining to maximal as well as fat-free biomass and TFA for all C/N-ratio’s tested have been summarized in Fig. 2. Fat-free biomass decreased, and TFA increased with decreasing amounts of N in the medium, but total biomass fell in a narrow range (30–38 g/L) with a statistically higher value for the lowest C/N-ratio tested (Fig. 2).
Figure 2.
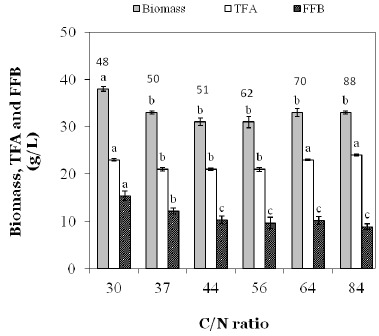
Maximal biomass, fatty acid free biomass (FFB) and total fatty acid (TFA) of Aurantiochytrium sp. B-072 as function of the C/N-ratio after growth in baffled flasks in a mineral glucose-MSG medium with glucose fixed at 90 g/L. A different letter indicates a statistical difference (p<0.05) within a parameter. Data at the top of the figure indicate cultivation time in h.
In a further experiment the effect of fixing the C/N-ratio but at the same time increasing absolute DHA-level was attempted. As in the experiment described above a C/N-ratio of 56 resulted in the highest FA synthetic rate (see section on fluxes below) this ratio was then selected and glucose and MSG levels were increased to 150 and 15 g/l, respectively. This condition resulted in 51±2 g/L biomass with a TFA content of 71±2 % w/w, statistically similar to the value of 69±2 % w/w observed at the same C/N-ratio but with 90 g/L glucose (Table 1). From Table 1 it can be calculated that a maximal level of ca 6 g DHA/L was obtained and that DHA constituted almost 20% w/w of total biomass. At this higher initial glucose level, volumetric and specific TFA-fluxes amounted to 0.47 g/(L x h) and 0.66 g/(L x h), respectively (Fig. 3a).
Table 1.
Fatty acid profiles (% TFA) of Aurantiochytrium sp. B-072 grown on a mineral glucose-MSG medium as a function of C/N-ratio with glucose fixed at 90 g/L. Samples were taken during the late lipogenic phase.
| C/N ratio | Fatty acid (% TFA) | ||||||
|---|---|---|---|---|---|---|---|
| C14:0 | C16:0 | C18:0 | C20:3n6 | C22:5n6 | C22:6n3 | TFA (%w/w) | |
| 30 | 7.7 | 61.2 | 1.1 | 1.3 | 3.1 | 25.6 | 60.1 |
| 37 | 10.6 | 57.6 | 1.1 | 1.2 | 3.0 | 26.5 | 64.6 |
| 44 | 10.8 | 58.7 | 0.9 | 1.2 | 3.2 | 25.2 | 66.8 |
| 56 | 12.0 | 58.2 | 0.9 | 1.2 | 3.1 | 24.5 | 68.5 |
| 56 a | 11.5 | 55.8 | 1.0 | 1.3 | 3.2 | 27.2 | 71.0 |
| 64 | 11.5 | 57.4 | 0.8 | 0.9 | 3.4 | 26.0 | 69.1 |
| 84 | 10.0 | 59.2 | 1.2 | 0.8 | 5.3 | 23.6 | 72.6 |
| 165 b | 5.9 | 60.0 | 1.2 | 0.4 | 7.6 | 25.0 | 65.7 |
with 150 g/L glucose
incomplete glucose utilization
Unidentified peaks accounted for less than 0.5% w/w of TFA and have been ignored. SD was less than 5%.
Figure 3.
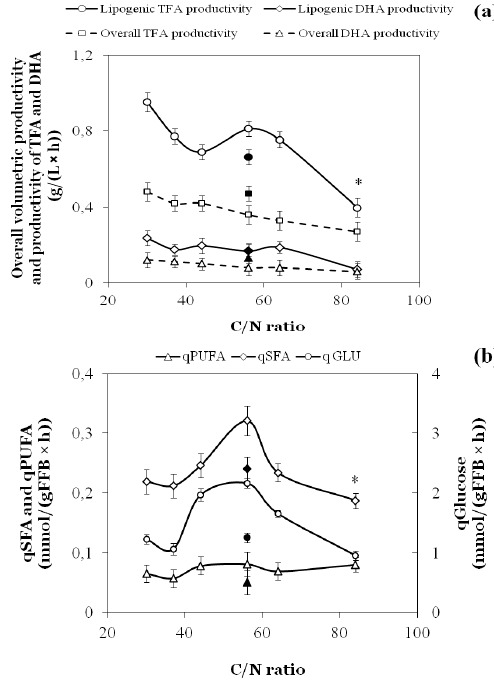
(a) Overall volumetric productivity and productivity during the lipogenic phase of total FAs and DHA (g/(L x h)) at different C/N-ratio’s in a glucose-MSG medium (glucose fixed at 90 g/L with an additional experiment at 150 g/L as marked by closed symbol). (b) Specific fluxes (q) of glucose, saturated FAs (SFAs) and PUFAs based on fat-free biomass (mmol/(g fat-free biomass x h)) during the lipogenic phase. For time point marked with * only the initial rate was calculated as fluxes were not linear under these conditions (see Fig 1b).
Assay of the FA-profile showed a simple pattern which was largely independent of either the time in the lipogenic phase where samples were taken (data not shown) or the C/N-ratio. Myristic acid (C14:0), palmitic acid (C16:0), docosapentaenoic acid (C22:5 n-6) and docosahexaenoic acid (C22:6 n-3) were the main components, with the latter making up ca 24.5–27.5% of the TFA (Table 1).
Glucose and fatty acid fluxes at different C/N-ratio’s
In commercial practice, the volumetric productivity is an important parameter, i.e. a high product level must be obtained in a short time. The overall volumetric productivity (from inoculation to point of glucose exhaustion) as well as the productivity during the main lipogenic phase for both TFA and DHA is shown in Fig. 3a. However, volumetric productivity depends strongly on the amount of fat-free biomass level and hence says little about the actual capacity of an organism to produce TFA or DHA and the mechanisms involved. Hence it is more useful to base data on fat-free biomass. Glucose, TFA and individual FA specific fluxes based on FA-free (FFB) biomass were calculated after depletion of N by linear regression of the concentrations of glucose and individual FAs in time. As at C/N-ratio >64 glucose and FA-levels tended to bend strongly (Fig. 1b), only the initial rate was calculated for these cases. To reduce data, all fluxes of saturated FAs, which are produced via a conventional fatty acid synthase (FAS) have been pooled. Similarly, fluxes through the PKS system(s) that lead to PUFAs have been combined. The three fluxes thus obtained had similar trends and went through a maximum at a C/N-ratio of ca. 56 where qTFA reached 0.402 mmol TFA/(gFFB x h) (Fig. 3b).
Metabolic networks and energetics during the lipogenic phase
In order to potentially improve product yield or specific flux, for instance by metabolic engineering, an understanding of the metabolic network(s) and energetic involved in lipogenesis and bioenergetics is important. A presumed key factor for lipogenesis in oleaginous microorganisms is the presence of a cytosolic ATP:citrate lyase (ACL) which splits citrate in acetyl-CoA and oxaloacetate (OAA) (13). Whereas the former is then used for FA synthesis, the latter has to be “re-cycled”. Based on a literature survey, three models were considered for the latter process. In model I, which was originally developed for oleaginous yeasts (8), OAA is converted into malate and then exchanged for citrate, thus leading to an electroneutral exchange. This reduces the overall ATP requirement by circumventing transport costs for either malate or citrate. Model II differs from model I in that malate is further converted into pyruvate by malic enzyme with production of NADPH. Pyruvate is subsequently transported back into the mitochondria (Fig. 4). Model III is based on the observation that some non-oleaginous yeasts, i.e. Saccharomyces cerevisiae (9) and plants (14) can directly transport OAA from the cytosol to the mitochondria. The exact mechanism has not been resolved, though it is conceivable that in the case of B-072 a citrate/OAA exchange might occur (Fig. 4b). Finally, model IV lacks ACL but uses transport of acetyl-CoA from mitochondria to cytosol by carnitine acetyltransferase (CAT). In this case, no OAA is formed (Fig 4a). This system possibly occurs in some yeasts (17) and filamentous fungi (31).
As an example, calculation for the redox- and energy balance employing model I is presented in Table 2. In this model, there is no direct formation of NADPH in the pathways leading to formation of the various FAs as evident from Fig. 4a. Hence a high flux through the transhydrogenase cycle is required to provide NADPH. However, this will simultaneously result in large drain of cytosolic NADH as the reaction involved can be written as [–NADH–ATP+NADPH =0], an aspect that has been ignored in the literature. Consequently, NADH would have to be transferred from the mitochondria to the cytosol by a “reverse” NADH-shuttle. Whether this occurs in vivo has not been established so far. Data for the other three models have been summarized in Table 3 with model I included for easier comparison. Model II has a lower requirement for NADPH provision than the other models (2.1 vs 5.5 mmol/(g FFB x h)) due to the involvement of malic enzyme in OAA recycling as shown in Fig. 4. However, it results in the highest calculated ATP requirement due to transport costs for citrate and pyruvate (cf. Fig. 4b). Model IV, however, results in a relatively low ATP-requirement as it does not require input of ATP for the reaction catalyzed by ATP:citrate lyase. This confirms earlier in silico calculations for NADPH production required for penicillin synthesis in Penicillium chrysogenum that suggest that ACL is a major energy drain and re-routing though CAT might be useful for provision of acetyl-CoA (16).
Table 2.
Example of redox and bioenergetic calculations for lipid synthesis in Aurantiochytrium sp. B-072 at a C/N-ratio of 56 with a transhydrogenase cycle for NADPH supply and OAA recycling via malate-citrate translocase (model I, see Fig. 4a). Data are expressed in mmol/(g fat-free biomass x h) with a minus sign indicating consumption.
| Component or process | Flux | Glucose a | NADPHcyt | NADHmit | NADHcyt | ATP |
|---|---|---|---|---|---|---|
| C14:0 | 0.060 | -0.210 | -0.720 | +0.840 | – | -0.360 |
| C16:0 | 0.261 | -1.044 | -3.654 | +4.176 | – | -1.827 |
| DPA (C22:5n6) | 0.012 | -0.066 | -0.180 | +0.264 | − | -0.120 |
| DHA (C22:6n3) | 0.069 | -0.380 | -0.966 | +1.518 | − | -0.690 |
| Glycerol | 0.136 | -0.068 | − | − | -0.136 | -0.136 |
| Triacylglycerol | 0.136 | − | − | − | − | -0.816 |
| Sum | -1.768 | -5.520 | +6.798 | -0.136 | -3.949 | |
| Transhydrogenase cycle | +5.520 | − | -5.520 | -5.520 | ||
| Pyruvate transport | − | − | − | -1.044 | ||
| Sum | 0 | +6.798 | -5.656 | -10.513 |
Overall measured glucose uptake rate was –2.156 mmol/(gFFB x h).
Table 3.
Overview of redox- and energy balance for lipid synthesis in Aurantiochytrium sp. B-072 at a C/N-ratio of 56 as calculated from various stoichiometric models (see Fig. 4). Data are expressed in mmol/(g fat-free biomass x h) with a minus sign indicating consumption. A refers to data for direct synthesis of lipids from glucose without balancing of NADPH or ATP, whereas these are included in B. For a detailed sample calculation refer to .
| NADPHmit | NADHcyt | NADH | ATP | |||
|---|---|---|---|---|---|---|
| Model | A* | A** | A | B | A | B |
| I | -5.520 | +6.798 | -0.136 | -5.656 | -3.949 | -10.513 |
| II | -2.121 | +3.399 | -0.136 | -2.257 | -7.348 | -13.645 |
| III | -5.520 | +3.399 | +3.263 | -2.257 | -3.949 | -10.513 |
| IV | -5.520 | +3.399 | +3.263 | -2.257 | -0.550 | -7.114 |
values for B are 0
values for A and B are identical (also see Table 2).
Apart from the “transhydrogenase cycle”, a further route of NADPH provision is the HMP pathway (hexose monophosphate pathway), which yields 2 NADPH/glucose if it operates in a non-cyclical manner. Hence if all glucose passed through the HMP pathway and with a measured glucose flux of ca 2.2 mmol/(g FFB x h), this might yield a maximum of 4.4 mmol NADPH/(g FFB x h). This would cover most of the flux of 5.5 as calculated for all but model II (Table 3). To check the feasibility of a major contribution of the HMP pathway, specific activity (s.a.) of glucose-6-P-dehydrogenase (G6PDH) and 6-P-gluconate dehydrogenase (6PGDH) was assayed. In contrast to yeasts (3), a low s.a. of G6PDH.was found which would limit the flux through HMP to only 1.4 mmol G6P/(g FFB x h), equivalent to 2.8 mmol NADPH/(g FFB x h) (Table 5.). Except for model II, this falls far short of the calculated flux, hence other sources of NADPH must be involved. Activity of malic enzyme was sufficient to account solely for the NADPH requirement (Table 5). This confirms various reports suggesting that apart from ACL, the presence of NADP-malic enzyme is another critical factor for lipogenesis (13, 30).
Table 5.
Specific activity (nmol/(mg protein x min)) of NADPH- and acetyl-CoA-producing enzymes for selected C/N-ratio’s during growth of Aurantiochytrium sp. B-072 on a glucose-MSG mineral medium. Samples were taken at the late lipogenic phase. Potential NADPH-flux (qpot, mmol/(g fat-free biomass x h)) through these enzymes assuming they operate at Vmax is also shown as is calculated flux.
| Enzyme | C/N-ratio | |||
|---|---|---|---|---|
| 37 | 56 | 84 | ||
| ATP:citrate lyase (ACL) | N.A. | 182 ± 7 | N.A. | |
| Carnitine acetyltransferase (CAT) | N.A. | 503 ± 9 | N.A. | |
| Glucose-6-P-DH | 63 ± 2 a | 94 ± 2 c | 80 ± 4 b | |
| 6-P-Gluconate DH | 214 ± 3 a | 233 ± 4 b | 233 ± 5 b | |
| Isocitrate-DH (ISDH) | ||||
| NADP+ | 84 ± 3 b | 132 ± 5 c | 45 ± 3 a | |
| NAD+ | 28 ± 2 a | 25 ± 3 a | 31 ± 3 a | |
| Malic enzyme (ME) | 246 ± 7 a | 320 ± 8 b | 330 ± 9 b | |
| qpotAcCoA from ACL | − | 2.7 | − | |
| qpotAcCoA from CAT | − | 7.5 | − | |
| qAcCoA calculated * | – | 3.4 | – | |
| qpotNADPH from HMP ** | 1.9 | 2.8 | 2.4 | |
| qpotNADPH from ISDH | 1.3 | 2.0 | 0.7 | |
| qpotNADPH from ME | 3.7 | 4.8 | 4.9 | |
| Sum | 6.9 | 9.6 | 8.0 | |
| qNADPH calculated * | 3.6 | 5.5 | 2.2 | |
N.A.: not assayed, DH: dehydrogenase. A different letter indicates a significant difference (p<0.05).
Data calculated assuming model I (also applicable for III and IV)
Maximal flux through G-6-P-DH multiplied with two to account for 6-P-G-DH.
A useful check for the feasibility of the various models is to calculate the in vivo P/O-ratio, i.e. the efficiency with which ATP is formed by oxidation of NAD(P)H. The calculated P/O varied considerably depending on the model, with a lowest value of 0.9 for model IV, 1.6 for models I and III and 2.2 for model II (Table 4). The low value for model IV can be attributed to the bypassing of ATP citrate lyase which, as its name implies, consumes one ATP per citrate formed. On the other hand, a value of 2.2 significantly exceeds that of estimated in vivo P/O-ratio’s in other microbes which range from ca. 1.0 to 1.8 (27, 28), suggesting that this model is not feasible. Non-growth associated maintenance is not yet included in these calculations which hence result in an underestimation of the in vivo P/O. Unfortunately no maintenance data appear to be available for thraustochytrids but using average literature data (0.023 glucose/gCDW/h or 0.128 mmol glucose/(g biomass x h) (27) for P. chrysogenum as an example), the calculated P/O-ratio would increase by ca 30%, resulting in values of 1.4 to 3.0 (Table 4). Except for model IV, these values are very high with respect to the literature, leaving open the possibility that in vivo a combination of metabolic networks is involved, for instance I plus IV. This would result in an intermediate P/O-ratio. In addition, the HMP might contribute to NADPH-supply which would reduce dependence on the cytosolic transhydrogenase cycle.
Table 4.
Calculated in vivo P/O-ratio’s for the main lipogenic phase in Aurantiochytrium sp. B-072 at a C/N-ratio of 56 for various stoichiometric models (see Fig 4).
| Model | ||||
|---|---|---|---|---|
| me | I | II | III | IV |
| 0 | 1.6 | 2.2 | 1.6 | 0.9 |
| 0.128 * | 2.2 | 3.0 | 2.2 | 1.4 |
Using maintenance (me) data in mmol glucose/(g DCW x h) for Penicillium chrysogenum (van Gulik et al. 2001)
Comparison with other DHA-producing microbes
A brief literature survey was performed in order to compare results for B-072 with other DHA-producing microbes. Autothrophs which have low specific substrate/product fluxes were excluded from this listing. The C/N-ratio’s used in previous experiment were either significantly lower or higher than the optimal value in the present study, though this may be partly due to strain differences. Furthermore, conditions have not necessarily been optimized. In terms of DHA-specific flux, values of 0.03 to 0.06 mmol DHA/(gFFB x h) were calculated for various aurantiochytrids, but C. cohnii performed poorly with a value of only 0.007, more than 10-fold lower than Aurantiochytrium sp. B-072 (Table 6). During the lipogenic phase, the yield of TFA on glucose in C. cohnii was only 0.07 g TFA/g glucose consumed, whereas at C/N-ratio of 56, it reached a value of 0.28 (calculated from Fig. 1a) for B-072. Possibly the low TFA yield in C. cohnii is caused by, among others, a loss of carbon via formation of extracellular polysaccharides as well as a high maintenance due to the high motility of this dinoflagellate, resulting in a high respiration. Hence less glucose would be available for lipid formation.
Table 6.
Comparison of media and data related to FA-formation in oleaginous, DHA-producing microorganisms under heterotrophic growth conditions.
| Organism | Medium (g/L) | C/N-ratio | Biomass (g/L) | TFA (%w/w) | TFA-flux | DHA-flux | Reference |
|---|---|---|---|---|---|---|---|
| (mmol/g FFB x h) | |||||||
| Crypthecodinium cohnii ATCC 30772 | G 84, YE 11.5 | 29 | 28 | 13 | 0.021 | 0.007 | (7) |
| Aurantiochytrium sp. G13/2S | G 40, MSG 2 | 111 | 15 | 35 | * | 0.030 | (10) |
| A. mangrovei Sk-02 | G 75, YE 10 | 30 | 23 | 53 | * | 0.061 | (25) |
| Aurantiochytrium sp. B-072 | G 90, MSG 9.4 | 56 | 31 | 69 | 0.402 | 0.071 | (This study) |
G=glucose, MSG=monosodium glutamate, YE=yeast extract.
insufficient data available All experiments were performed at 25–28 ˚C
Implications for optimization of DHA production by Aurantiochytrium sp.
A high C/N-ratio results in a prolonged period of N-starvation which presumably affects the physiology of the cells and results in a decrease in the lipid formation rate as also evidenced for B-072 at C/N-ratio >64 (cf Fig. 1b). In some oleaginous microbes this problem occurs irrespective of the C/N-ratio, assuming that sufficient glucose is available for lipogenesis. For instance, in fungi such as Mucor circinelloides a continuous decrease in the lipid formation rate is observed starting from the moment N-limitation is reached. Eventually this results in a cessation of glucose uptake and lipid formation at quite low TFA contents, i.e. 12% w/w for M. circinelloides. This is thought to be due to a rapid decrease in the specific activity of particularly malic enzyme, thereby limiting NADPH supply for lipid formation. Indeed, overexpression of ME (from ca 30 to 70 mU/mg protein at the start of lipogenesis) in recombinant strains of this fungus increased lipid content to 30% w/w (33). However, when malic- and other NADPH-producing enzymes were assayed in the late lipogenic phase in B-072 at several C/N-ratio’s (i.e. 37, 56 and 84), the only major difference at the high C/N was a large decrease in NADP+-isocitrate dehydrogenase(s) (Table 5). Furthermore it is noteworthy that activity of ME in B-072 (246–330 mU/mg protein, Table 5) exceeds that reported for various oleaginous microbes by a factor of three to five (for data see (4) and references therein).
The high conversion factor of glucose into TFA coupled with the high apparent in vivo P/O-ratio’s irrespective of the model used (Table 4) suggest that the overall yield of lipid in B-072 is close to its theoretical maximum. Hence medium development will of limited use. However, ammonium might be a cheaper alternative to glutamate. In theory, provision of cytosolic acetyl-CoA by removal of ACL and re-routing through CAT (as in model IV) should give a small increase in lipid yield due to the more favorable energetics (Table 3). The main aim, however, would be a relative increase in the %DHA/TFA. The latter is not greatly influenced by the C/N-ratio (Table 1). From literature data, it appears that higher DHA-fractions in the TFA can be achieved by lowering the process temperature (24) and/or oxygen limitation (5). Unfortunately these factors will also reduce the specific growth and lipid synthetic rates. Overexpression of the PKS system by random mutation would be useful in this and might prevent GMO issues.
It would be of great interest to attempt further analysis of metabolic pathways in Aurantiochytrium, for instance by 13C-NMR (9). The high volumetric and specific fatty acid fluxes attained in Aurantiochytrium sp. B-072 as compared to oleaginous yeasts and fungi makes it an interesting subject as a better understanding of its physiology might help to improve lipid formation in other microbes.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0241/2547) under supervision of CV and was partially supported by RA scholarship from the Faculty of Graduate Studies, Mahidol University Academic Year 2009. We would like to thank dr. Thunyarat Pongtharangkul for helpful discussions.
REFERENCE
- 1.Adler-Nissen J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food. Chem. 1979;27:1256–1262. doi: 10.1021/jf60226a042. [DOI] [PubMed] [Google Scholar]
- 2.Ashford A., Barclay W.R., Weaver C.A., Giddings T.H., Zeller S. Electron microscopy may reveal structure of docosahexaenoic acid-rich oil within Schizochytrium sp. Lipid. 2000;35:1377–1387. doi: 10.1007/s11745-000-0655-2. [DOI] [PubMed] [Google Scholar]
- 3.Bruinenberg P.M., van Dijken J.P., Scheffers W.A. An enzymic analysis of NADPH production and consumption in Candida utilis. J. Gen. Microbiol. 1983;129:965–971. doi: 10.1099/00221287-129-4-965. [DOI] [PubMed] [Google Scholar]
- 4.Certik M., Megova J., Horenitzky R. Effect of nitrogen sources on the activities of lipogenic enzymes in oleaginous fungus Cunninghamella echinulata. J. Gen. Appl. Microbiol. 1999;45:289–293. doi: 10.2323/jgam.45.289. [DOI] [PubMed] [Google Scholar]
- 5.Chi Z., Liu Y., Frear C., Chen S. Study of a two-stage growth of DHA-producing marine algae Schizochytrium limacinum SR21 with shifting dissolved oxygen level. Appl. Microbiol. Biotechnol. 2009;81:1141–1148. doi: 10.1007/s00253-008-1740-7. [DOI] [PubMed] [Google Scholar]
- 6.Chodchoey K, Verduyn C. Growth, fatty acid profile in major lipid classes and lipid fluidity of Aurantiochytrium mangrovei Sk02 as a function of growth temperature. Braz. J. Microbiol. 2011 doi: 10.1590/S1517-838220120001000020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Swaaf M.E., Rijk de T.C., Eggink G., Sijtsma L. Optimisation of docosahexaenoic acid production in batch cultivations of Crypthecodinium cohnii. J. Biotechnol. 1999;70:185–192. [Google Scholar]
- 8.Evans C.T., Scragg A.H., Ratledge C. A comparative study of citrate efflux from mitochondria of oleaginous and non-oleaginous yeasts. Eur. J. Biochem. 1983;130:195–204. doi: 10.1111/j.1432-1033.1983.tb07136.x. [DOI] [PubMed] [Google Scholar]
- 9.Fiaux J., Çakar Z.P., Sonderegger M., Wüthrich K.W., Szyperski T., Sauer U. Metabolic-flux profiling of the yeasts Saccharomyces cerevisiae and Pichia stipitis. Eukaryot. Cell. 2003;2:170–180. doi: 10.1128/EC.2.1.170-180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganuza E., Izquierdo M.S. Lipid accumulation in Schizochytrium G13/2S produced in continuous culture. Appl. Microbiol. Biotechnol. 2007;76:985–990. doi: 10.1007/s00253-007-1019-4. [DOI] [PubMed] [Google Scholar]
- 11.Garelli A., Rotstein N.P., Politi L.E. Docosahexaenoic acid promotes photoreceptor differentiation without altering crx expression. Invest. Ophthalmol. Vis. Sci. 2006;47:3017–3027. doi: 10.1167/iovs.05-1659. [DOI] [PubMed] [Google Scholar]
- 12.Grzebyk D., Sako Y., Berland B. Phylogenetic analysis of nine species of Prorocentrum (Dinophyceae) inferred from 18S ribosomal DNA sequences, morphological comparisons, and description of Prorocentrum panamensis, sp. nov.. J. Phycol. 1998;34:1055–1068. [Google Scholar]
- 13.Hamid A.A., Mokhtar N.F., Taha E.M., Omar O., Yusoff W.M.W. The role of ATP citrate lyase, malic enzyme and fatty acid synthase in the regulation of lipid accumulation in Cunninghamella sp. 2A1. Ann. Microbiol. 2010;61:463–468. [Google Scholar]
- 14.Hanning I., Baumgarten K., Schott K., Heldt H.W. Oxaloacetate transport into plant mitochondria. Plant. Physiol. 1999;119:1025–1031. doi: 10.1104/pp.119.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris D.M., Diedrich J.A., van der Krogt Z.A., Luttik M.A., Raamsdonk L.M., Bovenberg R.A., van Gulik W.M., van Dijken J.P., Pronk J.T. Enzymic analysis of NADPH metabolism in β-lactam-producing Penicillium chrysogenum: presence of a mitochondrial NADPH dehydrogenase. Metabolic. Eng. 2006;8:91–101. doi: 10.1016/j.ymben.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Henriksen C.M., Christensen L.H., Nielsen J., Villadsen J. Growth energetics and metabolic fluxes in continuous cultures of Penicillium chrysogenum. J. Biotechnol. 1996;45:149–164. doi: 10.1016/0168-1656(95)00164-6. [DOI] [PubMed] [Google Scholar]
- 17.Holdsworth J.E., Veenhuis M., Ratledge C. Enzyme activities in oleaginous yeast accumulating and utilizing exogenous or endogenous lipids. J. Gen. Microbiol. 1988;134:2907–2915. doi: 10.1099/00221287-134-11-2907. [DOI] [PubMed] [Google Scholar]
- 18.Kohlhaw G., Tan-Wilson A. Carnitine acetyltransferase: candidate for the transfer of acetyl groups through the mitochondrial membrane of yeast. J. Bacteriol. 1977;129:1159–1161. doi: 10.1128/jb.129.2.1159-1161.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postma E., Verduyn C., Scheffers W.A., van Dijken J.P. Enzymatic analysis of the crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1989;53:468–477. doi: 10.1128/aem.55.2.468-477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qui X. Biosynthesis of docosahexaenoic acid (DHA, 22:6–4,7,10,13,16,19): two distinct pathways. Prostaglandins. Leukot. Essent. Fatty Acids. 2003;68:181–186. doi: 10.1016/s0952-3278(02)00268-5. [DOI] [PubMed] [Google Scholar]
- 21.SanGiovanni J.P., Chew E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Srere P.A. Citrate cleavage enzyme. Methods Enzymol. 1962;5:641–644. [Google Scholar]
- 23.Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 24.Taoka Y., Nagano N., Okita Y., Izumida H., Sugimoto S., Hayashi M. Influences of culture temperature on the growth, lipid content and fatty acid composition of Aurantiochytrium sp. strain mh0186. Mar. Biotechnol. 2009;11:368–374. doi: 10.1007/s10126-008-9151-4. [DOI] [PubMed] [Google Scholar]
- 25.Unagul P., Assantachai C., Phadungruenluij S., Pongsuteeragul T., Suphantharika M., Verduyn C. Properties of the docosahexaenoic acid-producer Schizochytrium mangrovei SK2: effects of glucose, temperature and salinity and their interaction. Bot. Mar. 2005;48:387–394. [Google Scholar]
- 26.van Gulik W.M., de Laat W.T.A.M., Vinke J.L., Heijnen J.J. Application of metabolic flux analysis for the identification of metabolic bottlenecks in the biosynthesis of penicillin-G. Biotechnol. Bioeng. 2000;68:602–618. doi: 10.1002/(sici)1097-0290(20000620)68:6<602::aid-bit3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.van Gulik W.M., Antoniewicz M.R., de Laat W.T.A.M., Vinke J.L., Heijnen J.J. Energetics of growth and penicillin production in a high-producing strain of Penicillium chrysogenum. Biotechnol. Bioeng. 2001;72:185–193. doi: 10.1002/1097-0290(20000120)72:2<185::aid-bit7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Verduyn C., Stouthamer A.H., Scheffers W.A., van Dijken J.P. A theoretical evaluation of growth yields of yeasts. Antonie van Leeuwenhoek. 1991;59:49–63. doi: 10.1007/BF00582119. [DOI] [PubMed] [Google Scholar]
- 29.Ward O.P., Singh A. Omega-3/6 fatty acids: alternative sources of production. Proc. Biochem. 2005;40:3627–3652. [Google Scholar]
- 30.Wynn J.P., Hamid A.A., Midgley M., Ratledge C. Widespread occurrence of ATP:citrate lyase and carnitine acetyltransferase in filamentous fungi. World J. Microbiol. Biotechnol. 1998;14:145–147. [Google Scholar]
- 31.Wynn J.P., Hamid A.A., Ratledge C. The role of malic enzyme in the regulation of lipid accumulation in filamentous fungi. Microbiol. 1999;145:1911–1917. doi: 10.1099/13500872-145-8-1911. [DOI] [PubMed] [Google Scholar]
- 32.Yang H.-L., Lu C.-K., Chen S.-F., Chen Y.-M., Chen Y.-M. Isolation and characterization of Taiwanese heterotrophic microalgae: screening of strains for docosahexaenoic acid (DHA) production. Mar. Biotechnol. 2010;12:173–185. doi: 10.1007/s10126-009-9207-0. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Adam I.P., Ratledge C. Malic enzyme: the controlling activity for lipid production? Overexpression of malic enzyme in Mucor circinelloides leads to a 2.5-fold increase in lipid accumulation. Microbiol. 2007;153:2013–2025. doi: 10.1099/mic.0.2006/002683-0. [DOI] [PubMed] [Google Scholar]


