Abstract
Twenty seven bacterial isolates were isolated from superficial brown discolorations on the caps of cultivated Agaricus bisporus. After White Line Assay (WLA) and the assist of Biolog computer-identification system, isolates were divided into groups: (I) comprised ninteen bacterial isolates that positively responded to a Pseudomonas “reactans” reference strain (NCPPB1311) in WLA and were identified as Pseudomonas tolaasii, (II) comprised two isolates which were WLA+ towards the reference strain (JCM21583) of P. tolaasii and were proposed to be P. “reactans”. The third group comprised six isolates, two of which weakly responded to the strain of P. tolaasii and were identified as P. gingeri whereas the other four were WLA-and identified as P. fluorescens (three isolates) and P. marginalis (one isolate). Isolates of P. tolaasii showed high aggressiveness compared with those of P. “reactans” in pathogenicity tests. Cubes of 1 cm3 of A. bisporus turned brown and decreased in size when were inoculated with 10 µl of P. tolaasii suspension containing 108 CFU ml-1, whereas a similar concentration of P. “reactans” caused only light browning. Fifty µl of the same concentration of P. tolaasii isolates gave typical brown blotch symptoms on fresh mushroom sporophores whereas the two P. “reactans” isolates caused superficial light discoloration only after inoculation with 100 µl of the same concentration. Mixture from both bacterial suspensions increased the brown areas formed on the pileus. This is the first pathogenicity report of P. tolasii and P. “reactans” isolated from cultivated A. bisporus in Egypt.
Keywords: Button mushroom, Bacterial brown discoloration, Pseudomonads, Biolog, Pathogenicity
INTRODUCTION
Agaricus bisporus “button mushroom” is one of the most commonly cultivated mushrooms in more than 70 countries and accounts for 40% of the worldwide production (4). A. bisporus contains antioxidants (26), conjugated linoleic acid (5) and high amounts of vitamins D and B (14). A very recent study found that women consumed fresh mushrooms daily, were 64% less likely to develop breast cancer and risk was reduced by 90% (32). Mushrooms have a short postharvest shelf life compared to most vegetables as they have no cuticle to protect them from microbial attack and are highly susceptible to pathogens and to discoloration induced by storage (28). The high respiration rate and water content facilitates their microbial spoilage, their high tyrosinase and phenolic content makes them susceptible to enzymatic browning (2). Pseudomonads bacteria are present in compost and play a key role in the cultivation of Agaricus. Different studies explained the role of Pseudomonas species (e.g. P. putida) in the induction of mushroom fruiting body (6). However, bacterial pathogens present in most casing material, even after pasteurization, participate in disease occurrence which is found to be associated with bacterial population size on mushroom caps rather than on the casing (17). Bacterial blotch is endemic in mushroom houses, affecting mushroom quality and yield. The disease has typically been identified as being mainly caused by Pseudomonas specie. P. tolaasii causes Agaricus brown blotch whereas P. gingeri causes Agaricus ginger blotch (7). Blotch usually appears on mushrooms of any age even on refrigerated or over-wrapped ones. Spotting is observed at or near the cap edge and also on stems at the contact points between two mushrooms and the spots sometimes enlarge covering the entire cap (7). P. tolaasii produces pale yellow lesions, which later become rich chocolate and sometimes sunken associated with tissue collapse (29). Some saprophytic Pseudomonads in the compost may play a role through interacting with P. tolaasii and are known as P. “reactans” (29). This bacterium cause only light brown discoloration that sometimes gets darker with time (8). A study in Finland proved the pathogenicity of P. costantinii, as another causal agent of brown blotch disease in button mushroom (19). However, P. tolaasii is the major studied species which produces a low-molecular-weight extracellular toxin, tolaasin, the primary elicitor of disease symptoms, while the lipopolysaccharide lipid A is the disease-inducing principal in P. “reactans” (27). In Egypt, the bacterial brown blotch is not well known, and many mushroom farmers are not aware of the disease but concerns are now rising since blotched mushrooms are rejected by consumers and usually returned to suppliers.
Bacterial isolates
Pseudomonads bacteria described in the present study were isolated according to the method described by Lelliott and Stead (15) from the blotched sporophores of fresh A. bisporus, supplied by mushroom farms and local markets in Cairo. Sections of blotched outerlayer tissues were excised mainly from mushroom caps exhibiting brown discolorations and were placed in sterile water with vigourous shaking. Fifty µl samples of an appropriate dilution were spread on King’s B (KB) agar medium (13) and incubated at 25°C. Bacterial colonies developing fluorescent halo after 48 h of incubation were purified by streaking over the same medium and were maintained on nutrient agar plus glycerol 2% at 4°C for short-term use. The bacterial isolates were lyophilized for further use in pathogenicity, white line, nutritional and biochemical tests. Reference strain of P. tolaasii (JCM 21583) was obtained from the Japan Collection of Microorganisms (Riken, Japan). Whereas P. corrugata (NCPPB2445) and P. “reactans” (NCPPB1311) were obtained from the National Collection of Plant Pathogenic Bacteria (Harpenden, United Kingdom).
Production of fluorescent pigments
This is a key test for the identification of fluorescent Pseudomonads (15). The Pseudomonas isolates were grown on KB medium and after 48 h of incubation at 25°C, an aliquot from each prepared suspension was streaked over another Petri dish containing KB medium. Inoculated plates were incubated for five days at 25°C and the developed bacterial cultures were examined with UV light. The reference strain of P. tolaasii was used as positive control whereas that of P. corrugata was the negative control. The test was repeated three times.
LOPAT profile
Tests for the Pseudomonads were carried out to determine the “LOPAT” profile (Levan production, Oxidase production, Pectinolitic activity, Arginine dihydrolase production, and Tobacco hypersensitivity) for the isolated bacteria (15).
White line assay
The WLA was performed on KB medium according to the method of Wong and Preece (30) using two reference strains of P. tolaasii and P. “reactans” against the test isolates. Colonies of the bacterial isolates were toothpick-inoculated at a distance of 8 mm from streaks of the indicator bacteria. The appearance of the white line precipitate between patches was examined after 48 to 72 h of incubation at 25°C.
Nutritional profile using computerized Biolog system
Bacterial isolates were evaluated for different nutritional characteristics as reported by Goor et al. (8) for bacterial pathogens associated with cultivated mushroom. The nutritional profile was evaluated with the computer-assisted System Biolog (Biolog, Inc., Hayward, CA, USA) (1) based on the utilization of different carbon sources. Bacterial isolates were grown for 48 h at 30°C on nutritive agar then the bacterial growth was stopped in “gelling inoculating fluid” until reaching a transmittance of 590 nm Bacterial suspensions were dispensed in the wells of “GN2” Micro Plate (150 μl/well) containing different lyophilized nutritive sources except the first well which served as negative control. Plates were incubated at 30°C and after 24 h were analyzed by “Micro Station reader” using the software “Micro Log 4.01” to compare metabolic profile of bacterial isolates with that of the Biolog data base.
Tests of pathogenicity
Pathogenicity tests were performed according to the method described by Olivier et al. (22). Bioassays were performed using cubes (1 cm3) of 1-day-old A. bisporus excised with sterile scalpel blades from the cap and were placed into a sterile petri dish containing a 50-mm-pore-size filter paper dampened with 750 ml of sterile distilled water. Four cubes were placed 2 cm apart to eliminate cross-contamination by motile Pseudomonads. Bacterial isolates were cultured in KB medium to a density of 108 CFU ml−1 and 10 μl from the bacterial suspension of each isolate of P. tolaasii and of P. “reactans” isolates was spotted over the surface of three cubes whereas the fourth cube was inoculated with sterile distilled water (as control). Petri dishes were sealed with parafilm and incubated at 22°C and observed daily for three days. Suspensions used to inoculate fresh mushroom caps were as following: 50 μl of P. tolaasii; 50 μl and 100 μl of P. “reactans”; and 50 μl of a mixture of both suspensions. The degree of blotch discoloration was scored daily in comparison to controls inoculated with sterile distilled water after incubation at 22°C for three days. All bioassays were repeated three times in triplicates using different sources of fresh A. bisporus.
RESULTS
Bacterial isolates
Bacterial isolates described in this study were obtained over a period of one year from A. bisporus fruit bodies showing brown blotch symptoms collected from three mushrooms farms. Some mushroom samples were obtained from three local markets within the city of Cairo. Isolation from superficial brown spots and streaks of A. bisporus sporophores were always successful and these bacteria gave rise to colonies which mostly showed the typical morphology of Pseudomonas on nutritive agar after 48 h of incubation at 25°C: circular domed, 2-mm-diameter, whitish or yellowish white, with a creamy consistency. In addition, all the isolates were able to produce either yellow or yellowish-green pigments when cultured on KB media (Table 1).
Table 1.
Main differences in physiological and nutritional characteristics of P. tolaasii and P.” reactans” isolates using Biolog system
| P.tolaasii Isolate | FPP | D-trehalose | Malonica Acid | L-alanyl gylcine | Glycyl-L-glutanic Acid | L-threonine | D-trealose | WLA* | P.reactans Isolate | FPP | Malonico Acid | Propionic Acid | Acetic Acid | D-Sarbitol | D-manonine | L-treonine | WLA* | ||
| P.tolaasii JCM21583 | P.’reactans’ NCPPB1311 | P.tolaasii JCM 21583 | P.’reactans’ NCPPB1311 | ||||||||||||||||
| Biolog | + | + | + | + | + | + | Biolog | + | +- | +- | + | + | + | ||||||
| JCM 21583 | YG | + | + | +- | +- | +- | + | - | ++ | NCPPB1311 | Y | + | +- | +- | + | + | + | + | - |
| ECAGTI1 | YG | + | +- | - | + | + | + | - | + | ECAGTI1 | Y | + | + | + | + | + | + | ++ | + |
| ECAGTI2 | YG | + | +- | +- | - | -- | - | - | + | ECAGKI 2 | YO | +- | — | - | -- | - | - | - | - |
| ECAGTI3 | YG | - | +- | + | + | +- | - | - | + | ECAGTI3 | YG | - | + | + | + | + | + | - | - |
| ECAGTI4 | Y | - | + | +- | +- | + | - | - | ++ | ECAGTI4 | Y | + | + | - | - | - | - | - | - |
| ECAGTI5 | YG | + | +- | +- | +- | +- | + | - | + | ECAGTI5 | Y | +- | - | +- | +- | + | +- | - | - |
| ECAGTI 6 | Y | + | +- | +- | + | - | + | - | + | ECAGTI6 | Y | +- | - | - | - | +- | - | ++ | + |
| ECAGTI 7 | Y | - | + | + | + | + | - | - | + | ECAGTI 7 | Y | - | + | - | + | - | - | + | + |
| ECAGTI 8 | Y | + | +- | - | + | + | + | - | + | ECAGTI8 | Y | +- | - | - | - | + | + | + | + |
| ECAGTI 9 | YG | + | +- | +- | +- | +- | + | - | + | ||||||||||
| ECAGTI 10 | Y | + | +- | +- | + | - | + | - | + | ||||||||||
| ECAGTI11 | YG | + | +- | +- | +- | +- | + | - | ++ | ||||||||||
| ECAGTI12 | Y | + | +- | +- | + | - | + | - | + | ||||||||||
| ECAGTI 13 | YG | + | +- | + | + | + | + | - | ++ | ||||||||||
| ECAGTI 14 | Y | + | +- | + | + | + | + | - | ‘ | ||||||||||
| ECAGTI 15 | Y | + | - | +- | +- | +- | + | - | + | ||||||||||
| ECAGTI16 | Y | - | +- | - | - | - | - | + | |||||||||||
| ECAGTI 17 | YG | + | +- | +- | +- | +- | + | - | + | ||||||||||
| ECAGTI 18 | Y | + | +- | + | + | + | + | - | + | ||||||||||
| ECAGTI 19 | YG | + | +- | +- | - | +- | + | - | ++ | ||||||||||
FPP=Fluorescent pigment production, YG=Yellowish-green pigment, Y=Yellow pigment;
White line assay run on KB medium (Wong and Preece, 1979), - = absence of character whereas + = a weak WLA reaction and ++ = a strong WLA reaction.
“LOPAT” profile
All isolates of P. tolaasii and those proposed to be P. “reactans” showed the a similar “LOPAT” profile which is characteristic for the Pseudmonas group, tested isolates were: Levan production (-); Oxidase production (+); Pectinolitic activity (-); Arginine dihydrolase production (+); and Tobacco hypersensitivity (-).
White line assay
All the isolates referred to as ECAGTI demonstrated the ability to form white precipitate in agar when grown against the reference strain of P. “reactans” (NCPPB1311) but not against each other or against the reference strain of P. tolaasii (Table 1). Four of the eight isolates referred to as ECAGRI showed the same character when grown near P. tolaasii (JCM21583) (Table 1). These four isolates were proposed to be P. “reactans” but when streaked against the P. tolasii reference strain grown in the middle of the plate, they were different. Two of them, ECAGRI 1 and ECAGRI 6, strongly reacted (WLA ++) and were separated in the second group whereas the other two, ECAGRI 7 and ECAGRI 8, gave a weak reaction (WLA+) and were placed in a separate group (III) (Fig. 1). It is important to point out that conservation on agar substrates and sub-culturing resulted in the attenuation or total loss of this character for the two bacterial isolates in group (II).
Figure 1.
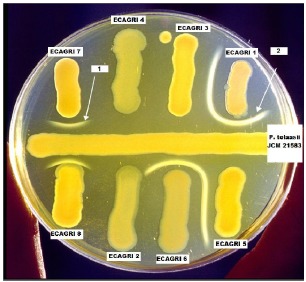
White line assay (WLA) on King’s B medium. The horizontal streak corresponds to P. tolasii reference strain (JCM21583) whereas the eight vertical bacterial colonies represent isolates proposed to be P. “reactans; 1 = weak white line reaction (WLA +) by P. gingeri (ECAGRI 7 and ECAGRI 8); 2 = strong white line reaction (WLA ++) by P.” reactans” (ECAGRI 1 and ECAGRI 6).
Biolog profile of Pseudomonas isolates
Test isolates referred to as ECAGTI in group (I) are proposed to be P. tolaasii and have confirmed the nutritional profile (8), in particular, those growing on media with minimal concentrations of sorbitol, 2-chetogluconate, L-arabitol and n-valerate but not on media containing D-tartrate or histamine. Whereas isolates proposed to be P. “reactans”, which is not a taxonomically characterized species, grew on media containing minimal concentrations of sorbitol, L-arabinose, L-arabitol, chetogluconate and n-valerate but not on media rich in D-tartarate or histamine. The major differences in the nutritional profile for the utilization of different carbon sources by all the isolates using the Biolog system is illustrated in Table (1). A similarity of 100% was confirmed among four of the nineteen isolates of P. tolaasii (no. 8, 12, 16 and 17). A similar identity could be also recorded in the same figure for other five isolates which shared a high degree of similarity as well, among which two of them (no. 11 and 13) were different from the other three (no. 1, 3 and 4). The rest of isolates scored more than 97% in relation to each other (Fig. 2). Three of the eight P. “reactans” isolates which are referred to as ECAGRI in the other two groups were identified using the same Biolog system as P. fluorescens, two as P. gingeri, one as P. marginalis, and they were placed in third group (Table 2).
Figure 2.
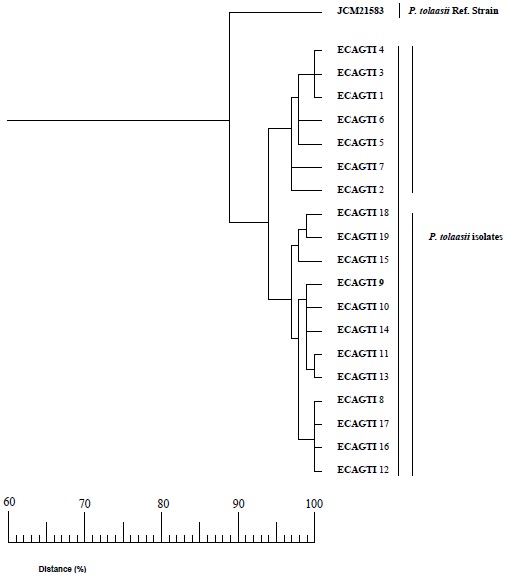
Dendrogram of carbon source utilization showing the relationship between the Biolog profile of Pseudomonas tolaas strain JCM21583 and the nineteen isolates using Biolog/UPGMA cluster analysis.
Table 2.
Identification of isolates of P. tolaasii and P.” reactans” using Biolog identification system
| P. tolaassii isolates | Identification | Probability (%) | Similitude | Distance | P. “reactans” isolates. | Identification | Probjbilily (%) | Similitude | Distance |
| JCM 21583* | P. tolaasii | 100 | 0,833 | 0,98 | NCPPB1311* | P. flourescens | 97 | 0:885 | 1,27 |
| ECAGTI 1 | “ | 90 | 0,610 | 4,86 | ECAGRI1 | Pseudomonas spp. | - | 0,296* | 6,04* |
| ECAGTI2 | “ | 100 | 0,933 | 1 | ECAGRI2 | P. flourescens | 93 | 0,827 | 2,02 |
| ECAGTI3 | “ | 100 | 1 | 0 | ECAGRI3 | P. flourescens | 96 | 0,754 | 3.38 |
| ECAGTI 4 | “ | 100 | 0,865 | 2 | ECAGRI4 | P. flourescens | 99 | 0,608 | 5,87 |
| BCAGTI 5 | “ | 89 | 0,635 | 4,06 | ECAGRI5 | P. marginalis | 100 | 0,861 | 2,05 |
| ECAGTI 6 | “ | 92 | 0,559 | 6,09 | ECAGRI6 | Pseudomonas spp. | - | 0,416** | 4,12* |
| ECAGTI 7 | - | 100 | 0,933 | 1 | ECAGRI7 | P. gingeri | 88 | 0,733 | 3,15 |
| ECAGTI 8 | “ | 100 | 0,853 | 2,19 | ECAGRI8 | P. gingeri | 70 | 0,507 | 2,49 |
| ECAGTI 9 | - | 100 | 0,953 | 0,7 | |||||
| ECAGTI 10 | “ | 99 | 0,886. | 1,63 | |||||
| ECAGTI 11 | “ | ]100 | 0,930 | 1,01 | |||||
| ECAGTI 12 | “ | 100 | 0,853 | 2,19 | |||||
| ECAGTI 13 | “ | 100 | 0,940 | 0,89 | |||||
| ECAGTI 14 | “ | 100 | 0,965 | 0,51 | |||||
| ECAGTI 15 | “ | 100 | 0,919 | 1,21 | |||||
| ECAGTI l6 | 100 | 0,865 | 2 | ||||||
| ECAGTI 17 | - | 100 | 0,894 | 1,53 | |||||
| ECAGTI 18 | “ | 100 | 0,884 | 1,73 | |||||
| ECAGTI 19 | 100 | 0,890 | 1,63 | ||||||
FPP=Fluorescent pigment production, YG=Yellowish-green pigment, Y=Yellow pigment; *White line assay run on KB medium (Wong and Preece, 1979), - = absence of character whereas + = a weak WLA reaction and ++ = a strong WLA reaction.
Pathogenicity of Pseudomonas isolates
Twenty one isolates including 19 isolates of P. tolaasii and 2 isolates proposed to be P. “reactans”, as well as the reference strains caused browning of A bisporus blocks. The browning scale varied and the incubation period which lasted for only 24 h at 22°C was sufficient to recognize the browning in Agaricus cubes inoculated with 10 μl of P. tolaasii suspension containing 108 CFU ml−1. Mushroom cubes treated under the same condition and with the same concentration of P. “reactans” were not affected. At 48 h of treatment, a slight decrease in cube size inoculated with P. tolaasii was recorded whereas those treated with P. “reactans” developed a light browning color (Fig. 3). In pathogenicity tests using the whole fresh sporophores, P. tolaasii isolates were also more aggressive, drops of 50 µl from suspensions of P. tolaasii applied to the surface of A. bisporus caps were sufficient to produce slightly sunken brown spots in the inoculation area (Fig. 3) after two days of incubation at 25°C, whereas suspensions of the two P. “reactans” isolates resulted in a superficial light discoloration when assayed at a higher concentration (100 µl). A 50 µl mixture from both bacterial suspensions increased spotting size on the pileus without change in the color intensity (Fig. 3).
Figure 3.
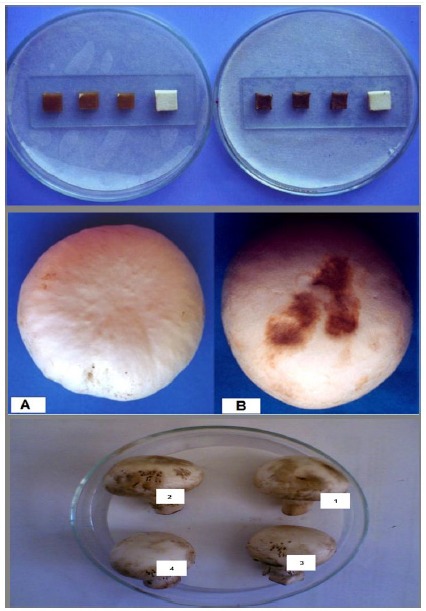
Upper image: Cube pathogenicity bioassays to determine isolates inducing brown discoloration in A. bisporus tissue with 10 μl of P.reactans (left plate) and P. tolaasii (right plate), fourth cube (white cube) in each slide is inoculated with distilled H2O (Control). Middle image: Browning on caps of Agaricus bisporus inoculated with drops (50 μl) of: [A] distilled H2O (Control); [B] P. tolaasii. Lower image: Symptoms on caps of Agaricus bisporus inoculated with drops of [1] 50 μl of P. tolaasii; [2] 50 μl of P. tolaasii and P. reactans; [3] 100 μl of P. reactans; [4] 50 μl of distilled H2O (Control).
DISCUSSION
The discoloration is an undesirable phenomenon in mushroom houses and the markets and is a major factor in selection by consumers (28). Most cultivated mushrooms are grown on sterilized substrates, and once a contaminant among hundreds of other fungi, bacteria, nematodes, gets a foothold, it flourishes in the absence of competition from other contaminants (3). Previous studies related pathogenic Pseudomonads to diseases affecting cultivated A. bisporus. Of the blotch-causing Peudomonads, the best characterized is P. tolaasii which enters the mushroom farm in peat and limestone used in the casing process (31). Once present, this bacterium is able to attach the mycelial surface of developing A. bisporus (23, 25). Under UV light, colonies of the isolated pseudomonas species produced yellow to yellowish-green fluorescent color. These isolates showed the “LOPAT” profile characteristic for that group and consistent with that reported by Lelliott and Stead (15). The WLA is an accurate method for recognizing P. tolaasii and P. “reactans” (29, 9) and this reaction results from a specific interaction between two diffusible lipodepsipeptides, tolaasin toxin by P. tolaasii (21, 24) and the so-called White Line Inducing Principal (WLIP) by some virulent Pseudomonads (12). This preliminary test divided the isolates obtained in this study according to their reaction towards the reference strains of P. tolaasii and P. “reactans” into three groups. The nutritional characteristics analyzed by the assistance of the Biolog system of thirteen utilized carbon sources nearly confirmed the three proposed groups. The nineteen isolates in group (I) conform to the commonly accepted identification criteria for P. tolaasii (8). Their metabolic fingerprint by the Biolog system was analyzed by un-weighed pair group method (UPGM) and isolates clustering in the Biolog/UPGM indicated their physiological similarities. These isolates shared a very high similarity (of 100% for some of them) to the reference strain of P. tolaasii except one which scored 89% and was weakly pathogenic to A. bisporus . The two Pseudomonas isolates in group (II) which produced typical white line (Characteristic to P. “reactans”) against P. tolaasii reference strain were identified as Pseudomonas spp. The inability to reach the species level for them analyzing their nutritional profile is attributed to a fact that P. “reactans” is still a non-classified bacterium in the data bank of the Biolog system and known to produces an extracellular substance called “WLIP” which plays a role in the interaction with cultivated mushrooms (16). The other two isolates with a weak WLA reaction against P. tolaasii, were identified as P. gingeri, Munsch and Alatossava (20) found that a strain of P. gingeri was able to produce a typical WLA when streaked towards a reference strain of P. tolaasii and they hypothesized that the bacterium produces some compounds behaving like the WLIP molecule and may be structurally identical or with similar biological function to the one produced by P. “reactans”. These two isolated were grouped together with other four, three of which were identified as P. fluorescence and one as P. marginalis. Some American strains associated with cultivated mushrooms were assigned to P. fluorescence biovars III and V (29) and a similar conclusion was obtained through cross studies which related some mushroom associated P. “reactans” isolates to representatives of P. fluorescence biovars II, III and V (18). Inoculating suspensions prepared from P. tolaasii isolates induced dark brown discoloration and produced sunken chocolate lesions on the caps of fresh A. bisporus. This characteristic pathogenicity distinguished P. tolaasii from other fluorescent Pseudomonads especially P. fluorescence (22). The two isolates in the second group which are proposed to be P. “reactans”, induced chestnut browning on mushrooms caps and this was evident only at higher concentrations. The remaining six isolates in group (3) exhibited normal discolorations that were consistent with that recorded on the negative controls and were therefore considered non-pathogenic isolates. The slight increase observed in the brown scale using a mixture from both bacterial suspensions confirms the previous finding of Iacobellis and Lo Cantore (10, 11) who suggested that the brown blotch of A. bisporus appears to be a complex disease caused mainly by P. tolaasii with a possible role of “P. reactans”. In conclusion, P. tolaasii is the main causal agent of the brown blotch affecting A. bisporus and a role of P. “reactans” in the disease progress may be present.
ACKNOWLEDGEMENTS
This work was supported by a grant from Ain Shams University. The author thanks the Agricultural Research Center (Giza, Egypt) for analysis of bacterial isolates using Biolog Automated Microplate System. I deeply acknowledge Prof. Y. Mikami (MMRC, Chiba University, Tokyo, Japan) for the gift of P. tolaasii strain (JCM 21583). I’m also grateful to Dr. Wael Samir (Ass. Prof. of Systematic Bacteriology, Department of Microbiology, Faculty of Science, Ain Shams University) for contribution during the Biolog analyses.
REFERENCES
- 1.Anonimo Biolog MicrologTM System, Release 4.01A User Guide, Biolog. Inc. 1999 [Google Scholar]
- 2.Brennan M., Le Port G., Gormley R. Postharvest treatment with citric acid or hydrogen peroxide to extend the shelf life of fresh sliced mushrooms. Lebensmittel-Wissenschaft und-Technologie. 2000;33:285–289. [Google Scholar]
- 3.Brosnan T., Sun D.W. Inspection and grading of agricultural and food products by computer vision systems. J. Food Eng. 2004;61:3–14. [Google Scholar]
- 4.Carluccio A. The Complete Mushroom Book. Savory recipes for wild and cultivated varieties with an illustrated field guide. Quadrille: Universe Promotional Books; 2003. p. 224. [Google Scholar]
- 5.Chen S. Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus) Cancer Res. 2006;66(24):12026–12034. doi: 10.1158/0008-5472.CAN-06-2206. [DOI] [PubMed] [Google Scholar]
- 6.Fermor T., Lincoln S., Noble R., Dobrovin-Pennington A. Microbiological properties of casing. In: van Greinsven L.J.L.D., editor. Science and cultivation of edible fungi. Rotterdam, The Netherlands: A.A. Balkema; 2000. pp. 447–454. [Google Scholar]
- 7.Gill W.M. Bacterial disease of Agaricus mushrooms. Tottori Mycological Institute, The Japan Kinoko Research Center Foundation. Japan Science and Technology Agency. 1995;33:34–55. [Google Scholar]
- 8.Goor M., Vandamme R., Swings J., Gillis M., Kersters K., De Ley J. Phenotypic and genotypic diversity of Pseudomonas tolaasii and white line reacting organisms isolated from cultivated mushrooms. J. Gen. Microbiol. 1986;132:2249–2264. [Google Scholar]
- 9.Hu F.P., Young J.M., Fletcher M.J. Preliminary description of biocidal (syringomycin) activity in fluorescent plant pathogenic Pseudomonas species. J. Appl. Microbiol. 1998;85:365–371. doi: 10.1046/j.1365-2672.1998.00516.x. [DOI] [PubMed] [Google Scholar]
- 10.Iacobellis N.S., Lo Cantore P. France: Montpellier; 1997. Bacterial diseases of cultivated mushrooms in Southern Italy. Proceedings of the 10th congress of the Mediterranean Phytopathological Union; pp. 33–37. [Google Scholar]
- 11.Iacobellis N.S., Lo Cantore P. Studi sull’eziologia dell’ingiallimento dell’ostricone (Pleurotus ostreatus) Agricoltura Ricerca. 1998;176:55–60. [Google Scholar]
- 12.Iacobellis N.S., Lo Cantore P. Pseudomonas “reactans” a new pathogen of cultivated mushrooms. In: Iacobellis N.S., editor. Pseudomonas syringae pathovars and related pathogens. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2003. pp. 595–605. [Google Scholar]
- 13.King E.O., Ward M.K., Raney D.E. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 14.Lee G.S., Byun H.S., Yoon K.H., Lee J.S., Choi K.C., Jeung E.B. Dietary calcium and vitamin D2 supplementation with enhanced Lentinula edodes improves osteoporosis-like symptoms and induces duodenal and renal active calcium transport gene expression in mice. Eur. J. Nutr. 2009;48(2):75–83. doi: 10.1007/s00394-008-0763-2. [DOI] [PubMed] [Google Scholar]
- 15.Lelliott R.A., Stead D.A. Methods for the diagnosis of bacterial diseases of plants. In: Preece T.F., editor. Methods in Plant Pathology. Oxford, UK: Blackwell Scientific Publications; 1987. p. 216. [Google Scholar]
- 16.Lo Cantore P., Lazzaroni S., Coraiola M., Dalla Serra M., Cafarchia C., Menestrina G., Evidente A., Iacobellis N.S. Biological characterization of WLIP produced by Pseudomonas “reactans” NCPPB1311. Mol. Plant-Microbe Interact. 2006;19(10):1113–1120. doi: 10.1094/MPMI-19-1113. [DOI] [PubMed] [Google Scholar]
- 17.Lukkasse L.J.S., Polderdijk J.J. Predictive modeling of postharvest quality evolution in perishables, applied to mushrooms. J. Food Eng. 2003;59:191–198. [Google Scholar]
- 18.Munsch P., Geoffroy V.A., Alatossava T., Meyer J.M. Application of siderotyping for characterization of Pseudomonas tolaasii and ‘Pseudomonas reactans’ isolates associated with brown blotch disease of cultivated mushrooms. Appl. Environ. Microbiol. 2000;66:4834–4841. doi: 10.1128/aem.66.11.4834-4841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munsch P., Alatossava T., Marttinen N., Meyer J.M., Christen R. Pseudomonas costantinii sp. nov., another causal agent of brown blotch disease, isolated from cultivated mushroom sporophores in Finland. Inter. J. Syst. Evol. Microbiol. 2002;52:1973–1983. doi: 10.1099/00207713-52-6-1973. [DOI] [PubMed] [Google Scholar]
- 20.Munsch P., Alatossava T. Several Pseudomonads associated with the cultivated mushrooms Agaricus bisporus or Pleurotus sp., are hemolytic. Microbiol. Res. 2002;157(4):311–315. doi: 10.1078/0944-5013-00159. [DOI] [PubMed] [Google Scholar]
- 21.Nair N.J., Fahy P.C. Toxin production by Pseudomonas tolaasii Paine. Aust. J. Biol. Sci. 1973;26:509–512. [Google Scholar]
- 22.Olivier J.M., Guillaumes J., Martin D. Angers, France: INRA; 1978. Study of a bacterial disease of mushroom caps. Proceedings of the 4th international conference on plant pathogenic bacteria; pp. 903–916. [Google Scholar]
- 23.Preece T.F., Wong W.C. Quantitative and scanning electron microscope observations on the attachment of Pseudomonas tolaasii and other bacteria to the surface of Agaricus bisporus. Physiol. Plant Pathol. 1982;21:251–257. [Google Scholar]
- 24.Rainey P.B., Brodey C.L., Johnstone K. Biological properties and spectrum of activity of tolaasin, a lipodepsipeptide toxin produced by the mushroom pathogen Pseudomonas tolaasii. Physiol. Mol. Plant Pathol. 1991;39:57–70. [Google Scholar]
- 25.Rainey P.B. The involvement of Pseudomonas putida in the process of basidiome initiation of the cultivated mushroom, Agaricus bisporus. Christchurch, New Zealand: Ph. D. Thesis. University of Canterbury; 1989. [Google Scholar]
- 26.Shi Y.L., James A.E., Benzie I.F., Buswell J.A. Mushroom-derived preparations in the prevention of H2O2-induced oxidative damage to cellular DNA. Teratogen. Carcinogen. Mutagen. 2002;22(2):103–111. doi: 10.1002/tcm.10008. [DOI] [PubMed] [Google Scholar]
- 27.Silipo A., Lanzetta R., Garozzo D., Lo Cantore P., Iacobellis N.S., Molinaro A., Parrilli M., Evidente A. Structural determination of lipid A of the lipopolysaccharide from Pseudomonas reactans. Eur. J. Biochem. 269:2498–2505. doi: 10.1046/j.1432-1033.2002.02914.x. [DOI] [PubMed] [Google Scholar]
- 28.Vízhányó T., Felföldi J. Enhancing color differences in images of diseased mushrooms. Comput. Electron. Agric. 2000;26:187–198. [Google Scholar]
- 29.Wells J.M., Sapers G.M., Fett W.F., Butterfield J.E., Jones J.B., Bouzar H., Miller F.C. Postharvest discoloration of the cultivated mushroom Agaricus bisporus caused by Pseudomonas tolaasii, P. ‘reactans,’ and P. ‘gingeri’. Phytopathology. 1996;86:1098–1104. [Google Scholar]
- 30.Wong W.C., Preece T.F. Identification of Pseudomonas tolaasii: the white line test in agar and mushroom block rapid pitting tests. J. Appl. Bacteriol. 1979;47:401–407. [Google Scholar]
- 31.Wong W.C., Preece T.F. Pseudomonas tolaasii in mushroom crops: a note on primary and secondary sources of the bacterium on a commercial farm in England. J. Appl. Bacteriol. 1980;49:305–314. [Google Scholar]
- 32.Zhang M., Huang J., Xie X., Holman C.D. Dietary intakes of mushrooms and green tea combine to reduce the risk of breast cancer in Chinese women. Int. J. Cancer. 2009;124(6):1404–1408. doi: 10.1002/ijc.24047. [DOI] [PubMed] [Google Scholar]


