Abstract
Representative strains of Serratia marcescens from an edible cactus plant and silkworms were characterized and a comparison based on their cellular fatty acid composition, 16S rRNA and groE gene sequence analysis as well as silkworm virulence and chitosan susceptibility was carried out. Results from this study indicate that there are no significant differences between the phenotypic and molecular characterization, virulence and chitosan susceptibility of the S. marcescens strains from the cactus plant and silkworms. Silkworms inoculated with S. marcescens from either plant or silkworm resulted in nearly 100% mortality. Chitosan solution exhibited strong antibacterial activity against S. marcescens. This activity increased with the increase of chitosan concentration and incubation time regardless of the strain source. Also, the results indicate that the plant associated S. marcescens maybe plays a possible role in the contamination of humans and animals, in particular silkworms, while chitosan showed a potential to control the contamination caused by S. marcescens.
Keywords: Serratia marcescens, Cactus, Silkworm, Characterization, Virulence, Chitosan susceptibility
INTRODUCTION
Various plant-associated roles have been put forward for Serratia marcescens, including that of a plant pathogenic bacterium (19); an herbicide degradation bacterium (24); a plant growth promoting rhizobacterium (23); an innocuous colonizer or endophyte of plants (25); and even a biocontrol agent (2, 20). However, the enteric bacterium S. marcescens is also an opportunistic human and insect, in particular silkworm, pathogen (5, 7, 9, 15). Therefore, it is necessary to examine the differences between S. marcescens strains from plants and that from humans or animals, in particular silkworms.
Silkworms have been used as model animals for studying bacterial pathogenicity in humans (10), which made it possible for us to evaluate the virulence potential of plant associated S. marcescens in human or animal hosts. In addition, the abilities of S. marcescens to cause animal and human infections and survive in the environment have been partially attributed to its high natural resistance to antimicrobials and cleaning agents (8). Interestingly, chitosan is been well known for its broad antimicrobial activity (3, 11) and has shown a potential to control bacterial septicemic disease of silkworms caused by S. marcescens (14). Therefore, the bactericidal activity of chitosan against plant associated S. marcescens was examined in this work.
The aim of this study is to examine and compare phenotypic and molecular characterization as well as the virulence potential and chitosan susceptibility between the S. marcescens strains from an edible cactus and from silkworms.
MATERIALS AND METHODS
Strains of S. marcescens
Two representative strains of S. marcescens were used in this study; one from a plant and the other from an animal. Strain ZJ-C0701 of S. marcescens was isolated from the healthy tissue of edible cactus plants (Opuntia Milpa Alta) grown in Zhejiang province of China. Strain ZJ-S0801 of S. marcescens was kindly provided by the College of Animal Science, Zhejiang University, which was isolated from diseased silkworms.
Phenotypic characterization
Classical bacteriological tests were conducted as described by Schaad et al. (22). The bacterial strains were then grown for 24 h at 28 °C on TSBA (13) for analyses of fatty acid methyl ester (FAME; MIDI, Inc., Newark, DE) and substrate utilization (BIOLOG, Inc., Hayward, CA) profiles, which were performed following manufacturers’ instructions (13). The BIOLOG tests were carried out using substrate plates designed for gram-negative bacteria. FAME testing was repeated a total of three times, and BIOLOG twice.
Molecular characterization
The 16S rRNA and groE gene of the bacterial strains were amplified and sequenced as described by Li et al. (12) and Harada and Ishikawa (6), respectively. Phylogenetic analysis was performed after including the consensus sequence in an alignment of small ribosomal subunit sequences collected from the international nucleotide sequence library GenBank. Nucleotides of the 16S rRNA and groE gene were aligned using CLUSTAL W. Phylogenetic and molecular evolutionary analyses were conducted using the genetic distance-based neighbor-joining algorithms within MEGA version 4.0 (http://www.megasoftware.net/). Bootstrap analysis for 1000 replicates was performed to estimate the confidence of tree topology.
Virulence potential against silkworm
Cultivation of bacteria:
The bacterial strains were cultured for 48 h on nutrient agar medium at 28 °C. After incubation, each bacterial suspension was prepared in sterilized water, and the initial concentration of bacteria was adjusted to approximately 108 colony forming units (CFU)/ml. All bacterial strains involved in this study were deposited in the culture collection of the Institute of Biotechnology, Zhejiang University, China.
Rearing of silkworms:
Hybrid strain larvae of silkworm (commercial name: Qingsong Haoyue) were reared at 25 °C in this study. Fresh mulberry leaves (average size: 10 cm × 20 cm) were obtained from a local mulberry farm (Hangzhou, China). Silkworm larvae were fed sufficient fresh mulberry leaves until the fifth instar and then were used for in vivo experiments.
The pathogenicity to silkworm:
The virulence potential of the S. marcescens strains from both plant and silkworms were examined by inoculating them in silkworms, which were used as model animals for studying bacterial pathogenicity. The healthy larvae of uniform size and age were inoculated by pricking them at the third abdominal segment with sterile needles that had been dipped into 1.0 ml of bacterial suspension (107 CFU/ml). The experiment was carried out in a randomized block design with three replicates of each treatment, with twenty larvae. Control larvae were inoculated with sterile water. Untreated healthy larvae were used as additional controls.
Chitosan susceptibility
Preparation of chitosan stock: Chitosan (degree of N-deacetylation no less than 85%, practical grade, from crab shells) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Stock solution of chitosan (5 mg/ml) was prepared in 1% acetic acid with pH being adjusted to 6.0 with NaOH (11). After stirring (160 rpm) for 24 h at room temperature, the stock solution was autoclaved at 121 °C for 20 min. Sterile deionized water of pH 6.0 was used as a control.
Surviving cells count:
Bacterial suspensions were ten-fold serially diluted and 10 μl samples were inoculated on nutrient agar medium in hexaplicate for each dilution and were incubated for 48 h at 28 °C. After incubation, the surviving cells on the agar were counted based on the colony forming units and then the mean value of the cells at the lowest dilution was calculated. Each experiment was carried out in duplicate and was replicated twice.
Effect of chitosan concentration:
Chitosan solutions of 5 ml in volume were prepared by adding chitosan stock to sterile deionized water to give a final chitosan concentration of 0.01, 0.05 and 0.10 mg/ml. Bacterial solution was added to 5 ml of chitosan solution to give a final bacterial concentration of 107 CFU/ml and then the mixture was incubated at 28 °C in a rotary shaker (Hualida Company, Taicang, China) at 160 rpm. In the control treatment chitosan stock was replaced with sterile deionized water of pH 6.0 in order to obtain the same pH. Two hours later, samples were collected from each cell suspension and bacterial counting was carried out as above.
Effect of incubation time:
Chitosan solutions of 5 ml in volume were prepared by adding 100 μl chitosan stock to 4.90 ml sterile deionized water to give a final chitosan concentration of 0.10 mg/ml. Bacterial strains were selected and inoculated into chitosan solution as described above. In the control treatment chitosan stock was replaced with sterile deionized water of pH 6.0 in order to obtain the same pH. Antibacterial activity of chitosan solution on the growth of S. marcescens was determined after 0.5, 1.0, 2.0 and 4.0 h of incubation.
Statistical analysis:
The software STATGRAPHICS Plus, version 4.0 (Copyright Manugistics Inc., Rockville, Md., USA) was used to perform the statistical analysis. Levels of significance (P<0.05) of main treatments and their interactions were calculated by analysis of variance after testing for normality and variance homogeneity.
RESULTS AND DISCUSSION
Characterization of S. marcescens
Results from this study indicated that there were no significant differences between the phenotypic and molecular characteristics of the S. marcescens strains from the cactus plant and silkworms. Both two strains formed red, smooth, convex, entire and round colonies on nutrient agar. Classical bacteriological tests showed that they were gram-negative, rod shaped and motile organisms. The fatty acid profiles of strain ZJ-C0701 were very similar to those of strain ZJ-S0801 (Table 1). Comparison of the fatty acid composition of strain ZJ-C0701 and ZJ-S0801 with species from the bacteria database of the Microbial Identification System (Microbial ID, Inc.) gave a similarity index value of 0.56 and 0.58 with S. marcescens, respectively. However, it seems that the Biolog profiles are not suitable for the characterization of this bacterium due to the influence of pigment. The partial 16S rRNA and groE gene sequence of strain ZJ-C0701 (EMBL accession No. FM883708, FM946180) and strain ZJ-S0801 (EMBL accession No. FM883709, FM946181) were determined and aligned to other known Enterobacteriaceae sequences deposited in GenBank (6). In the phylogenetic analysis, the two strains and S. marcescens were clustered within a group and were well separated from either the other Serratia species based on partial 16S gene sequences (Fig. 1) or the other genus of Enterobacteriaceae based on partial groE gene sequences (Fig. 2).
Table 1.
Analysis of cellular fatty acids of strain ZJ-C0701 of S. marcescens from edible cactus plant and comparison with strain ZJ-S0801 of S. marcescens from silkworm
| Fatty acid | Cellular fatty acids (%) | |
|---|---|---|
| ZJ-C0701 | ZJ-S0801 | |
| 10:0 | 0.23 | 0.25 |
| 10:0 3OH | 3.01 | 2.89 |
| 12:0 | 1.12 | 1.38 |
| 12:0 2OH | 0.40 | 0.51 |
| 12:0 3OH | 1.51 | 1.35 |
| 14:0 | 4.86 | 5.16 |
| 14:0 2OH | 3.80 | 2.10 |
| 14:0 3OH | 7.05 | 8.12 |
| 16:0 | 30.80 | 28.55 |
| 16:0 3OH | 0.18 | 0.22 |
| 16:1 w7c | 11.46 | 12.20 |
| 17:0 | 1.31 | 1.19 |
| 17:0 cyclo | 15.26 | 14.71 |
| 18:0 | 0.39 | 0.26 |
| 18:1 w7c | 15.12 | 16.58 |
Figure 1.
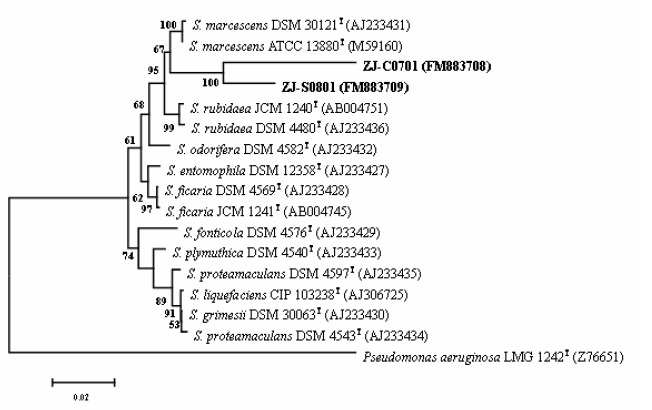
Phylogenetic tree derived from partial 16S rRNA gene sequence analysis on strain ZJ-C0701 and ZJ-S0801 as well as reference strains of each Serratia species. The tree was generated by the neighbor-joining method based on the two-parameter Kimura correction of evolutionary distances. Bootstrap analyses (1000 replicates) for node values from 50% are indicated. Pseudomonas aeruginosa was used as the outgroup.
Figure 2.
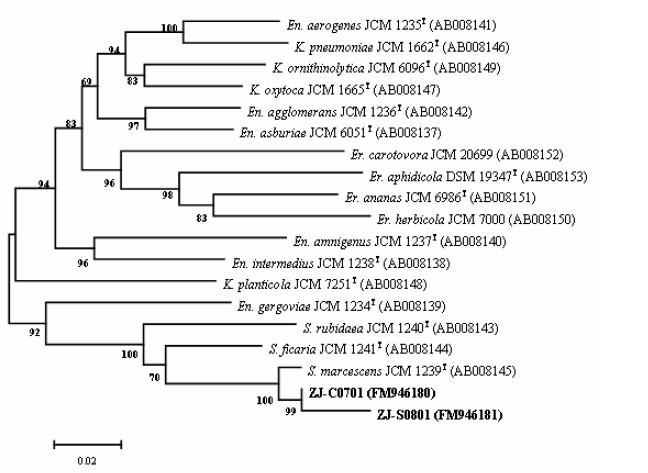
Phylogenetic tree derived from partial groE gene sequences analysis on strain ZJ-C0701 and ZJ-S0801 as well as reference strains of the genus Serratia (S.), Erwinia (Er.), Enterobacter (En.) and Klebsiella (K.). The tree was generated by the neighbor-joining method based on the two-parameter Kimura correction of evolutionary distances. Bootstrap analyses (1000 replicates) for node values from 50% are indicated.
Comparison of virulence potential
The silkworm larvae inoculated with strain ZJ-S0801 resulted in 73.3%, 86.7% and 93.3% mortality, while inoculation with strain ZJ-C0701 resulted in 93.3%, 96.7% and 96.7% mortality after 24, 48 and 72 h of rearing, respectively. In addition, the causal bacteria reisolated from inoculated silkworm larvae have morphology identical to those of the original inoculated culture of S. marcescens, which implied that there was no significant difference in virulence between the S. marcescens strains from the cactus plant and silkworms. The mortality of the larvae treated with sterile water and uninoculated control was 0% regardless of the rearing time. Thus, these results suggest that the plant strain maybe play a possible role in the contamination of humans and animals.
In agreement with the results of this study, vegetable plants as a habitat for beneficial and/or human pathogenic bacteria have received considerable attention (1, 17, 18). Indeed, increasing numbers of foodborne illness outbreaks have been traced to the consumption of plant-derived foods. While a number of outbreaks caused by Escherichia coli O157:H7, Listeria monocytogenes and Salmonella enterica have been linked to the consumption of contaminated fruit and vegetable produce (4, 16, 21). However, this is first report that plant associated S. marcescens maybe is a potential risk for humans and animal health.
Comparison of chitosan susceptibility
Chitosan solution at different concentrations showed effective antibacterial activity against S. marcescens strains isolated from both the cactus plant and silkworms. In addition, chitosan solutions up to 0.10 mg/ml showed stronger antibacterial activity against S. marcescens compared with the remainder treatment, which is consistent with the result of Li et al. (11), who found that the antibacterial activity of chitosan was influenced by its concentration in the solution. The surviving cell numbers of strain ZJ-C0701 in chitosan solution of 0.01 mg/ml decreased 1.74 log10 CFU/ml, while the surviving cell numbers in chitosan solution of 0.10 mg/ml decreased 2.24 log10 CFU/ml compared to the control (Fig. 3). Similarly, the surviving cell numbers of strain ZJ-S0801 in chitosan solution of 0.01 mg/ml decreased 1.92 log10 CFU/ml, while the surviving cell numbers in chitosan solution of 0.10 mg/ml decreased 2.61 log10 CFU/ml compared to the control (Fig. 4).
Figure 3.
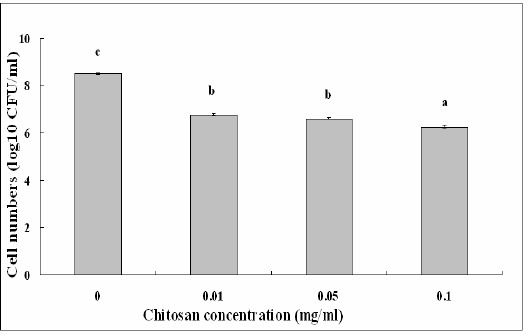
Effect of chitosan concentration on the antibacterial activity of strain ZJ-C0701 of S. marcescens. Columns with the same letters are not significantly different (P<0.05). Error bars represent the standard error of the mean (n = 6). Data are from a representative experiment repeated twice with similar results.
Figure 4.
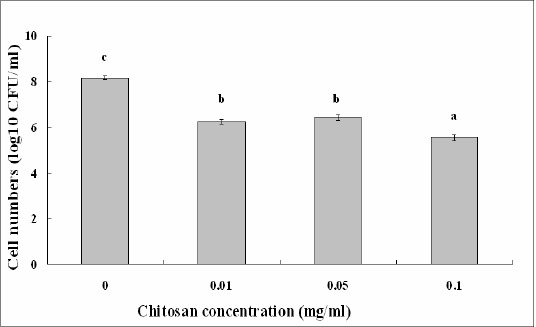
Effect of chitosan concentration on the antibacterial activity of strain ZJ-S0801 of S. marcescens. Columns with the same letters are not significantly different (P<0.05). Error bars represent the standard error of the mean (n = 6). Data are from a representative experiment repeated twice with similar results.
The antibacterial activity of chitosan against S. marcescens strains was affected by the incubation time. In the absence of chitosan, the surviving cell numbers of strain ZJ-C0701 in sterile deionized water decreased 0.18 log10 CFU/ml after 0.5 h of incubation compared with the starting value of 7.53 log10 CFU/ml. With the increase of incubation time, the surviving cell numbers remained stable (Table 2). In the presence of chitosan, the surviving cell numbers decreased significantly compared to the starting value. The antibacterial activity of the chitosan solution of 0.10 mg/ml increased with the incubation time through to 4.0 h. After 0.5 h of incubation, the surviving cell numbers in the chitosan solution decreased 1.20 log10 CFU/ml compared to the starting value and after 4.0 h of incubation, the surviving cell numbers in the chitosan solution decreased 2.78 log10 CFU/ml compared to the starting value (Table 2), which shows that a certain incubation time is required for the antibacterial activity of chitosan solution to take effect.
Table 2.
Effect of incubation time on the antibacterial activity of chitosan solution at 0.10 mg/ml against strain ZJ-C0701 of S. marcescens
| Incubation time (h) | Cell numbers (log10 CFU/ml) | |
|---|---|---|
| Control | Chitosan | |
| 0.0 | 7.53 ± 0.15a | 7.53 ± 0.15d |
| 0.5 | 7.35 ± 0.11a 6.33 ± 0.10c | 7.35 ± 0.11a 6.33 ± 0.10c |
| 1.0 | 7.20 ± 0.13a | 6.21 ± 0.09c |
| 2.0 | 7.22 ± 0.10a | 5.78 ± 0.10b |
| 4.0 | 7.27 ± 0.10a | 4.75 ± 0.09a |
The data were shown as means α standard error from a representative experiment repeated twice with similar results. All the means within a column followed by the same letter are not significantly different (P<0.05, Fisher’s LSD test). Each value represents the average of six replicates.
In agreement with the antibacterial effect of chitosan solution against strain ZJ-C0701, the surviving cell numbers of strain ZJ-S0801 remained stable in the absence of chitosan (Table 3). In the presence of chitosan, the surviving cell numbers decreased significantly compared to the starting value. The antibacterial activity of the chitosan solution of 0.10 mg/ml against strain ZJ-S0801 increased with the incubation time through to 4.0 h. The surviving cell numbers in the chitosan solution decreased 1.36 log10 CFU/ml after 0.5 h of incubation, and they decreased 2.40 log10 CFU/ml after 4.0 h of incubation compared to the starting value (Table 3).
Table 3.
Effect of incubation time on the antibacterial activity of chitosan solution at 0.10 mg/ml against strain ZJ-S0801 of S. marcescensa
| Incubation time (h) | Cell numbers (log10 CFU/ml) | |
|---|---|---|
| Control | Chitosan | |
| 0.0 | 7.69 ± 0.17a | 7.69 ± 0.17d |
| 0.5 | 7.37 ± 0.17a | 6.33 ± 0.12c |
| 1.0 | 7.59 ± 0.17a | 6.31 ± 0.10c |
| 2.0 | 7.53 ± 0.11a | 5.97 ± 0.09b |
| 4.0 | 7.53 ± 0.13a | 5.29 ± 0.10a |
The data were shown as means α standard error from a representative experiment repeated twice with similar results. All the means within a column followed by the same letter are not significantly different (P<0.05, Fisher’s LSD test). Each value represents the average of six replicates.
The results from this study indicate that there was no significant difference in chitosan susceptibility between the S. marcescens strains from the cactus plant and silkworms. In the last three decades there has been a steady increase in nosocomial S. marcescens infections that can be life-threatening to both animals and humans (7). As many S. marcescens strains are also resistant to multiple antibiotics (5, 7, 15), it represents a growing problem for animal and public health. Considering the absence of any sort of remedial measures for S. marcescens infections, the present investigation may prove helpful. The antibacterial activity of chitosan may be enhanced by combination with radiation processing in the control of S. marcescens.
CONCLUSION
In summary, our results clearly demonstrated that there was no significant difference between the phenotypic and molecular characterization, virulence and chitosan susceptibility of the S. marcescens strains from the cactus plant and silkworms, which indicated that the plant strain maybe play a possible role in the contamination of human and animals alike. In addition, results from this study showed that a chitosan solution has a strong antibacterial activity against plant associated S. marcescens, which will be helpful in the control of contaminated fruit and vegetable produce.
ACKNOWLEDGEMENTS
This project was supported by Zhejiang Provincial Natural Science Foundation of China (Y3090150), the Fundamental Research Funds for the Central Universities (KYJD09022), National Natural Science Foundation of China (30600475) and the Agricultural Ministry of China (nyhyzx200803010).
REFERENCES
- 1.Caggia C., Randazzo C.L., Di Salvo M., Romeo F., Giudici P. Occurrence of Listeria monocytogenes in green table olives. J. Food Prot. 2004;67:2189–2194. doi: 10.4315/0362-028x-67.10.2189. [DOI] [PubMed] [Google Scholar]
- 2.de Queiroz B.P.V., de Melo I.S. Antagonism of Serratia marcescens towards Phytophthora parasitica and its effects in promoting the growth of citrus. Braz. J. Microbiol. 2006;37:448–450. [Google Scholar]
- 3.Fang Y., Lou M.M., Li B., Xie G.L., Wang F., Zhang L., Luo Y.C. Characterization of Burkholderia cepacia complex from cystic fibrosis patients in China and their chitosan susceptibility. World J. Microb. Biot. 2010;26:443–450. [Google Scholar]
- 4.Gorski L., Palumbo J.D., Nguyen K.D. Strain-specific differences in the attachment of Listeria monocytogenes to alfalfa sprouts. J. Food Prot. 2004;67:2488–2495. doi: 10.4315/0362-028x-67.11.2488. [DOI] [PubMed] [Google Scholar]
- 5.Guimaraes M.A., Tibana A., Nunes M.P., dos Santos K.R.N. Disinfectant and antibiotic activities: A comparative analysis in Brazilian hospital bacterial isolates. Braz. J. Microbio. 2000;31:193–199. [Google Scholar]
- 6.Harada H., Ishikawa H. Phylogenetical relationship based on groE genes among phenotypically related Enterobacter, Pantoea, Klebsiella, Serratia and Erwinia species. J. Gen. Appl. Microbiol. 1997;43:355–361. doi: 10.2323/jgam.43.355. [DOI] [PubMed] [Google Scholar]
- 7.Hejazi A., Falkiner Falkiner, F.R. Serratia marcescens. J. Med. Microbiol. 1997;46:903–912. doi: 10.1099/00222615-46-11-903. [DOI] [PubMed] [Google Scholar]
- 8.Iranshahi M., Shahverdi A.R., Mirjani R., Amin G., Shafiee A. Umbelliprenin from Ferula persica roots inhibits the red pigment production in Serratia marcescens. Z. Naturforsch C. 2004;59:506–508. doi: 10.1515/znc-2004-7-809. [DOI] [PubMed] [Google Scholar]
- 9.Iwaya A., Nakagawa S., Iwakura N., Taneike I., Kurihara M., Kuwano T., Gondaira F., Endo M., Hatakeyama K., Yamamoto T. Rapid and quantitative detection of blood Serratia marcescens by a real-time PCR assay: Its clinical application and evaluation in a mouse infection model. FEMS Microbiol. Lett. 2005;248:163–170. doi: 10.1016/j.femsle.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 10.Kaito C., Akimitsu N., Watanab H., Sekimizu K. Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb. Pathog. 2002;32:183–190. doi: 10.1006/mpat.2002.0494. [DOI] [PubMed] [Google Scholar]
- 11.Li B., Wang X., Chen R.X., Huangfu W.G., Xie G.L. Antibacterial activity of chitosan solution against Xanthomonas pathogenic bacteria isolated from Euphorbia pulcherrima. Carbohyd. Polym. 2008b;72:287–292. [Google Scholar]
- 12.Li B., Xie G.L., Zhang J.Z., Janssens D., Swings J. Identification of the bacterial leaf spot pathogen of poinsettia in China. J. Phytopathol. 2006;154:711–715. [Google Scholar]
- 13.Li B., Xu L.H., Lou M.M., Li F., Zhang Y.D., Xie G.L. Isolation and characterization of antagonistic bacteria against bacterial leaf spot of Euphorbia pulcherrima. Lett. Appl. Microbiol. 2008a;46:450–455. doi: 10.1111/j.1472-765X.2008.02337.x. [DOI] [PubMed] [Google Scholar]
- 14.Li B., Su T., Chen X.L., Liu B.P., Zhu B., Fang Y., Qiu W., Xie G.L. Effect of chitosan solution on the bacterial septicemia disease of Bombyx mori (Lepidoptera: Bombycidae) caused by Serratia marcescens. Appl. Entomol. Zool. 2009;45:145–152. [Google Scholar]
- 15.Loureiro M.M., de Moraes B.A., Quadra M.R.R., Pinheiro G.S., Asensi M.D. Study of multi-drug resistant microorganisms isolated from blood cultures of hospitalized newborns in Rio de Janeiro city, Brazil. Braz. J. Microbiol. 2002;33:73–78. [Google Scholar]
- 16.Milillo S.R., Badamo J.M., Boor K.J., Wiedmann M. Growth and persistence of Listeria monocytogenes isolates on the plant model Arabidopsis thaliana. Food Microbiol. 2008;25:698–704. doi: 10.1016/j.fm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Rahme L.G., Ausubel F.M., Cao H., Drenkard E., Goumnerov B.C., Lau G.W., Mahajan-Miklos S., Plotnikova J., Tan M.W., Tsongalis J., Walendziewicz C.L., Tompkins R.G. Plants and animals share functionally common bacterial virulence factors. Proc. Natl. Acad. Sci. USA. 2000;97:8815–8821. doi: 10.1073/pnas.97.16.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahme L.G., Stevens E.J., Wolfort S.F., Shao J., Tompkins R.G., Ausubel F.M. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 19.Rascoe J., Berg M., Melcher U., Mitchell F.L., Bruton B.D., Pair S.D., Fletcher J. Identification, phylogenetic analysis, and biological characterization of Serratia marcescens strains causing cucurbit yellow vine disease. Phytopathology. 2003;93:1233–1239. doi: 10.1094/PHYTO.2003.93.10.1233. [DOI] [PubMed] [Google Scholar]
- 20.Roberts D.P., McKenna L.F., Lakshman D.K., Meyer S.L.F., Kong H., de Souza J.T., Lydon J., Baker C.J., Buyer J.S., Chung S. Suppression of damping-off of cucumber caused by Pythium ultimum with live cells and extracts of Serratia marcescens N4-5. Soil Biol. Biochem. 2007;39:2275–2288. [Google Scholar]
- 21.Samadpour M., Barbour M.W., Nguyen T., Cao T.M., Buck F., Depavia G.A., Mazengia E., Yang P., Alfi D., Lopes M., Stopforth J.D. Incidence of enterohemorrhagic Escherichia coli, Escherichia coli O157, Salmonella, and Listeria monocytogenes in retail fresh ground beef, sprouts, and mushrooms. J. Food Prot. 2006;69:441–443. doi: 10.4315/0362-028x-69.2.441. [DOI] [PubMed] [Google Scholar]
- 22.Schaad N.W., Jones J.B., Chun W. Laboratory guide for identification of plant pathogenic bacteria. 3rd Edition. St. Paul, Minn: American Phytopathological Society; 2001. [Google Scholar]
- 23.Selvakumar G., Mohan M., Kundu S., Gupta A.D., Joshi P., Nazim S., Gupta H.S. Cold tolerance and plant growth promotion potential of Serratia marcescens strain SRM (MTCC 8708) isolated from flowers of summer squash (Cucurbita pepo) Lett. Appl. Microbiol. 2008;46:171–175. doi: 10.1111/j.1472-765X.2007.02282.x. [DOI] [PubMed] [Google Scholar]
- 24.Silva T.M., Stets M.I., Mazzetto A.M., Andrade F.D., Pileggi S.A.V., Favero P.R., Cantu M.D., Carrilho E., Carneiro P.I.B., Pileggi M. Degradation of 2,4-D herbicide by microorganisms isolated from Brazilian contaminated soil. Braz. J. Microbiol. 2007;38:522–525. [Google Scholar]
- 25.Tan Z., Hurek T., Gyaneshwar P., Ladha J.K., Reinhold-Hurek B. Novel endophytes of rice form a taxonomically distinct subgroup of Serratia marcescens. Syst. Appl. Microbiol. 2001;24:245–251. doi: 10.1078/0723-2020-00002. [DOI] [PubMed] [Google Scholar]


