Abstract
To compensate for stress imposed by salinity, biofilm formation and exopolysaccharide production are significant strategies of salt tolerant bacteria to assist metabolism. We hypothesized that two previously isolated salt-tolerant strains Halomonas variabilis (HT1) and Planococcus rifietoensis (RT4) have an ability to improve plant growth, These strains can form biofilm and accumulate exopolysacharides at increasing salt stress. These results showed that bacteria might be involved in developing microbial communities under salt stress and helpful in colonizing of bacterial strains to plant roots and soil particles. Eventually, it can add to the plant growth and soil structure. We investigated the comparative effect of exopolysacharide and biofilm formation in two bacterial strains Halomonas variabilis (HT1) and Planococcus rifietoensis (RT4) in response to varying salt stress. We found that biofilm formation and exopolysaccharide accumulation increased at higher salinity. To check the effect of bacterial inoculation on the plant (Cicer arietinum Var. CM-98) growth and soil aggregation, pot experiment was conducted by growing seedlings under salt stress. Inoculation of both strains increased plant growth at elevated salt stress. Weight of soil aggregates attached with roots and present in soil were added at higher salt concentrations compared to untreated controls. Soil aggregation was higher at plant roots under salinity. These results suggest the feasibility of using above strains in improving plant growth and soil fertility under salinity.
Keywords: Biofilm, Cicer arietinum, exopolysaccharide, salinity, soil aggregates
INTRODUCTION
Worldwide increasing salinity greatly reduces the nodulation, growth and yield of leguminous crops i.e., faba-bean, soybean, chickpea (22, 32, 38). Chickpea, although frequently used over the world due to its higher nutritional value (33), is severely affected by salt stress. Having higher protein content compared to other cereal crops, chick-pea (Cicer arietinum L.) is also an important crop for providing nitrogen to the soil (32). Accumulation of extracellular exopolysaccharide and biofilm formation is commonly observed in bacteria. Significance of root-colonizing bacteria in improving plant growth has been variously reported (1). Biofilm is a complex association of bacterial cells attached to different biotic and abiotic surfaces that can retain moisture and protects plant roots from various pathogens (8). Association on surfaces involves different polymers of sugars called EPS that protects bacteria from stress (40). Exopolysaccharides production by bacteria in saline soil can be helpful against osmotic stress. Biofilms are established on various surfaces like roots and soil particles, respectively resulting in cementing of soil particles. This can improve crop productivity and physiochemical properties of soil (7, 8). Formations of these aggregates have water retaining capacity and sustain physiochemical properties of soil (7). The present research work aims to explore the exopolysaccharide accumulation in bacterial strains and consequences of their inoculation on plant growth stimulation and soil aggregate formation around roots.
MATERIALS AND METHODS
Two previously isolated and characterized (32) salt tolerant bacterial strains were used for the present research work, i.e., HT1 (accession no. DQ381961, Halomonas variabilis) and RT4 (accession no. JF794554 Planococcus rifietoensis). Strains were maintained on LB agar with 0.5 M NaCl added media.
Determination of Biofilm formation of bacterial cells
A qualitative assay for biofilm formation was done following (11). Briefly, bacterial cultures were grown in LB (21) and M9 broth (26) media with varying salt concentrations for 24 hours without agitation. After 24 hours, the liquid medium was removed, and the bacterial biofilm was visualized by staining test tubes with 0.01 % aqueous solution of 10 mL crystal violet for 20 minutes at room temperature. Excess stain was removed, and tubes were washed with sterile distilled water. Tubes were air dried in an inverted position and observed for biofilm formation. Biofilm formation was considered positive when a visible purple ring lined the wall and bottom of the tube.
Biofilm was quantified in terms of planktonic, loosely bound cells and tightly bound cells, at varying salt concentrations (11). Over night grown strains were inoculated (100 µL; OD600 nm of 0.3 A) in 10 ml of Lbroth (21) and M9 medium (26) supplemented with varying concentrations of salt (0, 0.5, 1, 1.5, 2, and 2.5 M). The tubes were incubated at 37˚C for 48 hours without agitation (conditions were optimized, data not shown). Cells were harvested from test tubes and planktonic, loosely bound and tightly bound cells/biofilm was quantified following Liaqat et al. (28).
Quantitative analysis of exopolysaccharide production
For exopolysaccharide determination, 250 mL flasks containing 100 mL of a medium suggested by Verhoef et al., (39) were supplemented with varying NaCl concentrations (0, 0.5, 1, 1.5, 2, 2.5 M). A medium was inoculated (1000 µl) with 24-hours old bacterial culture (OD 600 0.3) and incubated at 160 rpm shaker (orbital incubator Model I-4000 serial number 104 A IRMECO GmbH, Goesthacht /Germany) for 48 h at 37˚C. Bacterial growth was monitored by estimating OD600 nm (Model S-300 DL, R & M marketing, and England). In order to extract exopolysaccharide, we followed the method of De Vuyst et al. (14). Bacterial cultures were centrifuged (Sigma 3K30, Germany) at optimized conditions (10,000 rpm for 15 minutes at 4ºC). Exopolysaccharide fraction from the bacterial supernatant was precipitated (centrifugation 15000 rpm for 20 minutes at 4ºC) using three volumes of pre-chilled acetone (Merck). Weight of freshly precipitated exopolysaccharide was taken. Exopolysaccharide was dried at 58ºC for 24 hours in the same glass centrifuge tubes to minimize exopolysaccharide loss and dry weight (grams) was noted.
Exopolysaccharides were quantified in terms of total carbohydrates and measured by the phenol-sulfuric acid method using glucose as a standard (16). Experiments were performed in triplicates.
Plant inoculation, growth, and harvesting and biochemical analysis
Certified seeds of (Cicer arietinum Var. CM-98), obtained from Punjab seed corporation, Lahore, Pakistan. Seeds were surface sterilized with 0.1 % HgCl2 solution for 10 minutes, rinsed with sterile water two to three times and then inoculated with bacterial strains. Cells from bacterial cultures (24-hour old) were harvested by centrifugation. Cells were suspended in sterile distilled water to get bacterial suspension (OD 600 adjusted 108 mL-1 cfus). Sterilised seeds were inoculated for 30 minutes prior to sowing. For control, seeds were soaked in sterile water of same volume for the same period of time. Seeds were sown in plastic pots containing 120 g pot-1 sieved, autoclaved and air dried garden soil. Seeds were sown in soil filled pots added with four different concentrations of NaCl (0, 50, 100 and 200 mM) per gram weight of soil. Pots were placed in the dark at 28 ± 30˚C for three days. After three days of seed germination, pots were transferred to light intensity of 10 Klux, photoperiod of 16-hour light/dark and temperature 37˚C ± 1 for 15 days. After 15 days, the seedlings were harvested and different growth parameters i.e., seedling length (cm), fresh weight (mg per seedling) and dry weight (mg per seedling) was measured. Seedling length was measured of harvested plant. Harvested plants were weighed to get fresh weight (mg per seedling) Dry weight (mg per seedling) was noted after drying the harvested plant at 60˚C for 24 hours.
Total soluble protein (µg/g fresh weight) of plants was determined following Afrasayab et al., (1). Total soluble sugar per gram of dry weight of plant was measured by the phenol-sulphuric acid method (16) using glucose as a standard. In all experiments, reported values are the mean of three replicates. The difference between the means was tested using the least significant difference test (p<0.05).
RESULTS
Effect of varying salt concentrations on Biofilm formation
We have described the effect of varying salt concentrations in two different media on biofilm formation of two bacterial strains Halomonas variabilis (HT1) and Planococcus rifietoensis (RT4). Biofilm formation formed by both strains Halomonas variabilis (HT1) and Planococcus rifietoensis (RT4), was visualized as purple ring after staining with 0.01 % crystal violet in a qualitative analysis. Quantification of planktonic, loosely attached, and tightly bound cells in both strains showed that there was a trend of slight decrease in planktonic and loosely bound cells in LB and M9 medium after 1 M NaCl stress. However, in M9 medium a slight decrease was observed for loosely bound cells from 1.5 M to 2.5 M NaCl stress, pronounced for Halomonas variabilis (HT1) strain. Loosely bound cells were more in M9 medium as compared to LB medium. Loosely bound cells of Planococcus rifietoensis (RT4) seemed unaffected at increasing salt concentrations in M9 medium. Maximum amount of loosely bound cells were observed at 1 M and 1.5 M NaCl stress. Biofilm in terms of tightly bound cells was observed at comparable rates in LB medium for both strains at increasing salt stress. Biofilm formation was consistent at 2 M and 2.5 M NaCl stress for strain Planococcus rifietoensis (RT4) (Figure 1).
Figure 1.
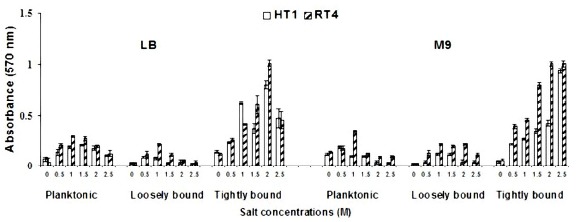
Effect of varying salt concentrations on planktonic, loosely bound and tightly bound cells of bacterial strains in LB and M9 media.
Effect of varying salt concentrations on exopolysaccharide production by bacterial strains
Effect of varying NaCl concentrations (0.5, 1, 1.5, 2 and 2.5 M) was also checked on the exopolysaccharide production of bacterial strains. Results showed that strain Planococcus rifietoensis (RT4) exhibited higher exopolysaccharide at increasing salt stress as compared to Halomonas variabilis (HT1). As observed from the fresh weight and dry weight (grams) values of exopolysaccharides (Figure 3), maximum increase in exopolysaccharide was observed at 1.5 M to 2 M NaCl but it slightly decreased thereafter. However, dry weight of exopolysaccharide was higher at 0.5 M to 1 M NaCl and decreased at 1.5 to 2.5 mM NaCl stress. Quantitative analysis of exopolysaccharide in terms of carbohydrate (Figure 2) was higher at 0.5 M and 1 M NaCl stress. However, at no salt stress or low concentration of NaCl exopolysaccharide production was reduced (Figure 3)
Figure 3.
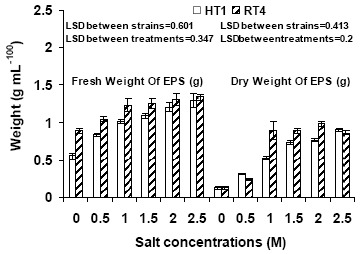
Effect of varying salt concentrations on fresh weight and dry weight of exopolysaccharide of bacterial strains
Figure 2.
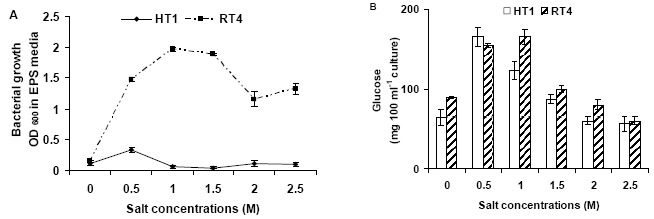
Effect of varying salt concentrations on (A) Bacterial growth in EPS medium (B) exopolysaccharide contents in terms of glucose (mg/100 ml culture) of bacterial strains.
Effect of Bacterial inoculation on plant growth
Increasing salt stress affect all growth parameters of plants. Results showed that in noninoculated plants, there was a little effect of NaCl stress up to 50 mM. However, reduction in all growth parameters of noninoculated plants, including germination (85 %), seedling length (50 %), fresh weight (52 %) and dry weight (48 %) parameters was observed at 200 mM NaCl stress. However, maximum increase in accumulation of total soluble sugars (82 %) and protein contents (9 %) was observed at 100 mM NaCl concentrations (Figure 4) in noninoculated plants. In all inoculated plants, growth parameters, significantly improved towards higher salinity. Inoculation stimulated the germination (152 % by HT1 and 178 % by RT4) of seedlings at 200 mM salt stress (Figure 4) compared to respective control treatment. Response of two strains on improving seedling length was pronounced at 200 mM NaCl stress. Strain RT4 and HT1 improved seedling length by 114 % and 51 %, respectively as compared to control without inoculation (Figure 4). Salt stress reduced the fresh weight of uninoculated plants. Both the strains HT1 and RT4 positively stimulated fresh weight (mg per seedling) 153 % and 177 % at 100 mM, respectively. Dry weight values were also significantly increased (1988 % and 3712 %) by both strains HT1 and RT4 at 200 mM NaCl stress, respectively (Figure 5). Maximum increase in the total soluble sugar content (mg g-1 fresh weight) was observed at 100 mM NaCl stress by HT1 (46 %) and at 200 mM NaCl stress by Planococcus rifietoensis (RT4) (256 %). Similar effect for protein content (µgg_1 fresh weight-1) (maximum 107 % increase by HT1 and 219 % increase by RT4) were observed at 100 mM NaCl stress (Figure 4).
Figure 4.
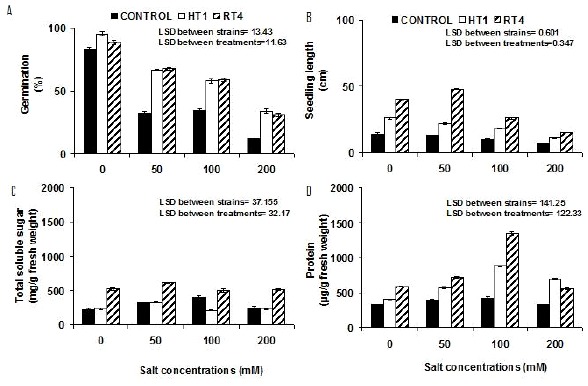
Effect of varying salt concentrations (mM) on the (A). Seed germination (B). Seedling length (cm) and biochemical parameters (C) Total soluble sugar (mg/g fresh weight) (D). Protein (µg/g fresh weight) contents of Cicer arietinum Var. CM-98
Figure 5.
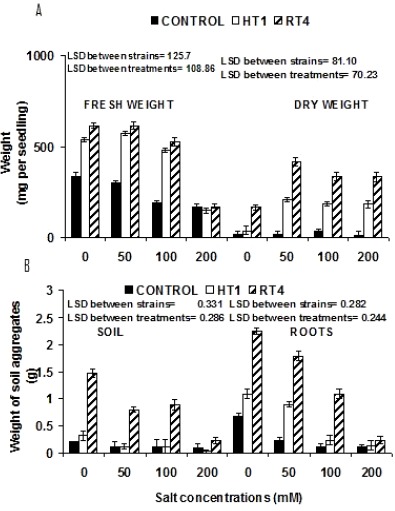
Effect of varying salt concentrations (mM) on the (A) Fresh weight and dry weight mg per seedling of (Cicer arietinum Var. CM 98) (B). Weight of soil aggregates (grams) present on soil and plant root.
Effect of bacterial inoculations on soil aggregates under varying salt stress
Soil aggregates attached to plant roots and present in soil were weighed to check the effect of bacterial inoculation under varying concentration of salt stress. Planococcus rifietoensis (RT4) caused maximum 808 % increase and Halomonas variabilis (HT1) caused maximum 666 % increase at 100 mM NaCl as compared to control plants. Increase in weight of aggregates at 200 mM NaCl was 130 % more with Planococcus rifietoensis (RT4) as compared to noninoculated control. Weight of aggregate formation in soil was less as compared to the roots. However, soil aggregation was more pronounced with Planococcus rifietoensis (RT4) where 75 % more soil aggregates were formed at 200 mM NaCl stress (Figure 5).
DISCUSSION
We choose two previously isolated salt tolerant strains Halomonas variabilis (HT1) and Planococcus rifietoensis (RT4) and studied the effect of varying NaCl concentrations (M) on biofilm formation and exopolysaccharide production. Biofilm was higher in M9 medium as compared to LB medium. It seems that biofilm development protected the cells at elevated stress of nutrients and salt with increasing time. Decrease in planktonic and loosely bound cells with a subsequent increase in tightly bound cells was observed. It may be speculated that bacterial cells fight back with stress to stay alive in the form of micro colonies (25). Environmental stress like nutrients and osmotic stress poses increased bacterial competition for available nutrients, and bacteria revert from the planktonic stage to sessile assemblages at various biotic and abiotic surfaces to protect them in the rhizosphere (19, 20). Moreover, increased production of exopolysaccharide against higher salt stress also favors biofilm formation and protects this mini assembly by retaining a water layer around the cells (9, 15, 20, 23). This is also in line with the previous findings that EPS of Staphylococcus was visible extending in various directions from the cells and help them to adhere to surfaces (18). Sticky nature of EPS depends on its composition, i.e., sugars, proteins and lipids (12). Our results are in line with previous studies where exopolysaccharide production reduced in the medium with the addition of salt (29). It may be speculated from previous reports that variable exopolysaccharide production in different bacterial genera Halomonas variabilis (HT1) and Planococcus rifietoensis (RT4) in response to salt stress may account for the success of bacterial strains in a wide variety of ecosystem. Previous reports also show that biofilm formation and exopolysaccharide production by bacterial strains significantly contribute to soil fertility and improve plant growth (6, 7, 13, 28).
Results of present study indicate that difference in fresh weight and dry weight values of EPS is significant at higher salt stress that shows its better water holding capacity. It is also in line with many previous studies showing that exopolysaccharide facilitates nutrient and water retention, (6, 15) cell adhesion, cell–cell signalling and protection of individual cells (25) from stress. Production of exopolysaccharide can also be beneficial in attachment of bacterial cells to biotic surfaces like plants (15, 19). We also tested the comparative effect of both strains on the growth of Cicer arietinum (Var. CM 98) seedlings. The results of present study are in agreement with several recent and previous reports where significant effect of salt on crops has been reported (10, 38). In general, it was observed that bacterial inoculation stimulated the plant grown with and without salt stress. The results are in line with many other previous and recent reports stating the role of bacterial inoculations in improving plant growth (3, 10, 38). Our results are consistent with the hypothesis that both strains have a tendency to form a biofilm at elevated stress so their presence on seedlings maintains sufficient moisture around seeds. This helps them to survive at their germination stage at higher levels of the NaCl stress. It is also important to mention (6, 34, 35) that excess of sodium ions in the soil decreases the fresh weight and dry weight values at higher salinity. Total soluble sugars and soluble protein contents are better indicators of osmotic adjustments in plants in response to stress (27). Both these parameters have also been reported to increase with increasing salt stress in noninculated plants under salt stress. Accumulation of total soluble sugars and soluble protein contents at higher level is associated with the maintenance of the plant turgor required for growth under salt stress.
Soil aggregation increased in inoculated soil at higher salt stress up to 100 mM. Aggregate formation was more pronounced around roots. This indicates that bacterial attachment in response to salinity is stimulated and leads to biofilm formation at root surfaces. Increased biofilm formation and higher EPS also helps the soil particles to stick together and with roots (7). The binding properties of their exopolysaccharide would cause soil particles to cement and strengthening aggregates formation (4, 5) and favour plant growth under salt stress.
PGPR traits are significantly important for crop development under saline soil. Although, as far as this study, the PGPR activities of these two strains Planococcus rifietoensis (RT4) and Halomonas variabilis (HT1) have not been tested yet. However, previous studies show that a salt tolerant bacterium Halomonas elongata has the ability to inhibit the fungal growth (17). Moreover, salt tolerant strains have also been reported to produce indoleacetic acid (IAA) resulting in the development of in vitro shoot formation (2). Inoculation of Halotolerant Planococcus rifietoensis RS18 isolated from rhizosphere on growth promotion of canola plant under salt stress has also been reported previously (36). Another study described the role of Planococcus rifietoensis M2–26 in secreting chitinase and b-1, 3-glucanase that can degrade the fungal cell walls components chitin and b-1, 3-glucan, respectively (41). Roles of plant growth promoting traits of bacteria are very significant for crop yield so these activities of salt tolerant strains must also be under consideration along with other growth promoting effects.
ACKNOWLEDGEMENTS
We are highly thankful to HEC Pakistan for funding this research.
REFERENCES
- 1.Afrasayab S., Faisal M., Hasnain S. Comparative study of wild and transformed salt tolerant bacterial strains on Triticum aestivum growth under salt stress. Braz. J. Microbiol. 2010;41:946–955. doi: 10.1590/S1517-838220100004000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali B., Hasnain S. Potential of bacterial indoleacetic acid to induce adventitious shoots in plant tissue culture. Lett. Appl. Microbiol. 2007;45:128–133. doi: 10.1111/j.1472-765X.2007.02158.x. [DOI] [PubMed] [Google Scholar]
- 3.Al-Sobhi O.A., Al-Zahrani H.S., Al-Ahmadi Effect of Salinity on Chlorophyll & Carbohydrate contents of Calotropis procera seedlings, Sci. J. King Faisal Univ. (Basic App Sci) 2006;7:105–114. [Google Scholar]
- 4.Ashraf M., Foolad M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007;59:206–216. [Google Scholar]
- 5.Ashraf M., Hafeez M. Thermotolerance of pearl millet and maize at early growth stages: growth and nutrient relations. Biol. Plant. 2004;48:81–86. [Google Scholar]
- 6.Ashraf M., Harris P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004;166:3–16. [Google Scholar]
- 7.Ashraf M., Hasnain S., Hussain F. Proc. Int. Conf. Environmentally Sustainable Development (ESDev-2005) 2005. Exo-polysaccharides (exopolysaccharide) producing biofilm bacteria in improving physico-chemical characteristics of the salt affected soils. [Google Scholar]
- 8.Batool R., Hasnain S. Growth stimulatory effects of Enterobacter and Serratia located from biofilms on plant growth and soil aggregation. Biotechnol. 2005;4(4):347–353. [Google Scholar]
- 9.Chan R., Lam J.S., Lam K., Costerton J.W. Influence of culture conditions on expression of the mucoid mode of growth of Pseudomonas aeruginosa. J. Clin.Microbiol. 1984:8–16. doi: 10.1128/jcm.19.1.8-16.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chookhampaeng S. The Effect of Salt Stress on Growth, Chlorophyll Content Proline content and antioxidative enzymes of Pepper (Capsicum Annuum L.) seedling. Eur. J. Sci. Res. 2011;49(1):103–109. [Google Scholar]
- 11.Christensen G.D., Simpson W.A., Younger J.J., Baddour L.M., Barrett F.F., Melton D.M., Beachey E.H. Adherence of coagulasenegative Staphylococci to plastic tissue culture plates: a quantitative model for the adherence of Staphylococci to medical devices. J. Clin. Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danhorn T., Fuqua C. Biofilm Formation by Plant-Associated Bacteria. Annu. Rev. Microbiol. 2007;61:401–422. doi: 10.1146/annurev.micro.61.080706.093316. [DOI] [PubMed] [Google Scholar]
- 13.Davey M.E., O’Toole G.A. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000;64:847–867. doi: 10.1128/mmbr.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Vuyst L., Vanderveken F., Van de Ven S., Degeest B. Production by and isolation of exopolysaccharides from Streptococcus thermophilus grown in milk medium and evidence for their growth-associated biosynthesis. J. Appl. Microbiol. 1998;84(6):1059–1068. doi: 10.1046/j.1365-2672.1998.00445.x. [DOI] [PubMed] [Google Scholar]
- 15.Dimkpa C., Weinand T., Asch F. Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell. Environ. 2009;32:1682–1694. doi: 10.1111/j.1365-3040.2009.02028.x. [DOI] [PubMed] [Google Scholar]
- 16.Dubois M., Gilles K.A., Hamilton J.K., Reberts P.A., Smiths F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;3:350–356. [Google Scholar]
- 17.Essghaier B., Fardeau M.L., Cayol J.L., Hajlaoui M.R., Boudabous A., Jijakli H., Sadfi-Zouaoui N. Biological control of grey mould in strawberry fruits by halophilic bacteria. J. Appl. Microbiol. 2009;106:833–845. doi: 10.1111/j.1365-2672.2008.04053.x. [DOI] [PubMed] [Google Scholar]
- 18.Franson T.R., Sheth N.K., Rose H.D., Sohnle P.G. Scanning electron microscopy of bacteria adherent to intravascular catheters. J. Clin. Microbiol. 1984;20:500–505. doi: 10.1128/jcm.20.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujishige N.A., Kapadia N.N., De Hoff P.L., Hirsch A. M. Investigations of Rhizobium biofilm formation. FEMS Microbiol. Ecol. 2006;56:195–206. doi: 10.1111/j.1574-6941.2005.00044.x. [DOI] [PubMed] [Google Scholar]
- 20.Fujishige N.A., Kapadia N.N., Hirsch A.M. A feeling for the micro-organism: structure on a small scale. Biofilms on plant roots. Bot. J. Linn. Soc. 2006;150:79–88. [Google Scholar]
- 21.Gerhardt P., Murray R. G. E., Wood W. A., Kreig N. R. Washington, D C.: American Society for Microbiology; 1994. In Methods for General and Molecular Bacteriology. [Google Scholar]
- 22.Hajlaouia H., Ayebb N. E., Garrecc J. P., Dendend M. Differential effects of salt stress on osmotic adjustment and solutes allocation on the basis of root and leaf tissue senescence of two silage maize (Zea mays L.) varieties. Ind. Crop Prod. 2010;31:122–130. [Google Scholar]
- 23.Ishii S., Koki J., Unno H., Hori K. Two Morphological Types of Cell Appendages on a Strongly Adhesive Bacterium, Acinetobacter sp. Strain Tol 5. Appl. Environmen Microbiol. 2004;70(8):5026–5029. doi: 10.1128/AEM.70.8.5026-5029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaleel C. A., Sankar B., Sriaharan R., Panneerselvam R. Soil salinity alters growth, chlorophyll content, and secondary metabolite accumulation in Catharanthus roseus, Turk. J. Biol. 2008;32:79–83. [Google Scholar]
- 25.Kawarai T., Furukawa S., Narisawa N., Hagiwara C., Ogihara H., Yamasaki M. Biofilm formation by Escherichia coli in hypertonic sucrose media. J. Bio. Sci. Bioeng. 2009;107(6):630. doi: 10.1016/j.jbiosc.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Ramant E., Helinski D.R. Vol. 86. Newyork: Academic Press; 1979. Plasmid cloning vehicles derived from plasmid colE1, R6K & RH4, In: Methods in Enzymology, (Wu, R., Ed.,) pp. 268–280. [DOI] [PubMed] [Google Scholar]
- 27.Khatkar D., Kuhad M.S. Short-term salinity induced changes in two wheat cultivars at different growth stages. Biol. Plant. 2000;43:629–632. [Google Scholar]
- 28.Liaqat I., Sumbal F., Sabri A. N. Tetracycline and chloramphenicol efficiency against selected biofilm forming bacteria. Curr. Microbiol. 2009;59:212–220. doi: 10.1007/s00284-009-9424-9. [DOI] [PubMed] [Google Scholar]
- 29.Lloret J., Wulff B.B.H., Rubio J.M., Downie J.A., Bonilla I., Rivilla R. Exoplysaccharide II production is regulated by salt in the halotolerant strain Rhizobium meliloti EFBI. Appl. Environ. Microbiol. 1998:1024–1028. doi: 10.1128/aem.64.3.1024-1028.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mack D., Fischer W., Krokotsch A., Leopold K., Hartmann R., Egge H., Laufs R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear ß-1, 6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mironescu M. Microbial polysaccharides production, characterisation and properties. Acta Universitatis Cibiniensis Series E. Food Technol. 2003;7(2) [Google Scholar]
- 32.Mirza T.Z., Sabri A.N., Hasnain S. Salt tolerant bacteria from rhizosphere, rhizoplane, histoplane and phylloplane of Mazus plant inhabitant of salt range. Sci. Int. 1998;10:151–156. [Google Scholar]
- 33.Mudgal V., Madaan N., Mudgal A., Mishra S. Changes in growth and metabolic profile of Chickpea under salt stress J. Appl. Biosci. 2009;23:1436–1446. [Google Scholar]
- 34.Nemati I., Moradi F., Gholizadeh S., Esmaeili M.A., Bihamta M.R. The effect of salinity stress on ions and soluble sugars distribution in leaves, leaf sheaths and roots of rice (Oryza sativa L.) seedlings. Plant Soil Environ. 2011;57(1):26–33. [Google Scholar]
- 35.Rajkumar M., Prasad M.N.V., Freitas H., Ae N. Biotechnological applications of serpentine soil bacteria for phytoremediation of trace metals. Crit. Rev. Biotechnol. 2009;29:120–130. doi: 10.1080/07388550902913772. [DOI] [PubMed] [Google Scholar]
- 36.Siddikee M.A., Chauhan P.S, Anandham R., Han G., Sa T. Isolation, Characterization, and Use for Plant Growth Promotion under Salt Stress, of ACC Deaminase-Producing Halotolerant Bacteria Derived from Coastal Soil. J. Microbiol. Biotechnol. 2010;20(11):1577–1584. doi: 10.4014/jmb.1007.07011. [DOI] [PubMed] [Google Scholar]
- 37.Sohrabi Y., Heidari G., Esmailpoor B. Effect of salinity on growth and yield of Desi and kabuli chickpea cultivars. Pak. J. Bio. Sci. 2008;11(4):664–667. doi: 10.3923/pjbs.2008.664.667. [DOI] [PubMed] [Google Scholar]
- 38.Soussi M., Ocan A., Lluch C. Effects of salt stress on growth, photosynthesis and nitrogen fixation in chick-pea (Cicer arietinum L.) J. Exp. Bot. 1998;49(325):1329–1337. [Google Scholar]
- 39.Verhoef R., Waard P.D., Schols H.A., Siika-aho M., Voragen A.G.J. Methylobacterium sp. isolated from a Finnish paper machine produces highly pyruvated galactan exopolysaccharide. Carbohydr Res. 2003;338(18):1851–1859. doi: 10.1016/s0008-6215(03)00261-1. [DOI] [PubMed] [Google Scholar]
- 40.Vyrides I, Stuckey D.C. Adaptation of anaerobic biomass to saline conditions: Role of compatible solutes and extracellular polysaccharides. Enzyme Microb. Technol. 2009;44:46–51. [Google Scholar]
- 41.Zouaoui N.S., Essghaier B., Hajlaoui M.R., Fardeau M.L., Cayaol J.L., Ollivier B., Boudabous A. Ability of moderately halophilic bacteria to control grey mould disease on tomato fruits. J. Phytopathol. 2008;156:42–52. [Google Scholar]


