Abstract
Vineyard soils are frequently polluted with high concentrations of copper due application of copper sulfate in order to control fungal diseases. Bioremediation is an efficient process for the treatment of contaminated sites. Efficient copper sorption bacteria can be used for bioremoval of copper from contaminated sites. In this study, a total of 106 copper resistant bacteria were examined for resistance to copper toxicity and biosorption of copper. Eighty isolates (45 from vineyard Mollisol, 35 from Inceptisol) were obtained from EMBRAPA (Empresa Brasileira de Pesquisa Agropecuária) experimental station, Bento Gonçalves, RS, Brazil (29°09′53.92″S and 51°31′39.40″W) and 26 were obtained from copper mining waste from Caçapava do Sul, RS, Brazil (30°29′43.48″S and 53′32′37.87W). Based on resistance to copper toxicity and biosorption, 15 isolates were identified by 16S rRNA gene sequencing. Maximal copper resistance and biosorption at high copper concentration were observed with isolate N2 which removed 80 mg L−1 in 24 h. Contrarily isolate N11 (Bacillus pumilus) displayed the highest specific copper biosorption (121.82 mg/L/OD unit in 24 h). GenBank MEGABLAST analysis revealed that isolate N2 is 99% similar to Staphylococcus pasteuri. Results indicate that several of our isolates have potential use for bioremediation treatment of vineyards soils and mining waste contaminated with high copper concentration.
Keywords: Copper contamination, vineyard soil; mining waste, copper biosorption; bioremediation
INTRODUCTION
Copper is a very important element. Living organisms require copper as an essential micronutrient. Prior to the recognition of the existence of microorganisms on Earth, the Egyptians, Greeks, Romans, and Aztecs used copper compounds for hygiene and for the treatment of diseases (21). Many fungicides, paints, antimicrobial medicines, oral hygiene products, hygienic medical devices, antiseptics and other products contain copper as an antimicrobial agent (21). However, at high copper concentrations, it is very toxic to most forms of life in addition to microorganisms (5).
Mining activities of modern societies, extensive industrial use of copper and its widespread use as a pesticide in crop production are major sources of copper pollution of soils and water. Toxic heavy metals pose a serious threat to human health, biodiversity and the ecosystem (3). In vineyards, copper pollution negatively impact grape production. Consequently, the development of methods to remove toxic heavy metals such as copper from water and soils is currently an area of intensive research (1, 6, 7, 8, 12, 14, 20, 22, 23, 25, 26, 27).
Treatment technologies such as ion exchange adsorption, electrodialyses, precipitation and chemical reduction can be used to remove heavy metals (17). These methods are conversely expensive compared to bioremediation processes. Biological removal of pollutants is attractive in this technology, and it is considered cost-effective and eco-friendly (4, 7, 8, 13, 14, 25). Contaminated environments select resistant microorganisms over pollutants (3). Microorganisms that are resistant to toxic and recalcitrant chemicals can be isolated from polluted sites as well as natural soils and used for bioremediation of environments contaminated with specific chemicals to which they are resistant (6, 7, 15, 27, 28). Biosorption is an important bioremediation process for removal of copper and other toxic heavy metals from the environments. In this study, in search for efficient strains for copper bioremoval, we examined a total of 106 copper resistant bacteria isolated from two copper contaminated vineyard soils and copper mining waste for copper biosorption at high concentration. We employed DNA-based methods to identify promising copper resistant isolates with potential for copper bioremoval from contaminated environments.
MATERIALS AND METHODS
Soil sample
Soil samples were collected from three contaminated soils from South of Brazil. Two of them were collected from copper contaminated vineyards areas of EMBRAPA experimental station, Bento Gonçalves, RS, Brazil (29°09′53.92″S and 51°31′39.40″W). These two soils were classified as Inceptisol and Mollisol. The copper mining waste sample was obtained from the copper mining area of Caçapava do Sul, RS, Brazil (30°29′43.48″S and 53′32′37.87W). Soil samples and copper mining waste were characterized. Table 1 presents the physico-chemical parameters analyzed in the Laboratory of Soil Analysis from Federal University of Rio Grande do Sul.
Table 1.
Chemical and physical properties of vineyard soils contaminated with copper (Inceptisol and Mollisol) and copper mining wastes (Waste).
| Tratamento | pH | CEC* | OM** | Clay | Cu | Zn | Mn |
|---|---|---|---|---|---|---|---|
| 1:1 | cmolc dm−3 | g dm−3 | % | ------------- mg dm−3 ------------- | |||
| Inceptisol | 6.3 | 17.2 | 2.6 | 19 | 207 | 19 | 55 |
| Mollisol | 6.0 | 13.9 | 2.5 | 29 | 142 | 18 | 35 |
| Waste | 7.9 | - | 0.9 | 2 | 576 | 0.8 | 2 |
| Ca | Al | Mg | H + Al | S | P | K | |
|---|---|---|---|---|---|---|---|
| --------------------cmolc dm−3-------------------- | -------------- mg dm−3 -------------- | ||||||
| Inceptisol | 10.9 | 0.0 | 3.1 | 2.8 | 6.1 | 28 | 142 |
| Mollisol | 7.8 | 0.0 | 2.1 | 3.5 | 5.9 | 27 | 167 |
| Waste | 24.2 | 0.0 | 1.7 | - | 12.3 | 32 | 32 |
CEC - cation exchange capacity.
OM - organic mater.
Enrichment and Isolation of Copper-Resistant Bacteria
Enrichment of copper resistant bacteria was in 100 mL of nutrient broth (NB) (5 g of Peptone and 3 g of Beef extract) in 250 mL Erlenmeyer flasks to which 300 mg L−1 of Cu(II) as copper sulfate (CuSO4.5H2O) was added and pH was adjusted to 7.0. NB was sterilized by autoclaving at 121°C for 20 min. The soil samples were independently used to inoculate (1%, w/v) sterile medium amended with Cu(II) and incubated for 24 h, with shaking (150 rpm) at 30°C. Subsequently, 1 mL of enrichment culture was used to inoculate 99 mL of sterile medium amended with Cu(II) and incubated for 24 h, with shaking (150 rpm, 30°C). This procedure was repeated two times. Cu(II)-resistant bacterial were thereafter purified by repeated streaking on nutrient agar (NA) plates containing Cu(II) (300 mg L−1). The isolates were coded with letter C for Mollisol isolates, letter N for Inceptisol isolates and letter R for waste from copper mining area.
Analysis of isolates for Cu(II)-resistance profile and biosorption
Monoculture isolates were evaluated for Cu(II)-resistance and biosorption as follows: Inoculants were prepared by transferring three loops of each isolate to NB medium amended with 300 mg L−1 of copper and incubated at 30°C for 24 h with shaking (150 rpm). After, each inoculum was adjusted with sterile saline solution (0.85%) to optical density of 0.85 (OD600) and 0.1 mL of each inoculum was added into 20 mL of NB medium containing 300 mg L−1 of Cu(II) in 50 mL Erlenmeyer and incubated (150 rpm, 24 h, 30°C). Biomass (cell density) was determined by measuring absorbance at OD600 of appropriately diluted cultures. Copper biosorption was determined by measuring copper remaining in the cell-free supernatant, using an atomic absorption spectrophotometer. Briefly, 5 mL of replicate cultures were subjected to centrifugation (10,000 rpm, 10 min). Total copper was analyzed using atomic absorption spectrometer (Perkin-Elmer 2380). Aliquots of culture supernatant (1000 µL aliquots) were diluted 20 times and injected into the atomic absorption spectrometer. Copper biosorption was calculated as the difference in total copper added to the medium and total copper remaining in the medium after different microbial treatments. (CuBiosor = CuTotal added – CuTotal after growth).
DNA based identification of isolates
Isolates were identified by 16S ribosomal RNA gene sequencing as follows. The isolates were grown by streaking on nutrient agar with incubation at 30°C for 24 h. DNA of each isolate was extracted from colonies forming units pooled from the nutrient agar plate using Promega Wizard Genomic DNA Purification Kit (Promega, Madison, WI) with slight modification. Briefly, cells were re-suspended in 300 μL of nucleic acid lyses solution, incubated at 80°C for 15 min and allowed to cool at room temperature. RNase solution (1.5 μL) was added and incubated at 37°C for 60 min. Protein precipitation solution (100 μL) was added and incubated on ice for 5 min. Following centrifugation, the supernatant was transferred to an ice cold tube with 95% ethanol. The precipitate was recovered by centrifugation. The pellet was washed with 70% ethanol at room temperature and re-suspended in sterile nuclease free distilled water. Two primers corresponding to E. coli positions 27F (5’-AGATTTGATCMTGGCTCAG-3’) and 1492R (5’-TACGGYTACCTTGTTACGAC TT-3’) were used for PCR amplification of the 16S ribosomal RNA (18). The PCR reaction mixture consisted of 12.5 μL of PCR master mix (Promega, Madison, WI), genomic DNA template (0.5 μL), primer 27F (2.5 μL=12.5 pmol), primer 1492R (2.5 μl=12.5pmol) and made up to 25 μl final volume with nuclease-free water. The 16S rRNA gene was amplified using a 35-cycle PCR (initial denaturation, 95°C for 5 min; subsequent denaturation, 95°C for 0.5 min; annealing temperature, 50°C for 1 min; extension temperature, 72°C for 1 min and final extension, 72°C for 5 min). The PCR amplification products were analyzed by electrophoresis on a 1% agarose gel. Millipore Montage PCR filter units (Millipore, Billerica, MA) were used to remove primers, salts, and unincorporated dNTPs according to the manufacturer’s instructions. DNA cycle sequencing was performed using BigDye terminator kit (Applied Biosystems, Foster City, CA) with sequencing primer 519r (5’-GWATTACCGCGGCKGCTG-3’) in independent reactions at the Institute of Integrative Genome Biology (IIGB) of UCR, Riverside, CA.
DNA Sequence Similarity and Phylogenetic Analysis
GenBank BLAST (N) was used for homology searches. Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 4.1 (30). Nucleotide sequence similarity searches were conducted by Genbank BLAST (N). The ribosomal RNA gene sequences were submitted to the GenBank database under accession numbers ranging from FJ577657 to FJ577671.
RESULTS
Biosorption of Cu(II) by isolates
Table 2 presents Cu(II) biosorption by 55 bacteria isolated from Mollisol collected from vineyard soil polluted with copper. Maximum biomass development at high copper concentration (300 mg L−1) was observed with isolate C28, C40, C41 and C44. Cu(II) biosorption was maximal in cultures of isolates C12 (62.21 mg L−1 in 24 h) and C14 (61.77 mg L−1 in 24 h). Isolate C34 displayed the lowest Cu(II) biosorption (6.48 mg L−1 in 24 h) although it grew luxuriantly (1.55 OD600) units at high concentration of Cu(II) (300 mg L−1).
Table 2.
Biomass levels and Cu(II) bioremoval in cultures of isolates from vineyard Mollisol incubated in NB medium contaminated with 300 mg L−1 of Cu(II) and incubated at 30°C for 24 h with orbital shaking.
| Isolates | Biomass | Cu(II) bioremoval | Specific Cu(II) bioremoval |
|---|---|---|---|
| ---- OD600 ---- | ---- mg L−1 ---- | --- mg L−1/OD units --- | |
| C1 | 1.16±0.0643* | 12.53±0.6330 | 11.73±0.096 |
| C2 | 1.53±0.0464 | 20.14±1.0338 | 13.25±0.848 |
| C3 | 1.71±0.0122 | 34.91±3.1758 | 20.41±1.890 |
| C4 | 1.14±0.0343 | 18.57±0.3165 | 15.76±0.712 |
| C6 | 1.57±0.0328 | 31.78±2.2971 | 20.24±1.301 |
| C7 | 1.16±0.0475 | 35.36±3.1652 | 32.10±1.762 |
| C8 | 1.45±0.0101 | 15.88±1.5826 | 11.12±1.146 |
| C9 | 1.22±0.0250 | 13.20±0.9496 | 10.88±0.494 |
| C11 | 1.31±0.0227 | 36.03±1.5826 | 26.66±1.208 |
| C12 | 1.19±0.0219 | 62.21±2.5322 | 53.71±1.773 |
| C14 | 1.16±0.0250 | 61.77±2.0675 | 53.72±2.900 |
| C15 | 0.83±0.0435 | 29.09±2.9805 | 36.83±6.043 |
| C16 | 0.85±0.0125 | 16.56±0.6330 | 19.82±0.490 |
| C17 | 0.80±0.0195 | 13.87±1.2661 | 17.00±1.020 |
| C18 | 0.64±0.0709 | 20.14±1.5826 | 33.07±2.614 |
| C20 | 0.93±0.0128 | 27.30±3.7983 | 28.83±4.064 |
| C21 | 1.58±0.0554 | 34.69±1.5826 | 21.75±0.068 |
| C22 | 0.89±0.0424 | 18.79±1.8091 | 21.13±1.781 |
| C24 | 1.01±0.0478 | 29.99±5.4273 | 29.09±5.062 |
| C28 | 1.92±0.0080 | 25.96±0.6330 | 13.59±0.371 |
| C30 | 0.84±0.0265 | 41.40±7.9131 | 51.04±8.672 |
| C31 | 1.75±0.0301 | 17.90±2.5322 | 10.59±1.560 |
| C34 | 1.55±0.0242 | 6.48±0.9496 | 4.07±0.534 |
| C35 | 1.59±0.0092 | 10.74±2.8429 | 6.71±1.746 |
| C36 | 1.67±0.0457 | 19.24±1.3675 | 11.01±0.139 |
| C37 | 1.60±0.0168 | 31.33±1.1265 | 19.81±0.194 |
| C38 | 1.03±0.0319 | 10.29±1.3675 | 9.91±1.303 |
| C39 | 1.52±0.1418 | 8.95±1.1265 | 5.79±0.223 |
| C40 | 1.91±0.0269 | 24.61±2.3260 | 12.99±1.365 |
| C41 | 1.89±0.0289 | 20.59±1.9512 | 10.90±1.057 |
| C42 | 1.65±0.1697 | 17.45±3.6458 | 10.12±2.210 |
| C43 | 1.52±0.1072 | 24.61±6.3305 | 15.68±2.827 |
| C44 | 1.91±0.0347 | 41.40±2.2157 | 22.13±1.710 |
| C45 | 1.16±0.0470 | 15.21±1.8991 | 12.20±1.367 |
| C46 | 1.00±0.0246 | 17.45±3.6458 | 17.75±3.899 |
| C47 | 1.24±0.0962 | 12.98±2.1154 | 9.27±0.596 |
| C48 | 1.63±0.0248 | 15.88±1.5826 | 9.67±0.454 |
| C49 | 1.56±0.0204 | 12.98±2.1154 | 8.30±1.341 |
| C50 | 1.39±0.0205 | 15.21±3.9787 | 11.32±0.902 |
| C52 | 1.45±0.1317 | 18.79±0.2584 | 13.85±1.607 |
| C53 | 1.45±0.0654 | 15.21±3.9787 | 11.05±3.050 |
| C54 | 1.63±0.0335 | 26.40±2.0675 | 16.12±1.155 |
| C55 | 1.72±0.0829 | 33.12±1.5720 | 19.79±1.941 |
Values are means ± standard error of the mean
Forty copper resistant bacteria were isolated from Inceptisol collected from copper contaminated vineyard area (Table 3). Growth of the isolates in media amended with 300 mg L−1 was not directly related to copper biosorption. Cell density was highest in cultures of isolate N18 (1.98 OD600 units). The highest biosorption of copper was recorded in culture of isolate N2 (80.22 mg L−1 in 24 h) while the lowest Cu(II) biosorption (5.85 mg L−1 in 24 h) was observed for the isolate N20 although cell density was high (1.41 OD600 units in 24 h).
Table 3.
Biomass levels, Cu(II) bioremoval, and specific copper bioremoval in cultures of isolates from vineyard Inceptisol incubated in NB medium contaminated with 300 mg L−1 of Cu(II) and incubated at 30°C for 24 h with orbital shaking.
| Isolates | Biomass | Cu(II) bioremoval | Specific Cu(II) bioremoval |
|---|---|---|---|
| ---- OD600 ---- | ------ mg L−1 ------ | --- mg L−1/OD units --- | |
| N1 | 1.46±0.0195* | 32.79±1.4768 | 22.01±0.957 |
| N2 | 1.45±0.0426 | 80.22±2.5696 | 53.40±2.950 |
| N3 | 1.36±0.0046 | 32.16±2.3257 | 23.75±1.776 |
| N4 | 1.43±0.0231 | 51.38±1.3427 | 36.02±1.280 |
| N5 | 1.13±0.0424 | 27.15±2.9239 | 23.65±1.838 |
| N6 | 1.20±0.0861 | 30.91±4.1770 | 27.84±5.232 |
| N7 | 1.41±0.0288 | 35.92±3.0121 | 25.31±1.773 |
| N8 | 1.38±0.0202 | 32.16±0.8861 | 23.14±0.030 |
| N9 | 1.33±0.0290 | 22.56±2.4116 | 16.97±1.805 |
| N10 | 1.40±0.0150 | 30.91±0.8980 | 22.11±0.234 |
| N11 | 0.67±0.1021 | 67.25±1.0070 | 121.83±0.900 |
| N12 | 1.44±0.0358 | 29.66±6.4979 | 21.20±4.386 |
| N13 | 1.50±0.2895 | 43.86±4.3875 | 33.68±2.649 |
| N14 | 1.61±0.0315 | 38.01±3.5030 | 23.53±2.093 |
| N16 | 1.61±0.0718 | 44.69±0.5907 | 27.22±1.822 |
| N17 | 1.46±0.0396 | 36.34±4.2869 | 24.97±2.920 |
| N18 | 1.98±0.3887 | 52.21±4.7257 | 26.10±1.003 |
| N20 | 1.41±0.0268 | 5.85±0.5000 | 4.17±0.112 |
| N22 | 1.43±0.0478 | 31.54±2.0675 | 22.54±2.503 |
| N23 | 1.40±0.2701 | 37.80±0.2954 | 26.96±0.419 |
| N24 | 1.38±0.2681 | 37.17±5.3165 | 26.44±2.927 |
| N25 | 1.38±0.2647 | 32.16±7.0886 | 23.27±5.036 |
| N26 | 1.33±0.0146 | 32.16±4.3609 | 23.96±3.019 |
| N27 | 1.30±0.0100 | 19.21±5.5151 | 14.64±4.152 |
| N28 | 1.31±0.0070 | 29.66±6.0242 | 22.75±4.735 |
| N29 | 1.36±0.0166 | 38.01±2.9338 | 28.10±2.380 |
| N30 | 1.41±0.0031 | 16.50±5.0211 | 11.72±3.553 |
| N32 | 1.68±0.0959 | 32.58±5.2559 | 18.68±2.279 |
| N33 | 1.44±0.0201 | 35.30±2.0675 | 25.07±1.261 |
| N34 | 1.39±0.0210 | 7.10±0.5907 | 4.98±0.366 |
| N35 | 1.31±0.0177 | 21.72±5.0356 | 16.28±3.634 |
| N36 | 1.29±0.0122 | 30.91±1.5060 | 23.92±1.201 |
| N38 | 1.26±0.0187 | 35.09±4.6576 | 27.56±3.245 |
| N39 | 1.39±0.0181 | 41.77±1.7390 | 30.03±1.059 |
| N40 | 1.18±0.0657 | 38.84±2.8432 | 33.48±2.701 |
Values are means ± standard error of the mean.
Copper biosorption and biomass levels in cultures of 30 bacterial isolates from copper mining waste are presented in Table 4. In general, no direct relationship was observed between amount of biomass in culture and biosorption of Cu(II) by the isolates. Bacterial cell density was highest in cultures of isolates R27 (1.20 OD600 units in 24 h), R17 (1.15 OD600 units in 24 h) and R8 (1.09 OD600 units in 24 h). Maximal Cu(II) biosorption occurred in cultures of isolates R17 (70.47 mg L−1 in 24 h) and R4 (68.34 mg L−1 in 24 h).
Table 4.
Biomass levels and Cu(II) bioremoval in cultures of isolates from copper mining waste incubated in NB medium contaminated with 300 mg L−1 of Cu(II) and incubated at 30°C for 24 h with orbital shaking.
| Isolates | Biomass | Cu(II) bioremoval | Specific Cu(II) bioremoval |
|---|---|---|---|
| ---- OD600 ---- | ----- mg L−1 ----- | --- mg L−1/OD units --- | |
| R1 | 0.81±0.0009* | 35.06±1.1920 | 43.45±1.528 |
| R2 | 0.92±0.0106 | 34.22±0.6438 | 37.48±1.236 |
| R3 | 0.94±0.0705 | 56.55±7.7480 | 36.84±0.900 |
| R4 | 0.80±0.0151 | 68.35±4.6992 | 79.70±5.918 |
| R5 | 0.84±0.0012 | 53.60±5.2693 | 67.21±6.448 |
| R6 | 0.94±0.0045 | 44.33±1.3547 | 53.06±3.562 |
| R7 | 0.82±0.0002 | 44.75±3.1631 | 49.54±1.826 |
| R8 | 1.09±0.0403 | 40.75±1.4900 | 28.98±0.993 |
| R9 | 0.89±0.0042 | 31.27±0.4214 | 35.46±2.351 |
| R10 | 0.89±0.0024 | 31.69±2.0789 | 53.35±3.408 |
| R11 | 0.84±0.0092 | 47.28±2.7094 | 62.70±3.122 |
| R12 | 0.85±0.0219 | 52.34±2.4331 | 52.10±1.854 |
| R17 | 1.15±0.0422 | 70.46±0.7299 | 76.49±3.448 |
| R18 | 0.84±0.0226 | 36.74±3.8007 | 44.77±5.862 |
| R19 | 0.73±0.0012 | 29.58±0.9733 | 40.79±1.439 |
| R20 | 0.86±0.0415 | 34.64±1.3547 | 41.18±3.019 |
| R21 | 0.82±0.0019 | 31.69±1.4800 | 38.70±1.845 |
| R22 | 0.86±0.0099 | 39.69±1.2875 | 45.96±1.089 |
| R23 | 0.67±0.1426 | 40.54±1.7032 | 44.00±1.299 |
| R24 | 0.90±0.0250 | 27.89±0.8773 | 31.30±0.851 |
| R25 | 0.85±0.0257 | 37.17±2.1212 | 44.01±3.490 |
| R26 | 0.80±0.0073 | 30.42±1.7546 | 38.03±2.596 |
| R27 | 1.20±0.0193 | 36.32±0.8429 | 30.26±0.718 |
| R28 | 0.78±0.0061 | 30.84±1.9003 | 39.43±2.634 |
| R29 | 0.79±0.0125 | 27.89±0.9733 | 36.38±0.935 |
| R30 | 0.85±0.0047 | 23.68±1.1150 | 27.96±1.447 |
Values are means ± standard error of the mean.
Specific copper removal capacity was generally high in isolates from mining waste. Isolate N11 from vineyard Inceptisol contaminated with copper, however, displayed the highest specific copper bioremoval (121.82 mg/L/OD unit) in 24 h (Table 3). This was followed by isolates R4, R5, R17 and R3 with specific copper bioremoval capacities of 79.70, 67.21, 61.46 and 64.61 mg/L/OD unit in 24 h respectively (Table 4). Statistical evaluation of copper removed by each isolate based on remaining copper in culture supernatant, however, showed that isolate N2 from copper contaminated vineyard Inceptisol was significantly higher than others isolates. Isolates N11, R4 and R17 removed similar levels of copper from the culture.
Identity of selected isolates based on 16S rRNA gene sequence
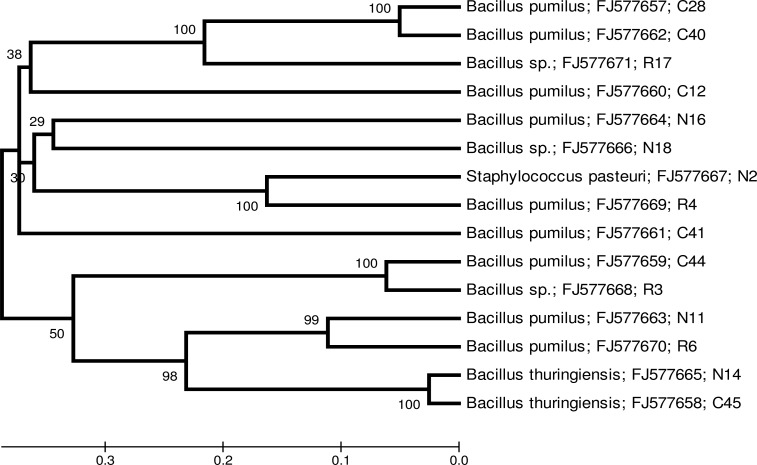
Fifteen bacterial isolates selected on the basis of copper biosorption and resistances to high copper concentration were identified by 16S rRNA gene sequence analysis. Nucleotide sequences in the range of 471-506 nucleic acid bases (Table 5) were used for Genbank blast analysis and construction of phylogenetic tree. Most of the isolates were identified as Bacillus species in the phylum Firmicutes. Nine isolates (C28; C44; C12; C40; C41; N11; N16; R4 and R6) were identified as Bacillus pumilus. Two isolates (C45 and N14) were identified as Bacillus thuringiensis and three (N18; R3 and R16) as Bacillus sp. One isolate was identified as Staphylococcus pasteuri (N2). Blast analysis revealed that isolates C44, C41, C40, N11, N2, R4 and R16 were 99% similar to the Genbank match. Isolates C28, C45, C12, N16, N14, N18 and R3 were 98% similar to the Genbank match and 97% similarity was observed for isolate R6. Figure 1 presents the phylogenetic relationship among selected isolates from three different copper contaminated areas in study.
Table 5.
DNA-based identification of isolates from different contaminated soils vineyard Mollisol (C), vineyard Inceptisol (N) and copper mining waste (R).
| Isolates | Source | 16S rDNA Nucleotides | GenBank Submition | GenBank Match | Identity (%) |
|---|---|---|---|---|---|
| C28 | Mollisol (Vineyard) | 494 | FJ577657 | EU102277.1 | B. pumilus (98) |
| C45 | Mollisol (Vineyard) | 472 | FJ577658 | EU037097.1 | B. thuringiensis (98) |
| C44 | Mollisol (Vineyard) | 476 | FJ577659 | EF528292.1 | B. pumilus (99) |
| C12 | Mollisol (Vineyard) | 478 | FJ577660 | DQ412563.1 | B. pumilus (98) |
| C41 | Mollisol (Vineyard) | 471 | FJ577661 | FJ032017.1 | B. pumilus (99) |
| C40 | Mollisol (Vineyard) | 474 | FJ577662 | AY792029.1 | B. pumilus (99) |
| N11 | Inceptisol (Vineyard) | 503 | FJ577663 | EU102277.1 | B. pumilus (99) |
| N16 | Inceptisol (Vineyard) | 506 | FJ577664 | EU855197.1 | B. pumilus (98) |
| N14 | Inceptisol (Vineyard) | 502 | FJ577665 | EU037097.1 | B. thuringiensis (98) |
| N18 | Inceptisol (Vineyard) | 505 | FJ577666 | EU821778.1 | Bacillus sp. (98) |
| N2 | Inceptisol (Vineyard) | 494 | FJ577667 | EU373331.1 | S. pasteuri (99) |
| R3 | Mining Waste | 502 | FJ577668 | EU821778.1 | Bacillus sp. (98) |
| R4 | Mining Waste | 495 | FJ577669 | EU855197.1 | B. pumilus (99) |
| R6 | Mining Waste | 495 | FJ577670 | FM179663.1 | B. pumilus (97) |
| R17 | Mining Waste | 472 | FJ577671 | DQ122328.1 | Bacillus sp. (99) |
Figure 1.
Phylogenetic tree showing evolutionary distance among selected isolates from three copper contaminated areas based on 16S rRNA gene sequence. The number at each node is the bootstrap from 100 replicates. The scale is the evolutionary distance value.
DISCUSSION
Copper is one of the toxic heavy metals of concern in the environment. Toxicity of heavy metals is largely due to their presence in aqueous systems in ionic forms, which are easily absorbed by living organisms (2, 3). There is increasing interest in the use of microbial biomass for biosorption of heavy metals from the environment. Biosorption of heavy metals involve accumulation of the metals in microbial biomass with subsequent recovery and remediation through bioremediation or chemical technologies. Copper resistant microorganisms with the capacity to adsorb copper on biomass can be used as bioremediation tools to remove copper from contaminated terrestrial and aquatic environments (1).
Several bacteria isolated in this study demonstrated tolerance to extremely high copper concentration. An Agrobacterium tumefaciens, strain CCNWRS33-2 grew in 2.0 mM or 127 mg L−1 of copper in TY liquid medium (5 g tryptone, 3 g yeast extract, and 0.7 g CaCl2·2H2O per liter), (YMA) (29) but our isolates resisted up to 300 mg L−1 of Cu(II) in nutrient broth medium, in which they have almost the same composition. The isolate A. tumefaciens CCNWRS33-2 removed 6.35 mg L−1 of Cu(II) after 36 h (29), and our best isolate (N2) removed 80 mg L−1 after 24 h, being much more efficient on copper bioremoval. One isolate was identified as Staphylococcus pasteuri. There is little or no published information on copper biosorption by the genus Staphylococcus. However, Staphylococcus warneri was isolated from sediment slurry contaminated with Se (24). Microbial communities are affected by environmental pressures which decrease natural populations and select microbes resistant to contaminants (3). Selenate reducing Bacillus species were abundant in sediment slurries from an evaporation pond heavily polluted with Se and salt (24). Bacillus is an important bacterial genus for bioremediation of heavy metals in different heavy metal contaminated areas (13, 16).
In general, the isolates from the copper mining area showed stronger capacity for copper biosorption. Isolates from copper mining waste area, R4, R17, R3 and R6 removed as much as 70 mg L−1 from liquid medium in 24 h. A P. putida CZ1 (6) and Pseudomonas sp. NA (1) removed from liquid medium 20 to 25 mg L−1 in 24 h. Staphylococcus pasteuri N2 isolated in our work from vineyard soil polluted with Cu(II) displayed the highest copper biosorption capacity and removed as high as 80 mg L−1 of Cu(II) in 24 h. On the contrary, isolate N11 (B. pumilus) showed the highest specific rate of copper biosorption calculated relative to cell density. This parity in copper removal capacity of the two isolates indicates that copper biosorption can be directly related to biomass as observed with S. pasteuri N2.
High sorptive capacity of prokaryotic (29) and eukaryotic (19) microorganisms are due to components of cell walls that offer an array of functional groups with metal binding capacity. Metals can also be accumulated in the cell cytoplasm (3), but some studies show that higher copper concentrations are linked to the cell wall than the cytoplasm in one prokaryote specie (29). Copper is one of the metals with great potential for bioremoval from contaminated environments through biosorption. In a comparative study (11) on selective binding of different metals to the cell wall of Pseudomonas sp., Cu(II) had much more affinity than other heavy metals like Ni(II), Co(II) and Cd(II) when evaluated together.
In summary, we evaluated copper biosorption by several environmental isolates of copper resistant bacteria. DNA-based methods were employed to characterize selected highly copper resistant isolates. Our results showed that several of the isolates have good potential for copper bioremoval from complex environments contaminated with copper.
Acknowledgments
Thanks to Brazilian National Research Council (CNPq) for a scholarship to Robson Andreazza and to Auburn University for the opportunity given to Robson to conduct part of his Ph.D. research. An equipment grant to Benedict Okeke to purchase a thermal cycler was received from Auburn University at Montgomery.
REFERENCES
- 1.Andreazza R., Pieniz S., Wolf L., Lee M., Camargo F.A.O., Okeke B.C. Characterization of copper biosorption and bioreduction by a highly copper resistant bacterium isolated from copper-contaminated vineyard soil. Sci. Total Environ. 2010;408(7):1501–1507. doi: 10.1016/j.scitotenv.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Arica M.Y., Bayramoglu G. Cr(VI) biosorption from aqueous solutions using free and immobilized biomass of Lentinus sajor-caju: preparation and kinetic characterization. Physicochem. Eng. A. 2005;253:203–211. [Google Scholar]
- 3.Atlas R.M., Bartha R. Microbial Ecology: Fundamentals and Applications. 4th edn. USA: Benjamin/Cummings Science Publishing; 1997. p. 656. [Google Scholar]
- 4.Camargo F.A.O., Bento F.M., Jacques R.J.S., Roesch L.F.W., Frankenberger W.T. Uso de microrganismos para a remediação de metais. Tópicos Especiais em Ciência do Solo. 2007;5:467–496. [Google Scholar]
- 5.Cervantes C., Gutierrez-Corona F. Copper resistance mechanisms in bacteria and fungi. Microbiol. Rev. 1994;14:121–138. doi: 10.1111/j.1574-6976.1994.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen X.C., Wang Y.P., Lin Q., Shi J.Y., Wu W.X., Chen Y.X. Biosorption of copper (II) and zinc (II) from aqueous solution by Pseudomonas putida CZ1. Colloid. Surface B. 2005;46:101–107. doi: 10.1016/j.colsurfb.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Chen H., Yang B., Chen X. Identification and characterization of four strains of Acidithiobacillus ferrooxidans isolated from different sites in China. Microbiol. Res. 2007 doi: 10.1016/j.micres.2007.09.002. doi:10.1016/j.micres.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Chen X.C., Wu W.X., Shi J.Y., Xu X.H., Wang H., Chen Y.X. Adsorption of copper and zinc on Pseudomonas putida CZ1: Particle concentration effect and adsorption reversibility. Colloid. Surface B. 2007;54:46–52. doi: 10.1016/j.colsurfb.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y.Q., Ren G.J., An S.Q., Sun Q.Y., Liu C.H., Ahuang J.L. Changes of bacterial community structure in copper mine tailings after colonization of reed (Phragmites communis) Pedosphere. 2008;6:731–740. [Google Scholar]
- 10.Chen X.C., Hu S.P., Shen C.F., Dou C.M., Shi J.Y., Chen Y.X. Interaction of Pseudomonas putida CZ1 with clays and ability of the composite to immobilize copper and zinc from solution. Bioresource Technol. 2009;100:330–337. doi: 10.1016/j.biortech.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 11.Choudhary S., Sar P. Characterization of a metal resistant Pseudomonas sp. isolated from uranium mine for its potential in heavy metal (Ni2+, Co2+, Cu2+, and Cd2+) sequestration. Bioresource Technol. 2009;100:2482–2492. doi: 10.1016/j.biortech.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Cojocaru C., Diaconu M., Cretescu I., Savic J., Vasic V. Biosorption of copper(II) ions from aqua solutions using dried yeast biomass. Colloid. Surface A. 2009;335:181–188. [Google Scholar]
- 13.Desai C., Jain K., Madamwar D. Evaluation of In vitro Cr(VI) reduction potential in cytosolic extracts of three indigenous Bacillus sp. isolated from Cr(VI) polluted industrial landfill. Bioresource Technol. 2008;99:6059–6069. doi: 10.1016/j.biortech.2007.12.046. [DOI] [PubMed] [Google Scholar]
- 14.Freitas O., Delerue-Matos C., Boaventura R. Optimization of Cu(II) biosorption onto Ascophyllum nodosum by factorial design methodology. J. Hazard. Mater. 2009;167(1):449–454. doi: 10.1016/j.jhazmat.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 15.He Z., Gao F., Sha T., Hu Y., He C. Isolation and characterization of a Cr(VI)-reduction Ochrobactrum sp. strain CSCr-3 from chromium landfill. J. Hazard. Mater. 2009;163:869–873. doi: 10.1016/j.jhazmat.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 16.Jiang C., Sun H., Sun T., Zhang Q., Zhang Y. Immobilization of cadmium in soils by UV-mutated Bacillus subtilis 38 bioaugmentation and NovoGro amendment. J. Hazard. Mater. 2009;167:1170–1177. doi: 10.1016/j.jhazmat.2009.01.107. [DOI] [PubMed] [Google Scholar]
- 17.Krishna K.R., Philip L. Bioremediation of Cr(VI) in contaminated soils. J. Hazard. Mater. 2005;121:109–117. doi: 10.1016/j.jhazmat.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Lane D. 16S/23S sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial sistematics. New York, USA: John Wiley and Sons; 1991. pp. 171–244. [Google Scholar]
- 19.Macfie S.M., Welbourn P.M. The cell wall as a barrier to uptake of metal ions in the unicellular green alga Chlamydomonas reinhardtii (Chlorophyceae) Arch. Environ. Contam. Toxicol. 2000;39:413–419. doi: 10.1007/s002440010122. [DOI] [PubMed] [Google Scholar]
- 20.Melgar M.J., Alonso J., García M.A. Removal of toxic metals from aqueous solutions by fungal biomass of Agaricus macrosporus. Sci. Total Environ. 2007;385:12–19. doi: 10.1016/j.scitotenv.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Michels H.T. Anti-microbial characteristics of copper. 2006. Accessed in July 29th of 2009, < http://www.astm.org/SNEWS/OCTOBER_2006/michels_oct06.html>.
- 22.Özer A., Gürbüz G., Çalimli A., Körbahti B.K. Biosorption of copper(II) ions on Enteromorpha prolifera: Application of response surface methodology (RSM) Chem. Eng. J. 2009;146:377–387. [Google Scholar]
- 23.Sari A., Mendil D., Tuzen M., Soylak M. Biosorption of palladium(II) from aqueous solution by moss (Racomitrium lanuginosum) biomass: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2009;162:874–879. doi: 10.1016/j.jhazmat.2008.05.112. [DOI] [PubMed] [Google Scholar]
- 24.Siddique T., Zhang Y., Okeke B.C., Frankenberger W.T. Characterization of sediment bacteria involved in selenium reduction. Bioresource Technol. 2006;97:1041–1049. doi: 10.1016/j.biortech.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 25.Uzel A., Ozdemir G. Metal biosorption capacity of the organic solvent tolerant Pseudomonas fluorescens TEM08. Bioresour. Technol. 2009;100:542–548. doi: 10.1016/j.biortech.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 26.Vilar V.J.P., Martins R.J.E., Botelho C.M.S., Boaventura R.A.R. Removal of Cu and Cr from an industrial effluent using a packed-bed column with algae Gelidium-derived material. Hydrometallurgy. 2009;96:42–46. [Google Scholar]
- 27.Umrania V.V. Bioremediation of toxic heavy metals using acidothermophilic autotroph. Bioresour. Technol. 2006;97:1237–1242. doi: 10.1016/j.biortech.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 28.Voss M., Thomas R.W.S.P. Sorção de cobre e manganês por bactérias rizosféricas do trigo. Ciência Rural. 2001;31:947–951. [Google Scholar]
- 29.Wei G., Fan L., Zhu W., Fu Y., Yu J., Tang M. Isolation and characterization of the heavy metal resistant bacteria CCNWRS33–2 isolated from root nodule of Lespedeza cuneata in gold mine tailings in China. J. Hazard. Mater. 2009;162:50–56. doi: 10.1016/j.jhazmat.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 30.Tamura K., Dudley J., Nei M., Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]