Abstract
Streptomyces sp. CH7 was found to efficiently produce glucose(xylose) isomerase when grown on either xylan or agricultural residues. This strain produced a glucose(xylose) isomerase activity of roughly 1.8 U/mg of protein when it was grown in medium containing 1% xylose as a carbon source. Maximal enzymatic activities of about 5 and 3 U/mg were obtained when 1% xylan and 2.5% corn husks were used, respectively. The enzyme was purified from a mycelial extract to 16-fold purity with only two consecutive column chromatography steps using Macro-prep DEAE and Sephacryl-300, respectively. The approximate molecular weight of the purified enzyme is 170 kDa, and it has four identical subunits of 43.6 kDa as estimated by SDS-PAGE. Its Km values for glucose and xylose were found to be 258.96 and 82.77 mM, respectively, and its Vmax values are 32.42 and 63.64 μM/min/mg, respectively. The purified enzyme is optimally active at 85°C and pH 7.0. It is stable at pH 5.5–8.5 and at temperatures up to 60°C after 30 min. These findings indicate that glucose(xylose) isomerase from Streptomyces sp. CH7 has the potential for industrial applications, especially for high-fructose syrup production and bioethanol fermentation from hemicellulosic hydrolysates by Saccharomyces cerevisiae.
Keywords: agricultural residues, glucose(xylose) isomerase, production, purification, Streptomyces
INTRODUCTION
Glucose(xylose) isomerase catalyzes the reversible isomerization of glucose to fructose and that of xylose to xylulose. It is an important enzyme used in the industrial production of high-fructose corn syrup (HFCS) (3). Apart from the food industry, this enzyme has recently gained more interest due to its potential applications in the biofuel industry. Currently, ethanol is the major form of biofuel, and numerous technologies have been employed to improve its production (1, 19, 26). Furthermore, fuel ethanol production from hemicellulosic hydrolysates by Saccharomyces cerevisiae is of great economic interest as an alternative to fossil fuel (18). Whereas wild-type S. cerevisiae can ferment xylulose to ethanol via the pentose-phosphate pathway (28), it cannot ferment xylose. Xylose is a major monosaccharide in plant hemicellulosic hydrolysates that can account for up to 30% of total sugars in some plant biomasses such as that of hardwoods and agricultural residues (8). Therefore, glucose(xylose) isomerase indirectly plays an important role in the ethanol fermentation of plant biomass hydrolysates by S. cerevisiae.
Many microorganisms have been reported to produce glucose isomerase, and most of them require xylose as an inducer for enzyme synthesis (2, 6, 9). Hemicelluloses are heterogeneous plant polymers consisting of pentoses, hexoses and sugar acids in which xylan, a backbone of 1,4-linked xylopyranosyl residues, is found as the major polymeric compound (28). As hemicelluloses are abundantly available in nature, they are good renewable resources for biofuel production, and they are cheap substrates for the production of glucose isomerase by microorganisms capable of growing on xylan-containing materials. We previously reported that Streptomyces sp. CH7 is capable of growing on xylan and that it efficiently produces β-xylosidase (20, 27). Furthermore, we also showed that β-xylosidase from this strain acts cooperatively with other xylanolytic enzymes from Streptomyces sp. PC22 to efficiently hydrolyze agricultural residues simply prepared as milled particles with no requirement for prior pretreatment with either chemicals or steam explosion (21). Here we report the ability of Streptomyces sp. CH7 to produce glucose isomerase when grown on agricultural residues. The ability of Streptomyces sp. CH7 to use low cost substrates for glucose isomerase production will reduce enzyme production costs and subsequently make both HFCS and ethanol production from hemicellulosic hydrolysates more cost effective and sustainable. The purification and characterization of this enzyme are also presented.
MATERIALS AND METHODS
Microorganism
Streptomyces sp. CH7 (GenBank accession number DQ385868) was used. It was maintained as a spore suspension at -20°C and prepared according to Kieser et al. (11).
Growth conditions and enzyme preparation
A seed culture was prepared by inoculating 100 μl of spore suspension (∼ 108 spores ml-1) into 30 ml of Tryptic Soy Broth (TSB) adjusted to pH 7.0 and incubated for 24 h with shaking at 200 rpm and 40°C. Ten percent (v/v) of the seed culture was inoculated into production medium described by Raweesri et al. (21) consisting of (w/v) 0.5% polypeptone, 0.1% yeast extract, 0.4% K2HPO4, 0.02% KCl, 0.1% MgSO4.7H2O, and 0.002% FeSO4.7H2O but using xylose, oat spelt-xylan or agricultural residues (milled and sieved to approximately 200 μm-particle size) at the concentrations indicated in the results as a carbon source. The pH of the medium was adjusted to 7.0. After cultivation, mycelia were removed from the culture by centrifugation at 5000 rpm for 20 min and washed twice with 50 mM sodium phosphate buffer, pH 7.0. The culture supernatant was used to determine xylanase activity. Mycelial extract was prepared by permeabilizing the mycelia with detergent as described by D’Cunha (5) with some modifications. Briefly, mycelia were suspended in 0.1% cetyl trimethyl ammonium bromide (CTAB) at a ratio of 1 ml packed cells to 3 ml of the CTAB solution, and permeabilization was carried out for 24 h at 40°C. After removing cell debris by centrifugation at 10,000 ×g for 20 min, the supernatant was used for the determination of D-glucose/D-xylose isomerase, β-xylosidase activities and protein content.
Protein content
Protein content was determined by the Lowry method (17) using bovine serum albumin as a standard.
Enzyme assays
D-Glucose isomerase was assayed in a reaction mixture that contained 0.5 M D-glucose, 150 mM sodium phosphate buffer, pH 7.0, 5 mM MgSO4, 0.1 mM CoCl2 and enzyme solution in a final volume of 2 ml. After incubation at 85°C for 20 min, the amount of fructose formed was determined by the cysteine-carbazole method (7). One unit (U) of enzyme activity was equal to the formation of 1 μmol of fructose per min under the assay conditions employed. D-Xylose isomerase was assayed under the same conditions as those for D-glucose isomerase except that D-glucose was replaced by D-xylose and the product formed, D-xylulose, was determined by the same colorimetric assay. Xylanase activity was determined by measuring the release of reducing sugars from xylan as previously described (29). β-Xylosidase was determined by measuring the amount of p-nitrophenol released from p-nitrophenyl-β-D-xylopyranoside as previously described (20).
Purification of glucose isomerase
The mycelial extract referred to as the crude enzyme from Streptomyces sp. CH7 was used for glucose isomerase purification. The crude enzyme was loaded onto a Macro-Prep DEAE ( Bio-Rad Laboratories ) column (1.5×30 cm) and the bound protein was eluted with a linear gradient of NaCl (0- 0.8 M) in 0.05 M Tris-HCl buffer (pH 7.5) at a flow rate of 30 ml h-1. The active fractions were pooled, concentrated by ultrafiltration using a 10 kDa-molecular weight cut-off membrane filter, - dialyzed against 50 mM sodium phosphate buffer (pH 7.0) containing 5 mM MgSO4.7H2O and 0.1 mM CoCl2.6 H2O 2 times and finally against the same buffer containing 30% (v/v) glycerol. The concentrated solution was loaded onto a Sephacryl S-300 column (1×30 cm) previously equilibrated with 50 mM sodium phosphate buffer (pH 7.0) containing 100 mM NaCl, 5 mM MgSO4.7H2O and 0.1 mM CoCl2.6 H2O and eluted with the same buffer at a flow rate of 15 ml h-1. The active fractions were pooled, concentrated, and glycerol was added to a final concentration of 20% (v/v). The purified enzyme was kept at -20°C for prolonged storage.
Influence of temperature, pH, metal ions and enzyme kinetics
Glucose isomerase activity was determined under standard conditions except that different temperatures were assayed within the range 35–90°C. The enzyme activity was then determined at various pH values ranging from 4.0 to 9.0 at the predetermined optimal temperature. The thermostability of the enzyme was evaluated by measuring the residual activity after a 30 min pre-incubation of the enzyme in the absence of substrate at various temperatures between 35 and 90 °C, and pH stability was studied by pre-incubating the enzyme in the presence of substrate at pHs ranging from 4.0 to 9.0 at 85 °C for 30 min and assaying the remaining enzymatic activity under standard conditions.
For the influence of metal ions, the enzyme was pretreated with EDTA by dialyzing overnight against 100 mM EDTA in 50 mM sodium phosphate buffer (pH 7.0), and after that it was dialyzed twice against the same buffer without EDTA. The effect of metal ions was assessed by pre-incubating the enzyme with the test compound at 0 °C for 30 min and then assaying the residual enzymatic activity under standard conditions. The Michaelis-Menten kinetic parameters Km and Vmax were determined from Lineweaver-Burk plots using glucose and xylose at concentrations ranging from 100 to 800 mM and 20 to 160 mM, respectively, as substrate.
Estimation of molecular mass
The apparent molecular mass of the purified enzyme in native form was determined by gel filtration on Sephacryl S-300 using ferritin (450 kDa), catalase (250 kDa) and globulin (150 kDa) as molecular mass standards. Subunit molecular masses were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 12% (w/v) acrylamide gel by the method of Laemmli (13).
RESULTS AND DISCUSSION
Effects of xylose and glucose on glucose isomerase production
The present work demonstrated that Streptomyces sp. CH7 is capable of producing glucose isomerase when grown on either xylose or glucose (Fig. 1). Enzyme activity was roughly five-fold higher in the presence of xylose, indicating that the synthesis of this enzyme is constitutive in the presence of glucose and inducible by xylose. The constitutive biosynthesis of this enzyme by CH7 is similar to that of Arthrobacter nicotianae reported recently by Sapunova et al. (25). However, the glucose isomerase producing ability of CH7 is different from that of either Streptomyces sp. EC10 (2) (in which no enzyme activity is detected when grown on glucose) or Arthrobacter ureafaciens (24) (in which glucose inhibits enzyme synthesis when grown on xylose). A glucose isomerase activity level of about 1.8 U/mg of protein was obtained from CH7 when grown on 1% xylose, which is quite high compared to Arthrobacter nicotianae (0.5 U/mg), E. coli (0.5 U/mg) and Erwinia carotovora subsp. Atroseptica (0.8 U/mg) (23) grown on the same concentration of xylose. At 0.5% xylose, CH7 produced roughly 0.8 U/mg of enzyme, which is still higher than Bacillus thermoantarcticus (0.18 U/mg) (14) and Arthrobacter ureafaciens (0.06 U/mg) (24) grown on the same concentration of xylose. Therefore, Streptomyces sp. CH7 is a strong glucose isomerase producer and an attractive candidate for industrial applications.
Figure 1.
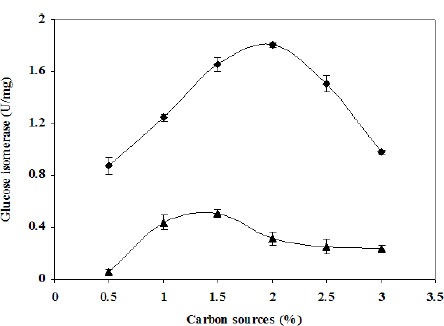
Effects of glucose (▲) and xylose (◆) on glucose isomerase production by Streptomyces sp. CH7 cultivated at 40°C with shaking at 200 rpm for 24 hours.
Effects of xylan and agricultural residues on glucose isomerase production
Because CH7 can grow on xylan and produce β-xylosidase (20), we investigated its ability to produce glucose isomerase when grown on xylan. As shown in Figure 2, CH7 efficiently produced the enzyme in the presence of xylan. This capability will lead to the discontinuation of xylose as an inducer. The maximum glucose isomerase production (of about 5 U/mg) was obtained at 1% xylan when grown for 3 days. The enzymatic activity was about 3-fold higher when xylan was used as the carbon source as compared with the use of xylose as a carbon source. This finding is in agreement with those of Belfaquih and Penninckh (2) who studied Streptomyces sp. EC10 and found that higher levels of xylose isomerase were produced using xylan, from either birchwood or oat-spelt, as the carbon source than was produced using xylose as the carbon source. Streptomyces sp. EC10 provided roughly 1.3- and 1.7-fold higher enzyme levels with xylan from oat-spelt and birchwood, respectively.
Figure 2.
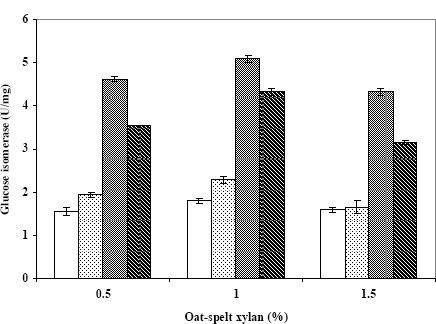
Effect of oat-spelt xylan on glucose isomerase production by Streptomyces sp. CH7 grown at 40°C with shaking at 200 rpm for 1 day (□), 2 days (▧), 3 days (▨) and 4 days (▩).
We further investigated the ability of CH7 to use different hemicellulosic agricultural residues as a carbon source for the simultaneous production of xylanolytic enzymes including xylanase, β-xylosidase and glucose isomerase. The results shown in Table 1 indicate that corn husks are the best carbon source for enzyme production, and the optimal concentration of corn husks is shown in Figure 3. This finding shows that cheap and abundantly available agricultural residues can replace the more expensive xylose and xylan for glucose isomerase production by CH7.
Table 1.
Effect of different hemicellulosic agricultural residues (1%, w/v) on the production of xylanase, β-xylosidase and glucose isomerase by Streptomyces sp. CH7 grown for 3 days.
| Carbon sources | Glucose Isomerase (U/mg) | Xylanase (U/ml) | β-Xylosidase (U/mg) |
|---|---|---|---|
| corn cob | 1.45 ± 0.02 | 0.13 ± 0.01 | 0.37 ± 0.01 |
| corn husk | 1.85 ± 0.02 | 0.23 ± 0.01 | 0.49 ± 0.02 |
| cotton seed husk | 0.41 ± 0.09 | 0.11 ± 0.03 | 0.37 ± 0.03 |
| rice bran | 0.41 ± 0.02 | 0.06 ± 0.01 | 0.14 ± 0.11 |
| wheat bran | 1.07 ± 0.01 | 0.49 ± 0.08 | 0.33 ± 0.06 |
Figure 3.
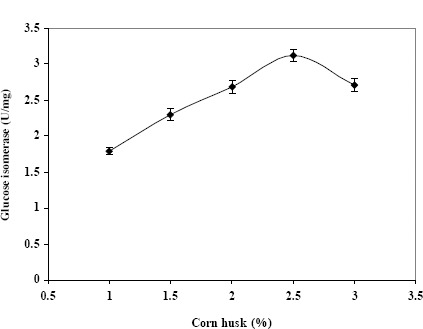
Effect of concentrations of corn husk on glucose isomerase production by Streptomyces sp. CH7 grown for 3 days.
Purification, molecular characteristics and properties of glucose isomerase
Glucose isomerase from Streptomyces sp. CH7 was purified to homogeneity from the mycelial extract by only two column chromatography steps. A summary of the purification procedures is presented in Table 2. The final purification resulted in a considerably high yield (64.5%) with about 16-fold purity. The molecular mass of the enzyme was estimated by gel filtration on Sephacryl-300 to be approximately 170 kDa (data not shown), and it consists of four identical subunits with a molecular mass of 43.6 kDa as estimated by SDS-PAGE (Fig 4). These values are slightly different from those of the glucose isomerases from Streptomyces sp. EC10 (163 kDa with 4 identical subunits of 42 kDa) (2) and from Streptomyces sp. SK strain (180 kDa with four 43-kDa subunits) (4).
Table 2.
Summary of purification of glucose isomerase from Streptomyces sp. CH7.
| Purification step | Total protein (mg) | Total activity (U) | Specific activity (U/mg) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Crude extract | 34.2 | 92.8 | 2.71 | 100.0 | 1.0 |
| Macro-prep DEAE | 2.2 | 71.6 | 32.55 | 77.2 | 12.0 |
| Sephacryl S-300 | 1.4 | 59.9 | 42.78 | 64.5 | 15.9 |
Figure 4.
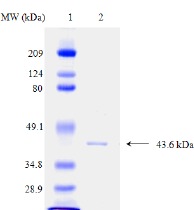
SDS-polyacrylamide gel electrophoresis of purified glucose isomerase. Lane 1, molecular mass standard; myosin (209 kDa), β-galactosidase (124 kDa), bovine serum albumin (80 kDa), ovalbumin (49.1 kDa), carbonic anhydrase (34.8 kDa), soy bean trypsin inhibitor (28.9 kDa); Lane 2, purified glucose isomerase from Streptomyces sp. CH7(1.4 μg).
As shown in Figure 5, the enzyme had maximal activity at 85°C and was still quite active at 90°C. It was found to be stable at temperatures up to 60°C and to retain about 50% of its activity at 75°C, but it completely lost activity at 80°C. The thermostable properties of this enzyme are similar to those of other thermostable microorganisms including Thermoanaero bacterium strain JW/SL-YS 489 (16), Bacillus thermoantarticus (14), E. coli K12 (22) and Thermus thermophilus (30); thus, it is a thermostable enzyme. Its optimal pH is 7.0, and it is stable across a broad pH range (from 5.5 to 8.5) when tested by preincubating the enzyme in the absence of substrate at different pH values at 4°C for 30 min (Fig. 6).
Figure 5.
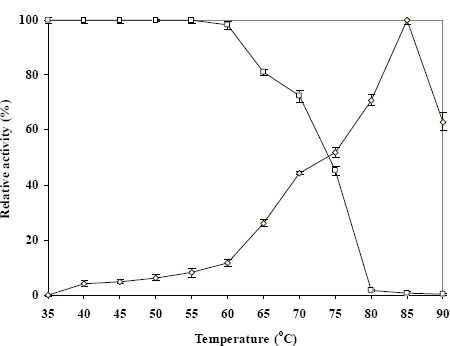
Effects of temperature on activity (⋄) and stability (□) of the enzyme.
Figure 6.
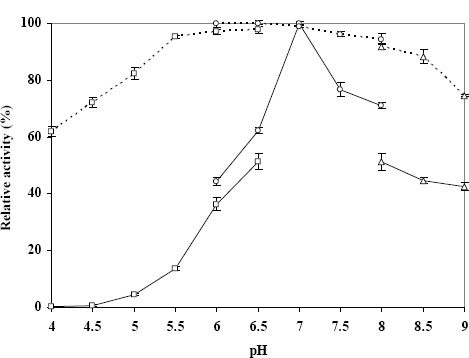
Effects of pH on activity (—) and stability (┄) of the enzyme. (□), 100 mM acetate buffer; (◿), 100 mM phosphate buffer; (△), 100 mM Tris-HCl buffer.
Regarding substrate specificity, under standard assay conditions the Michealis-Menten constant (Km) values of the enzyme for glucose and xylose were found to be 258.96 and 82.77 mM, respectively, and the Vmax values were 32.42 and 63.64 μM/min/mg, respectively. The lower Km values for xylose, as compared to glucose, indicates that this glucose isomerase has a preference for xylose as a substrate over glucose, which is in agreement with glucose isomerases from other microorganisms. However, its Km values for both substrates were found to be lower than those of Bacillus sp. Tx-3 (Km values of 290 and 100 mM for glucose and xylose, respectively) whereas its Vmax values are higher (Vmax values of 1.6 and 28.6 μM/min/mg for glucose and xylose, respectively) (12). Its Km value for glucose is lower than that of Streptomyces sp. PLC (400 mM), but for xylose it is slightly higher (35 mM) (10). Due to its preference for xylose and its lower Km value for glucose when compared to those of the other strains mentioned above, glucose isomerase from CH7 has the potential for industrial applications such as high-fructose syrup production and xylose isomerization for substrate preparation for ethanol fermentation by S. cerevisiae. It was also found that this enzyme has no activity against β-D-xylopyranoside, which distinguishes it from glucose isomerase from Streptomyces sp. EC10 (2) and indicates that it is not a bifunctional β-xylosidase/ xylose isomerase protein.
Effect of metal ions
There have been reports that glucose isomerases typically require divalent metal ions such as Mg2+, Co2+ or Mn2+ as cofactors (14, 15). Glucose isomerase from Streptomyces sp. CH7 was found to be similar to the enzyme from Bacillus thermoantarcticus (14), for which the combination of Mg2+ and Co2+ is essential for enzymatic activity. However, the enzyme from Streptomyces sp. CH7 reached maximum activity at 10 mM Mg2+ and 0.1 mM Co2+ (Table 3), whereas that of B. thermoantarcticus requires a much higher Co2+ concentration (1 mM) but the same Mg2+ concentration. Because Co2+ is a heavy and toxic metal ion, glucose isomerase from CH7 has superior properties because it needs only a trace amount of Co2+ for its optimal activity.
Table 3.
Effect of metal ions on activity of EDTA-treated glucose isomerase from Streptomyces sp CH7.
| Metal | Relative activity (fold) |
|---|---|
| No metal added | 1.0 |
| Mg2+ (1.0 mM) | 3.6 |
| Mn2+ (1.0 mM) | 2.8 |
| Ca2+ (1.0 mM) | 2.5 |
| Fe2+ (1.0 mM) | 0.1 |
| Co2+ (1.0 mM) | 2.1 |
| Co2+ (0.1 mM) | 4.4 |
| Mg2+ (1.0 mM) + Co2+ (0.1 mM) | 6.2 |
| Mg2+ (5.0 mM) + Co2+ (0.1 mM) | 26.5 |
| Mg2+ (10.0 mM) + Co2+ (0.1 mM) | 44.2 |
| Mg2+ (15.0 mM) + Co2+ (0.1 mM) | 23.3 |
CONCLUSION
Streptomyces sp. CH7 was found to be capable of producing glucose(xylose) isomerase efficiently when grown in medium containing corn husks, simply prepared as milled particles, as a carbon source. This cheap and abundantly available carbon source will result in low enzyme production costs. The enzyme was found to have optimal activity at 85°C and pH 7.0, and it is stable across a broad pH range (5.5–8.5). Therefore, it has the potential for industrial applications, especially for high-fructose syrup production and for substrate preparation for bioethanol fermentation from xylose (a major component in hemicellulosic hydrolysates) by Saccharomyces cerevisiae.
ACKNOWLEDGEMENTS
This work was supported in part by CU.GRADUATE SCHOOL THESIS GRANT, Chulalongkorn University, Thailand.
REFERENCES
- 1.Bangrak P., Limtong S., Phisalaphong M. Continuous ethanol production using immobilized yeast cells entrapped in loofa-reinforced alginate carriers. Braz. J. Microbiol. 2011;42(2):676–684. doi: 10.1590/S1517-838220110002000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belfaquih N., Penninckx M.J. A bifunctional β-xylosidase-xylose isomerase from Streptomyces sp. EC10. Enzyme Microl. Technol. 2000;27:114–121. doi: 10.1016/s0141-0229(00)00182-4. [DOI] [PubMed] [Google Scholar]
- 3.Bhosale S.H., Rao M.B., Deshpande V.V. Molecular and industrial aspects of glucose isomerase. Microbiol. Rev. 1996;60:280–300. doi: 10.1128/mr.60.2.280-300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borgi M.A., Srih-Belguith K., Ali M.B., Mezghani M., Tranier S., Haser R., Bejar S. Glucose isomerase of the Streptomyces sp. SK strain : purification, sequence analysis and implication of alanine 103 residue in the enzyme thermostability and acidotolerance. Biochimie. 2004;86:561–568. doi: 10.1016/j.biochi.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 5.D’Cunha G.B. Enrichment of phenylalanine ammonia lyase of Rhodotolura yeast. Enzyme and Microb.Tech. 2005;36:498–502. [Google Scholar]
- 6.Deshmukh S.S., Deshpande M.V., Shankar V. Medium optimization for the production of glucose isomerase from thermophilic Streptomyces thermonitrificans. World. J. Microbiol. Biotechnol. 1994;10:264–267. doi: 10.1007/BF00414859. [DOI] [PubMed] [Google Scholar]
- 7.Dische Z., Borenfreund E. A new spectrophotometric method for the detection of keto sugar trioses. J. Biol. Chem. 1951;192:583–587. [PubMed] [Google Scholar]
- 8.Ericksson K. E. L., Blanchette R. A., Ander P. Microbial and enzymatic degradation of wood and wood components. Berlin, Germany: Ozach GmbH and Co.; 1990. pp. 181–222. [Google Scholar]
- 9.Givry S., Duchiron F. Optimization of culture medium and growth conditions for production of L-arabinose isomerase and D-xylose isomerase by Lactobacillus bifermentans. Microbiology. 2007;77(3):281–287. [PubMed] [Google Scholar]
- 10.Inyang C.U., Gebhart U., Obi S.K.C., Bisswanger H. Isolation and characterization of a D-glucose/xylose isomerase from a new thermophilic strain Streptomyces sp. PLC. Appl. Microbiol. Biotechnol. 1995;43:632–638. [Google Scholar]
- 11.Kieser T., Bibb M.J., Buttner M., Chater K.F., Hopwood D.A. Practical Streptomyces genetics. Norwich, England: The Jonh Innes Foundation, Jonh Innes Centre; 2000. pp. 43–47. [Google Scholar]
- 12.Kitada M., Dobashi Y., Horikoshi K. Enzymatic properties of purified D-xylose isomerase from a thermophilic alkalophile, Bacillus TX-3. Agric. Biol. Chem. 1989;53(6):1461–1468. [Google Scholar]
- 13.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Lama L., Nicolaus B., Calandrelli V., Romano I., Basile R., Gambacorta A. Purification and characterization of thermostable xylose(glucose) isomerase from Bacillus thermoantarcticus. J. Ind. Microbiol. Biotecnol. 2001;27:234–240. doi: 10.1038/sj.jim.7000182. [DOI] [PubMed] [Google Scholar]
- 15.Lehmacher A., Bisswangher H. Isolation and characterization of an extremely thermostable D-xylose isomerase from Thermus aquaticus HB8. J. Gen. Microbiol. 1990;136:679–686. [Google Scholar]
- 16.Liu S.Y., Wiegel J., Gherardine F.C. Purification and cloning of a thermostable xylose(glucose) isomerase with an acidic pH optimum from Thermoanaerobacterium sp. JW/SL-YS 489. J. Bacteriol. 1996;178:5938–5945. doi: 10.1128/jb.178.20.5938-5945.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:267–275. [PubMed] [Google Scholar]
- 18.Mabee W.E. Policy options to support biofuel production. Adv. Biochem. Eng. Biotechnol. 2007;108:329–357. doi: 10.1007/10_2007_059. [DOI] [PubMed] [Google Scholar]
- 19.Mohammadi A., Razavi S.H., Mousavi S.M., Rezaei K. A comparative between sugar consumption and ethanol production in wort by immobilized Saccharomyces cerevisiae, Saccharomyces ludwigii and Saccharomyces rouxii on brewer’s spent grain. Braz. J. Microbiol. 2011;42(2):605–615. doi: 10.1590/S1517-838220110002000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinphanichakarn P., Tangsakul T., Thongnumwon T., Talawanich Y., Thamchaipenet A. Purification and characterization of β-xylosidase from Streptomyces sp. CH7 and its gene sequence analysis. World. J. Microbiol. Biotechnol. 2004;20:727–733. [Google Scholar]
- 21.Raweesri P., Riangrungrojana P., Pinphanichakarn P. α-L-Arabinofuranosidase from Streptomyces sp. PC22: purification, characterization and its synergistic action with xylanolytic enzymes in the degradation of xylan and agricultural residues. Biol. Technol. 2008;99:8981–8986. doi: 10.1016/j.biortech.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Rozanov A.S., Zagrebelny S.N., Beklemishev A.B. Cloning of Escherichia coli K12 xylose isomerase(glucose isomerase) gene and studying the enzymatic properties of its expression product. Appl. Biochem. Microbiol. 2009;45(1):38–44. [PubMed] [Google Scholar]
- 23.Sapunova L.I., Lobanok A.G., Kazakevich I.O., Shlyakhotko E.A., Evtushenkov A.N. Biosynthetic features and properties of xylose isomerases from Arthrobacter nicotianae, Escherichia coli, and Erwinia carotovora subsp. Atroseptic. Appl. Microbiol. Biotechnol. 2006;42(3):246–251. [PubMed] [Google Scholar]
- 24.Sapunova L.I., Tamkovich I.O., Lobanok A.G. Catabolite repression of xylose isomerase synthesis in Arthrobacter ureafaciens. Microbiology. 2008;77(3):268–274. [PubMed] [Google Scholar]
- 25.Sapunova L.I., Tamkovich I.O., Lobanok A.G. Some aspects of xylose isomerase constitutive biosynthesis in Arthrobacter nicotianae. Appl. Biochem. Microbiol. 2010;46(4):438–442. [PubMed] [Google Scholar]
- 26.Tao N.G., Gao Y.M., Liu Y.J. Isolation and characterization of a Pichia anomala strain: a promising candidate for bioethanol production. Braz. J. Microbiol. 2011;42(2):668–675. doi: 10.1590/S1517-838220110002000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ungchaithum S., Pinphanichakarn P. Optimization of xylanase production by Streptomyces sp. PC22 growing on agricultural residues. J. Sci. Res. Chula. Univ. 1998;23(1):45–50. [Google Scholar]
- 28.van Maris A.J.A., Abbott D.A., Bellissimi E., van den Brink J., Kuyper M., Luttik M.A.H., Wisselink H.W., Scheffers W.A., van Dijken J.P., Promk J.T. Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: current status. Antonie van Leeuwenhoek. 2006;90:391–418. doi: 10.1007/s10482-006-9085-7. [DOI] [PubMed] [Google Scholar]
- 29.Wateewuthajarn K., Pinphanichakarn P. Purification and characterization of xylanase from Streptomyces sp. PC22. J. Sci. Res. Chula. Univ. 2000;25(2):245–256. [Google Scholar]
- 30.Xu W., Yan M., Xu L., Ding L., Ouyang P. Engineering the activity of thermophillic xylose isomerase by site-directed mutation at subunit interfaces. Enz. Microb. Tech. 2009;44:77–83. [Google Scholar]


