Abstract
Valencia orange (Citrus sinensis) peel was employed in this work as raw material for the production of citric acid (CA) by solid-state fermentation (SSF) of Aspergillus niger CECT-2090 (ATCC 9142, NRRL 599) in Erlenmeyer flasks. To investigate the effects of the main operating variables, the inoculum concentration was varied in the range 0.5·103 to 0.7·108 spores/g dry orange peel, the bed loading from 1.0 to 4.8 g of dry orange peel (corresponding to 35-80 % of the total volume), and the moisture content between 50 and 100 % of the maximum water retention capacity (MWRC) of the material. Moreover, additional experiments were done adding methanol or water in different proportions and ways. The optimal conditions for CA production revealed to be an inoculum of 0.5·106 spores/g dry orange peel, a bed loading of 1.0 g of dry orange peel, and a humidification pattern of 70 % MWRC at the beginning of the incubation with posterior addition of 0.12 mL H2O/g dry orange peel (corresponding to 3.3 % of the MWRC) every 12 h starting from 62 h. The addition of methanol was detrimental for the CA production. Under these conditions, the SSF ensured an effective specific production of CA (193 mg CA/g dry orange peel), corresponding to yields of product on total initial and consumed sugars (glucose, fructose and sucrose) of 376 and 383 mg CA/g, respectively. These results, which demonstrate the viability of the CA production by SSF from orange peel without addition of other nutrients, could be of interest to possible, future industrial applications.
Keywords: Orange peel, citric acid, Aspergillus niger, solid-state fermentation
INTRODUCTION
Today, citrus is unrolling in almost all regions of the world inside the strip bounded by a line of latitude 40 degrees N and S. The citrus processing industry yearly generates tons of residues, including peel and segment membranes, from the extraction of citrus juice in industrial plants. The management of these wastes, which produce odor and soil pollution, represents a major problem for the food industry (33). Orange peel contains soluble sugars and pectin as the main components. According to Rivas et al. (42), the orange peel is in fact constituted by soluble sugars, 16.9 % wt; starch, 3.75 % wt; fiber (cellulose, 9.21 % wt; hemicelluloses, 10.5 % wt; lignin 0.84 % wt; and pectins, 42.5 % wt), ashes, 3.50 % wt; fats, 1.95 % wt; and proteins, 6.50 % wt.
The total sugar content of orange peel varies between 29 and 44 % (21), soluble and insoluble carbohydrates being the most abundant and economically interesting constituents of this residue (26). Approximately 50 % of the dry weight of orange is soluble in alcohol (47), and soluble sugars are the major components also of this fraction. Glucose, fructose and sucrose are the main sugars, although xylose can also be found in small quantities in orange peel. Insoluble polysaccharides in orange peel are composed of pectin, cellulose and hemicelluloses. Pectin and hemicelluloses are rich in galacturonic acid, arabinose and galactose, but they also contain small amounts of xylose, glucose, and perhaps rhamnose (16,33). Glucose is the dominant sugar in the cellulosic fraction, which also contains some quantities of xylose and arabinose, traces of galactose and uronic acids, and in some instances mannose. On the other hand, lignin seems to be absent in these tissues. Consequently, a mixture of cellulases and pectinases is needed to complete the conversion of all polysaccharides to monosaccharides (15,16).
Citric acid (CA), an intermediate of the tricarboxylic acid cycle, is found in a variety of acidic fruit juices, particularly in the citric ones, although its extraction from natural sources, primarily lemon, was gradually replaced by biological procedures, mainly based on the use of microfungi, which are currently the most widely used. The production of CA was described in 1893 by Wehmer as a result of the metabolism of the fungus Penicillium glaucum (49). In 1913, it was obtained the first patent in the United States for a method of producing CA by Aspergillus niger in sugar solutions. Recent estimates put the global production of CA in over 1.4 million tons per year (49) with rising trend in demand. More than 50 % of this volume is being produced in China. It is traditionally used in the food industry thanks to its high solubility, extremely low toxicity, and palatability; moreover, examples are given of some recent CA applications in the industry of detergents and cosmetics, or as the active ingredient in some bathroom and kitchen cleaning solutions (56).
The low cost and the high carbohydrate content and susceptibility to fermentation make citrus byproducts attractive raw materials for CA biotechnological production (42). In most cases, the industrial production of CA by fermentation is done using A. niger strains, but also many other microorganisms are capable of accumulating CA, including other species belonging the same genus, Penicillium janthinellum, Penicillium restrictum, Trichoderma viride, Mucor pirifromis, Ustulina vulgaris and various species of the genera Botrytis, Ascochyta, Absidia, Talaromyces, Acremonium and Eupenicillium (25). There are some processes that use various species of yeast (mainly belonging to the genus Candida) or bacteria and a wide range of carbon sources, including sucrose, glucose, molasses, alcohol, fatty acids, natural oils, acetate, and hydrocarbons (4). Additionally, some attempts have been made to induce CA overproduction by mutations of different microorganisms, particularly A. niger strains (31,46). Aravantinos-Zafiris et al. (5) examined three different strains of A. niger and found that the strain NRRL 599 was the best CA producer, followed by NRRL 364 and NRRL 567, respectively.
CA has been successfully produced using submerged, liquid surface or solid state fermentation (SSF), with the best results being obtained in this last case (36). In spite the SSF was the first process proposed for the production of CA using different absorbing materials (beet pulp, sugar cane bagasse, pineapple pulp) with embedded solutions of carbohydrates (mainly sucrose-rich solutions), CA has been conventionally produced by submerged fermentation, mainly by A. niger. However, because of several advantages over the submerged fermentations such as solid waste management, biomass energy conservation, production of high value products and little risk of bacterial contamination (44), the SSF methods have recently gained attention using agroresidues like sugarcane or cassava bagasse (29, 30, 38, 46), carob pod (44), areca husk (36), beet molasses (1), soy residues (27), sugar cane bagasse, coffee husk and cassava bagasse (55) and waste of food processing industries including pineapple wastes (6, 11, 18, 22, 52, 53), apple pomace (20, 48), grape pomace (19), or different fruit peels, including kiwi (17), orange (43) or prickly pear (12).
The main characteristics of SSF that differentiate it from submerged cultures are the low water content, which is usually related to low values of water activity, especially for hydrophylic supports, and the enhanced aeration. The O2 and CO2 exchange between the gas phase and the substrate depends on the intra- and inter-particle mass transfer in SSF systems, which is influenced by various factors (8,14): a) the matrix porosity that depends on its physical characteristics and water content; b) the pore size and particle diameter that influence the area of interchange; c) the system geometry; and d) the aeration and the agitation, especially when the fermentation is advanced.
Following a previous study (42), where the bioproduction of CA by A. niger NRRL 599 was studied in submerged culture using a medium prepared after sugars solubilization by orange peel autohydrolysis, in this work we investigated the potential of such a residue as a substrate for CA production by solid-state fermentation by the same microorganism. To this purpose, we investigated the effects of inoculum concentration, bed loading, and water and methanol addition on CA production and culture performance. In comparison to other related works, no nutrients were added to the fermentation broth in order to minimize the costs of production. Finally, the results of CA production by SSF were compared with those previously obtained in submerged culture.
MATERIALS AND METHODS
Raw material
Samples of Valencia orange (Citrus sinensis) peel obtained from a national citrus processing plant were dried at 40 °C to reach a final moisture lower than 10 %, milled to a particle size less than 2 mm, homogenized in a single lot to avoid any variation in composition, and stored at 4 °C in a cold chamber until use.
Microorganism
Aspergillus niger CECT-2090 (ATCC 9142, NRRL 599), obtained from the Spanish Collection of Type Cultures (Valencia, Spain), was used in this work.
Inoculum
The fungus was grown on slants of potato dextrose agar (Scharlau Chemie, Barcelona, Spain) at 33 °C for 5 days. A spore inoculum was prepared by adding sterile distilled water to the slant, shaking vigorously for 1 min with the help of sterile glass balls to prepare the spore suspension, and filtering to eliminate mycelium particles. Spores were quantified by optical density measurement at 750 nm using a calibration curve.
Maximum water retention capacity
Before SSF, an experiment was carried out in triplicate to determine the maximum water retention capacity (MWRC) of the dry orange peel under saturated conditions, which resulted to be 3.6 ± 0.1 mL of water absorbed per gram of dry material. This result was taken into account when the liquid phase was added to the substrate to promote the microbial growth.
Culture media and sterilization
Dried and milled samples of orange peel were dispensed into 50 mL Erlenmeyer flasks provided with aluminum caps with 24-26 mm diameter, model Sero-Tap (Selecta, Abrera, Spain), without any additional nutrients. Different bed loadings from 1.0 to 4.8 g/Erlenmeyer were assayed according to the experimental design described later, which corresponded to minimum and maximum loadings of 35 and 80 % of the flask working volume, respectively. The material was moistened by two-step addition of the liquid phase. In a first step, and for all cases, 1.6 mL of water/g, corresponding to 45 % of the MWRC, was added before sterilization to protect the material from thermal degradation. Then, the rest of water necessary to reach the level of moisture desired for each experiment was added together with the inoculum. Sterilization was made by autoclaving at 100 °C for 1 h.
Incubation
Flasks were incubated at 30 °C in a stationary incubator where a saturated NaCl solution allowed to maintain the level of air moisture needed to prevent drying of the material. Each flask was stirred every 24 h with a sterile spatula inside a laminar flow chamber to reduce compactation and diffusion restrictions. All experiments were done in triplicate.
In selected experiments performed with variable degrees of humidification, sterile distilled water was added at different rates and proportions, as indicated in Table 1. For the study of the effect of methanol on citric acid production, experiments were done adding methanol at the beginning of the cultivation at variable proportions (0, 2, 4, and 6 % v/w). Methanol, of analytical grade, was purchased from Sigma (Switzerland). In both cases, the content of each flask was shaken by a sterile spatula after every addition.
Table 1.
Conditions of SSF with variable water addition.
| Runs | Amount added in each addition (mL/g) | Frequency of addition (h) | Time of addition (h) | Number of additions | mL water/g dry solid accumulated at the end of incubation | increase of % saturation at the end of incubation |
|---|---|---|---|---|---|---|
| A (control) | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 0.08 | 12 | 48 | 6 | 0.48 | 13.3 |
| C | 0.12 | 12 | 48 | 6 | 0.72 | 20.0 |
| D | 0.12 | 12 | 62 | 5 | 0.60 | 16.7 |
Sampling
The whole content of a flask was used for each sample. The material was homogenized carefully with the help of a spatula or even of a mortar, especially when the abundant cell growth hampered the homogenization of the samples. Amounts of about 0.5-1.0 g were used to determine the moisture content in oven at 105 °C. According to the sample consistency, aqueous extracts were obtained from the remaining sample by addition of distilled water up to a 10:1 (v/w) ratio. The extraction was assisted mechanically with an Ultra-Turrax homogenizer, model T25 (IKA-Labortechnik, Staufen, Germany) for 30 s, and centrifugation at 8,000 rpm for 10 min to eliminate the solid particles.
Analytical methods
Samples of the aqueous extracts were filtered through 0.45 μm-pore membranes and assayed for glucose, fructose, sucrose, citric acid, and galacturonic acid concentrations by HPLC, model 1100 (Agilent, Palo Alto, CA) with a Refractive Index detector. Standards were prepared from the corresponding reagents purchased from Sigma (Switzerland). Separation was performed using a ION-300 column (Transgenomic Inc., San Jose, CA), thermostated at 50 °C and eluted with 0.01 M H2SO4 at 0.4 mL/min flow rate. All analyses were carried out in triplicate, and the error was less than 3 %.
Total sugars (including neutral and acid sugars) were analyzed by the method of Dubois et al., modified according to Strickland and Parsons (51), which is based on the phenol-sulfuric acid reaction that allows determining the reducing sugars after acid hydrolysis of polysaccharides. Glucose, from Sigma (Switzerland), was used as a standard.
Experimental design
The effect of the bead loading and water content on orange peel SSF was studied by means of a second-order rotatable experimental design with α?= 1.414 and five replicates in the center of the domain, according to Akhnazarova and Kafarov (2) and Box et al. (7). Experimental domain and coding criteria are given in Table 2. The significance of the coefficients of the models was calculated using Student’s t test (α?< 0.05) as the acceptance criterion. Model consistency was verified by Fisher’s F test (α?< 0.05) applied to the following mean square (QM) ratios:
model/total error (QMM/QME) (F ≥ Fdennum)
(model + lack of fitting)/model (QM(M + LF)/QMM) (F ≤ Fdennum)
total error/experimental error (QME/QMEe) (F ≤ Fdennum)
lack of fitting/experimental error (QMLF/QMEe) (F ≤ Fdennum)
Table 2.
Experimental domain and codification of the independent variables analyzed by means of a second-order rotatable factorial design applied to citric acid production by SSF on orange peels.
| Actual values | |||
|---|---|---|---|
| Coded values | Water content(% of saturation) | Water content(mL/g dry solid) | Bed loading(g/flask) |
| -1.414 (-α) | 50.0 | 1.80 | 1.0 |
| -1 | 57.3 | 2.06 | 1.6 |
| 0 | 75.0 | 2.70 | 2.9 |
| +1 | 92.7 | 3.34 | 4.2 |
| +1.414 (+α) | 100.0 | 3.60 | 4.8 |
Codification: Vc = (Vn-Vo)/△Vn. Decodification: Vn = Vo+(△Vn×Vc). Vn, natural value; Vc, coded value; Vo, natural value in the center of the domain; △Vn, increment of Vn corresponding to one unit of Vc.
RESULTS AND DISCUSSION
Preliminary experiments: inoculum concentration
A preliminary set of solid-state fermentations was performed using orange peel as substrate and Aspergillus niger at different inoculum concentrations. The objective of these runs was to evaluate the suitability of this system for citric acid production and to get a first insight of the kinetics of this fungus on this substrate in view of future optimization of this production.
Considering the aeration requirements of this microorganism, a low bed loading of 2 g of dry orange peel (corresponding to 46.8 % of the flask working volume) was selected as a condition of reduced depth of the matrix in the flask able to provide an aerobic environment suitable for cell growth. Taking into account that inoculum concentrations in the range 103-108 spores/g substrate are usually employed for CA production by A. niger (32,45) and that an increase in the inoculum level is well known to reduce the lag phase, three different spore concentrations were tested, namely 0.5·103, 0.5·106 and 0.7·108 spores/g dry orange peel, the last being the maximum value that could be easily achieved using the procedure described in Materials and Methods. The solid was moistened to reach 75 % saturation, and incubations were done at 30 °C.
The results illustrated in Fig. 1 demonstrate the feasibility of using orange peel as a substrate for CA production by A. niger by SSF, with no need of supplying any additional nutrient and using the sterilization as the only pretreatment. Moreover, they clearly show that the intermediate inoculum level (0.5·106 spores/g of orange peel) ensured the highest product concentration (170.5 mg of CA/g dry orange peel).
Figure 1.
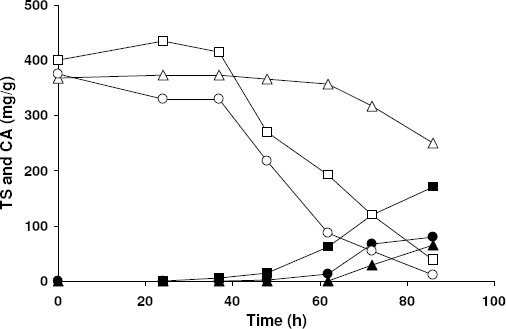
Effect of the inoculum concentration on CA production from orange peel by A. niger SSF. Inoculum concentration (spores/g dry orange peel): 0.5·103 (?), 0.5·106 (A), 0.5·108 (?). Results are the average of three independent experiments. Standard deviations were below 2.2 % of the mean.
It should be noted that the lag phase was very long at the lowest inoculum level (0.5·103 spores/g of dry orange peel). Both the consumption of sugars and the production of CA (Fig. 1) did in fact start no sooner than 60 h. As a consequence, after 86 h the CA concentration was only 65 mg/g dry orange peel, and a large portion of sugars remained unconsumed in the medium. Also the highest inoculum level (0.7·108 spores/g dry orange peel), used with the aim of accelerating the fermentation, revealed to be unadvisable from an industrial point of view, owing not only to the long time required to produce CA, but also to the final CA concentration reached (100.4 mg/g dry orange peel after 72 h), which was considerably lower than that obtained at the intermediate inoculum level.
In order to get a more detailed view of the kinetics, Fig. 2 illustrates the evolution of the solubilized sugars and CA production during the best run (0.5·106 spores/g of orange peel). It is noteworthy the occurrence of two distinct phases of the culture, determining a fermentation pattern quite different from the trophophase-idiophase one usually observed in submerged culture. A first phase, which occurred approximately between 23 and 47 h, was characterized by a) a low rate of CA production coinciding with the disappearance of sucrose, b) an initial increase in glucose and fructose concentrations as the mainly result of sucrose hydrolysis at higher rate than its metabolization, and c) the net solubilization of sugars. A second one corresponded to an intense CA production and the net consumption of sugars.
In addition to sucrose, glucose and fructose, also pectins were analyzed since they are the main component of orange peel (42). Pectins are a heterogeneous group of acidic structural polysaccharides, consisting mainly of galacturonic acid units. There are many references describing the ability of this microorganism to produce pectinases that catalyze the partial or total hydrolysis of pectins, leading to their solubilization and the release of galacturonic acid. The production of pectinases is referenced either in solid-state or in submerged cultures (34,50), and even in semisolid lemon pulp culture (10), which is similar to the orange peel SSF. It is also described the production of pectinolytic enzymes by A. niger even on materials with low pectin content including wheat bran and soy (9). Whereas the addition of sucrose, glucose, or galacturonic acid reduced the production of pectinases in submerged cultures, during SSF the addition of these sugars even increased their levels in the broth (50).
With the aim of evaluating the possible solubilization and hydrolysis of pectins from orange peel cultures, the concentration of total sugars (TS) was followed throughout the fermentation by the phenol-sulfuric method that allows quantifying neutral and acidic sugars from pectins together (Fig. 2). It can be observed that TS was actually higher than the sum of neutral sugars determined by HPLC. Such a difference became more evident at 48 h, when TS achieved a maximum value coinciding with the beginning of the net consumption of glucose and fructose and the intensive production of CA, which suggests the occurrence of a significant solubilization of pectines. After 48 h, TS decreased drastically together with the levels of neutral monosaccharides. Nevertheless, the rate of TS reduction was lower than that of neutral sugars, reflecting a gradual accumulation of pectins and/or their products of hydrolysis. According with that, galacturonic acid (GA) started to accumulate progressively in the medium coinciding with the increase of total sugars at 48 h and until the end of the culture. These data seem to provide an indirect confirmation of the ability of A. niger to produce pectinases in these conditions.
Figure 2.
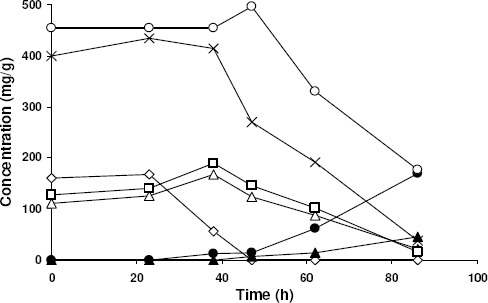
Time behavior of sugars consumption, CA production and galacturonic acid release during SSF of orange peel by A. niger using an inoculum concentration of 0.5·106 spores/g dry orange peel. Sucrose (◊), glucose (△), fructose (□), sucrose + glucose + fructose (×), total sugars (○), galacturonic acid (▲), citric acid (•). Results are the average of three independent experiments. Standard deviations were below 2.4 % of the mean.
Finally, it must be highlighted the progressive acidification of the medium during these runs from about 4.5 to approximately 2.7, as the consequence of CA accumulation. Although A. niger has higher tolerance to low pH than other microorganisms, excess acidification is well known to affect both growth and CA production. Rivas et al. (2) did in fact found an increase in the CA production from 4.9 to 8.3 g/L when the pH was controlled by the addition of CaCO3 during the submerged culture of this microorganism. On the contrary, the control of pH during the SSF process is difficult; therefore, this variable was only monitored in this study.
CA yields achieved in SSF of fig fruits practically remained constant when initial pH values ranged from 4 to 8 (45), whereas Selvi et al. (46) found the best results in terms of CA yield using sugarcane bagasse as a substrate at initial pH of 4. Nevertheless, it has been reported that CA is accumulated in significant amounts only when the pH is below 2.5 (28). Although the reasons for the requirement of a low pH are not clear, it is known that at pH>4 the gluconic acid produced by the reaction catalyzed by glucose oxidase accumulates at the expense of CA (40). Moreover, due to its extracellular localization, this enzyme is directly susceptible to the external pH and is inactivated at pH < 3.5 (57). On the basis of these considerations, we think that the low pH values reached in this work likely favored the CA production.
Influence of the bed loading and water content
The O2 supply is a limiting factor of submerged aerobic cultures because, due to its low solubility in water, its concentration in a saturated aqueous medium is usually lower than the microorganism requirements. This aspect is of a great concern for CA production because A. niger is an aerobic microorganism, and the oxygenation is essential for its growth (35). Although in SSF the oxygen availability is many orders of magnitude higher than that found in submerged cultivations, several reports demonstrated the importance of aeration also in SSF. For example, even though forced aeration at the beginning of the solid-state fermentation of Kumara by A. niger favored the CA production in a packed-bed reactor compared to flasks, too high air flow rates exerted adverse shear stress to the fungus (32). Vandenberghe et al. (55) reported that an air flow rate of 60 mL/min improved cassava fermentation by A. niger, achieving 265 g citric acid/kg dried cassava.
According to Lu et al. (32), the bed loading (B) is the most important factor affecting the CA production by SSF because it influences the degree of aeration in the system. It is also related to heat transfer, which is affected by higher restrictions in the solid state than in submerged cultures. Optimal B is therefore necessary to ensure the suited supply of oxygen and heat exchange necessary for efficient growth and CA production.
The water content (W) of the medium is another important operating variable for CA production by SSF because it influences growth and metabolism of the microorganism as well as the mass transfer phenomena primarily related to the diffusion of nutrients, oxygen and toxic metabolites (41). In addition, it causes swelling of the substrate, facilitating the penetration of the mycelium for its effective utilization (39), and affects heat transfer since its molecules occupy the interparticular spaces and/or causes aggregation of the solid particles. Therefore, the optimal moisture content depends on the specific requirements of the microorganism, the desired production, and the nature of the material, with particular concern to its hydrophilicity and porosity.
Considering all of that, a second order factorial design was done, as described in Materials and Methods, to better investigate the effect of these two operational variables on CA production. The best inoculum concentration (0.5·106 spores/g dry orange peel) was applied, and the time of incubation was fixed at 62 h to avoid possible substrate limitations under some of the tested conditions.
As a result, the following significant model (Table 3), expressed in codified values, was obtained:
| [1] |
whose response surface within the experimental domain is illustrated in Fig. 3.
Table 3.
Experimental results and analysis of variance of the model describing the effect of the water content (W) and the bed loading (B) on SSF citric acid (CA) production by A. niger on orange peels. (SS: sum of squares, FD: freedom degrees, QM: mean squares).
 |
Figure 3.
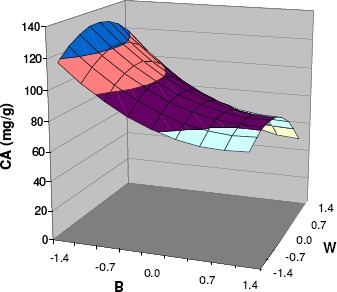
Response surface obtained from equation [1], showing the effect of the bed loading (B) and the water content (W) of the solid on the citric acid (CA) production from orange peel by SSF.
The absolute values of the coefficients of the equation, representing 44 % of the absolute value of the independent term, confirm, first of all, the strong effect of these two operating variables considered. The maximum predicted response (133.8 mg CA/g), corresponding to codified values of W=-0.305 and B=-1.414 and natural values of W=70 % of saturation and B=1 g/flask, was in fact 2.5-fold the minimum predicted one (53.5 mg CA/g).
Considering now the behaviour of these two variables inside the experimental domain (Fig. 3), it is possible to make a mechanistic interpretation of the model in terms of the real variables: aeration and water activity (aw). The convex profile of the CA response surface with regard to W, with a maximum inside the domain, reflects the need of increasing the amount of water to improve aw and, consequently, fungal metabolism and nutrients diffusion. From this maximum threshold, aeration restrictions become relevant as a consequence of the decrease of the substrate porosity and the interparticular void space.
With respect to B, and taking into account the influence of this operating variable on gas transfer, the high value of the first order B term indicates the very important effect of aeration on this system and reduces the relative importance of the quadratic term.
Water addition during incubation
Although the model allowed enhancing CA production at 62 h of incubation, at this time there were still sugars remaining that could lead to higher levels of CA if the culture were continued until substrate depletion. When thinking about prolonging the time of incubation in solid state cultures, it must be taken into account that water activity can decrease as a consequence of water evaporation and consumption by the fungus metabolism, and reach low and harmful levels. As a consequence, the optimum W value defined by the model for 62 h of incubation could not be directly extrapolable to longer times.
To allow the incubation to continue without water limitations in order to consume the substrate and increase CA production, it seemed necessary to supply water along the culture without affecting negatively the aeration by an excess of the liquid. Consequently, 4 fermentations were performed starting at the best conditions defined by the model (W=70 % of saturation and B=1 g/flask) and adding water in different proportions and rates, as specified in the Materials and Methods section, whose results, in terms of CA production, are illustrated in Fig. 4.
Figure 4.
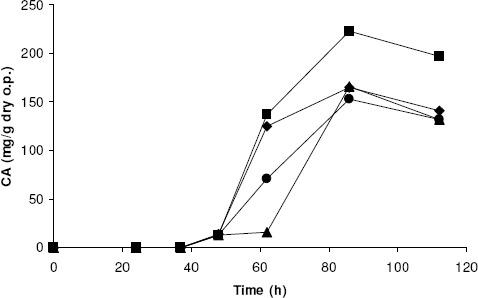
Evolution of CA throughout SSF after water addition: A) control without addition of water (υ); B) addition of 0.08 mL/flask every 12 h starting from 48 h (?); C) addition of 0.12 mL/flask every 12 h starting from 48 h (?); and D) addition of 0.12 mL/flask every 12 h starting from 62 h (A). Results are the average of three independent experiments. Standard deviations were below 2.1 % of the mean value.
The highest CA production (193.2 mg CA/g dry) was obtained at 86 h of incubation adding water every 12 h starting from 62 h (run D), a value 17 % higher than that obtained in the reference run (A). Moreover, it is interesting to highlight that, in spite of the negative effect at 62 h on CA production expected in series C by the use of W values higher than the optimum predicted by the model, the productions of run C and D at 86 h were practically coincident, as the likely consequence of excess water evaporation at this time. However, run B was the worst one ever, probably due to an excess of water at 62 h and to an insufficient water content at longer incubation times. These results strongly suggest the need of adding water progressively along the SSF and then controlling carefully the maintenance of adequate aw and aeration.
Effect of methanol (MeOH) addition on citric acid production by SSF
The addition of methanol at low concentrations can improve the yield of citric acid in cultures of A. niger, although contradictory effects have been reported. For example, Zhang (59) added 2 % MeOH to the solid residue of an orange juice factory in fermentations carried out by A. niger 999. Meanwhile, Kang et al. (24) found the optimal conditions in terms of maximum yield of citric acid (80.4 %) from skins of mandarin by A. niger, using semisolid fermentations, with the addition of 0.2 % NH4NO3, 0.1 % of MgSO4·7H2O, 2.5 % MeOH or 1.5 % of ethanol. Tran et al. (52), obtained the highest CA yield (194 g/kg) adding 3 % methanol and 5 ppm de Fe2+ during fermentations conducted using pineapple waste and A. niger ACM 4992. De Lima et al. (11) added 4 % methanol using A. niger ATCC 1015 and pineapple waste in solid-state fermentation to achieve the highest CA production (132 g/kg). Recently, Rodrigues et al. (43) obtained the best results (445.4 g of CA/kg of citric pulp) with sugarcane molasses and 4 % methanol (v/w) in SSF by A. niger LPB BC mutant. Finally, Roukas (44) reported that the addition of 6 % (w/w) methanol into the substrate increased the concentration of citric acid from 176 to 264 g/Kg dry pod during SSF. The same stimulatory effect of methanol was observed by Kumar et al. (30) using a mixture of different fruit wastes and bagasse in SSF by A. niger DS1. Conversely, some authors have reported a decreased synthesis of CA after methanol addition. For instance, Hang et al. (17) observed that the supplementation of 0.74 mmol methanol/L diminishes the CA production during the SSF of kiwifruit peel by A. niger ATCC 9142, and Tsay and To (54) reported that methanol inhibited mycelial growth of A. niger TMB 2022 as well as CA production. Similar findings were reported by Navaratnam et al. (37), Ali et al. (3) and Xie and West (58).
In a previous study, Rivas et al. (42), adding 4 % (v/w) methanol to an orange peel aqueous extract as culture medium, increased 20-fold the maximum CA production with the same strain of Aspegillus niger in submerged fermentation, although this was accompanied by an increase in the duration of the lag phase. To study the possibility of improving the production also in this system, four fermentations were done in the presence of methanol under the best conditions previously defined by the model, with water addition according to the protocol earlier defined for run D. To this purpose, methanol was added at the beginning of the incubation and after sterilization in proportions (0, 2, 4, and 6 % v/w) usually applied in submerged and solid state cultures (18, 43). Nevertheless, in all the cases methanol had a negative effect on the cultivation, leading not only to an increase in the lag time, but also to a strong decrease in the maximum CA production for fermentations with 4 % and 6 % (v/w) methanol.
These results suggest that the positive effects attributed to methanol due to the reduction of the toxicity of some metal ions, the positive alteration of the cell wall and the membrane, and the modification of the fungal morphology (23), are not relevant in this system. The intrinsic effect of the conditions defined by the solid state culture system on fungal morphology and metabolism could explain the lack of an important favorable effect of methanol on CA production in this case. On the other hand, the low values of aw that usually characterize the solid state cultures performed with low water contents and hydrophilic supports as orange peel, could have emphasized the methanol toxicity as a consequence of a high local methanol activity.
Comparison between SSF and submerged culture for CA production
Recently, there have been an increasing number of reports on the use of SSF processes because they exhibit a series of advantages over submerged fermentations. Since the culture conditions are more similar to the natural habitat of filamentous fungi, these are in fact able to grow and excrete large amounts of hydrolytic enzymes and, consequently, product concentrations after extractions are usually higher, and the amounts of liquid and solid wastes generated are lower (16, 17, 30). Furthermore, in SSF the degree of aeration is higher, the low water activity reduces the risk of bacterial contaminations, and the energy requirements are lower. On the other hand, SSF also shows some disadvantages such as a greater challenge for control of some important operating variables such as pH and temperature.
In a previous work, Rivas et al. (42) submitted orange peel to autohydrolysis at 130 °C at liquid/solid ratio of 8.0 g/g. Without additional nutrients, 40 g of the liquors generated were employed as media for submerged CA production by A. niger in 100 mL-Erlenmeyer flasks at 30 °C and 200 rpm. Table 4 allows comparing the results of the best fermentations of dry orange peel in terms of CA produced by the same strain in submerged fermentation (in the presence of 40 mL/kg methanol and 20 g/L calcium carbonate) and in SSF (W = 70 %; B = 1 g; addition of 0.12 mL H2O/Erlenmeyer flask every 12 h starting from 62 h). All the data refer to the time of the highest CA concentration in each fermentation. The first remarkable differences between these two culture systems were the different state of the substrate and the sugar availability, which made unavoidable the use of different units. Nevertheless, to make easier the comparison between them, the concentrations of sugars and CA in the submerged culture were expressed as mg per g of dry orange peel considering the early-mentioned 8.0 g/g water/solid ratio employed for preparing the extract. All the sugars contained in the solid substrate were available for SSF, whereas only those solubilized by autohydrolysis were so for the submerged fermentation. Thus, sucrose was the most abundant sugar in the SSF and fructose in the submerged one. It is also important to notice that the authoydrolysis led to more diluted fermentation media in comparison with SSF.
Table 4.
Comparison of the main results of submerged fermentation and SSF of dry orange peel by A. niger.
| Reference | Submerged culture (g/L)Rivas et al., 2008 | SSF (mg/g)This work |
|---|---|---|
| Initial sucrose | 6.6 | 156.6 |
| Initial glucose | 9.6 | 137.3 |
| Initial fructose | 13.6 | 145.2 |
| TS0 | 29.8 | 439.1 |
| Residual sucrose | 0.0 | 0.0 |
| Residual glucose | 4.1 | 7.6 |
| Residual fructose | 8.3 | 0.0 |
| Total residual sugars | 12.4 | 7.6 |
| TSc | 17.4 | 431.5 |
| CaCO3 | 20 | - |
| Methanol | 40 | - |
| Fermentation time (h) | 72 | 85 |
| Citric acid | 9.2 | 223.2 |
| YTSc (g citric acid/g TSc) | 0.53 | 0.52 |
| YTSo (g citric acid/g TS0) | 0.31 | 0.51 |
TS0 = Total initial neutral sugars.
TSC = Total consumed neutral sugars.
Y = Yield of CA on the initial amount of dry orange peel.
YTSo = Yield of CA on TS0.
YTSc = Yield of CA on TSc.
Ymax = Experimental conversion of neutral sugars to CA with respect to the theoretical yield (one CA mol per mol of glucose or fructose consumed; two CA moles per mol of sucrose consumed).
Another notable difference between the two methods concerned the time behavior of the concentrations of the different sugars. In both cases sucrose was the first sugar to disappear. However, while in submerged culture glucose and fructose were gradually consumed throughout the cultivation, the levels of monosaccharides in SSF increased slightly during sucrose depletion. Several species belonging to the genus Aspergillus are reported to hydrolyze extracellular sucrose to be used later as a carbon source in the form of glucose and fructose (13). These results suggest that the rate of sucrose hydrolysis was higher than that of consumption of the resulting monosaccharides, thus leading to their net accumulation at the beginning of cultivation in the case of the SSF.
The SSF and the submerged cultures also differed markedly in terms of the yield of CA per gram of dry orange peel, which reached a value in SSF (193.2 mg/g dry orange peel) about 3-fold that observed in submerged culture (73.6 mg/g dry orange peel). In addition, it was comparable with those reported for dry carob pod (176 mg/g) (44) and higher than for kiwifruit peel (100 mg/g) (17), and dry fig (64 mg/g) (45).
On the other hand, the yields of CA referred to sugar consumption (YTSc = 0.61 and 0.58 g/g in SSF and submerged culture, respectively) were comparable for the two systems, suggesting that the metabolic dysfunctions responsible for CA accumulation were ensured to the same extent in both culture modalities. However, the potential of SSF is highlighted by the fact that no methanol is required to stimulate CA production as for the submerged culture (42). This seems to be confirmed by the values of the yield referred only to the neutral sugars available at the beginning. This parameter was in fact substantially lower in submerged culture (YTSo = 0.32 g/g) than in SSF (YTSo = 0.59 g/g), likely due to the interruption of CA production when sugars were not yet completely consumed.
CONCLUSIONS
The results of this study point out the viability of Valencia orange (Citrus sinensis) peels as substrate for the production of citric acid (CA) by Aspergillus niger CECT-2090 in solid-state fermentation (SSF). Compared to previous results obtained in submerged culture, the SSF proved to be very versatile and did not need any additional nutrients or treatment besides sterilization. The highest CA concentration (193.2 mg/g dry orange peel) was obtained at 85 h of incubation using an inoculum concentration of 0.5·106 spores/g of dry orange peel, a bed loading of 1.0 g/Erlenmeyer, an initial water content of 2.52 mL/g of orange peel, corresponding to a 70 % saturation, and a water addition of 0.12 mL H2O/Erlenmeyer flask every 12 h starting from 62 h. Methanol addition did not show to improve CA production. During SSF the microorganism likely produced pectinases, but pectins were not metabolized in any appreciable extent. Finally, SSF ensured yields of product on total initial sugars and consumed sugars of 0.59 g CA/g TS0 and 0.61 g CA/g TSC, respectively. These results are considerably better than those previously obtained in submerged culture.
ACKNOWLEDGMENTS
We are grateful to the Spanish Government (project CT Q2006-02241/PPQ), which partially financed this work through the FEDER funds of the European Union, the FPI grant to Belén Max and the MAEC-AECID grant to Belinda P. Bibbins.
REFERENCES
- 1.Adham, N.Z. (2002). Attempts at improving citric acid fermentation by Aspergillus niger in beet-molasses. Bioresour. Technol. 84(1), 97–100. [DOI] [PubMed] [Google Scholar]
- 2.Akhnazarova, S.; Kafarov, V. (1982). In: Experiment Optimization in Chemistry and Chemical Engineering, Mir, Moscow, Russia. [Google Scholar]
- 3.Ali, S.; Ashraf, H.; Ikram, U. (2002). Enhancement in citrate production by alcoholic limitation. J. Biol. Sci. 2, 70–72. [Google Scholar]
- 4.Anastasssiadis, S.; Morgunov, I.G.; Kamzolova, S.V.; Finogenova, T.V. (2008). Citric acid production patent review. Recent. Pat. Biotechnol. 2, 107–123. [DOI] [PubMed] [Google Scholar]
- 5.Aravantinos-Zafiris, G.; Tzia, C.; Oreopoulou, V.; Thomopoulos, C.D. (1994). Fermentation of orange processing wastes for citric acid production. J. Sci. Food Agric. 65, 117–120. [Google Scholar]
- 6.Babu, I.S.; Rao, G.H. (2006). Citric acid production by Yarrowia lipolytica NCIM 3589 in solid state fermentation using pineapple waste as a novel substrate. Asian J. Microbiol. Biotechnol. Environ. Sci. 8(4), 799–802. [Google Scholar]
- 7.Box, G.E.P.; Hunter, W.G.; Hunter, J.S. (1989). In: Estadística para Investigadores, Reverté, Barcelona, Spain. [Google Scholar]
- 8.Cannel, E.; Moo-Young, M. (1980). Solid-state fermentation systems. Process Biochem. 15(6), 24–8. [Google Scholar]
- 9.Castilho, L.R.; Medronho, R.A.; Alves, T.L.M. (2000). Production and extraction of pectinases obtained by solid state fermentation of agroindustrial residues with Aspergillus niger. Bioresour. Technol. 71(1), 45–50. [Google Scholar]
- 10.De Gregorio, A.; Mandalari, G.; Arena, N.; Nucita, F.; Tripodo, M.M.; Lo Curto, R.B. (2002). SCP and crude pectinase production by slurry-state fermentation of lemon pulps. Bioresour. Technol. 83(2), 89–94. [DOI] [PubMed] [Google Scholar]
- 11.de Lima, V.L.A.G.; Stamford, T.L.M.; Salgueiro, A.A. (1995). Citric acid production from pineapple waste by solid-state fermentation using Aspergillus niger, Arquivos de Biologia e Tecnologia 38(3), 773–783. [Google Scholar]
- 12.Flores, J.L.; Gutiérrez-Correa, M.; Tengerdy, R.P. (1994). Citric acid production by solid state fermentation of prickly pear peel with Aspergillus niger. Agro-Food-Industry Hi-Tech 5(1), 18–20. [Google Scholar]
- 13.Friedrich, J.; Cinierman, A.; Steiner, W. (1994). Concomitant biosynthesis of Aspergillus niger pectolytic enzymes and citric acid on sucrose. Enzyme Microb. Technol. 16(8), 703–707. [Google Scholar]
- 14.Ghildyal, N.P.; Ramakrishna, M.; Lonsane, B.K.; Karanth, N.G. (1992). Gaseous concentration gradients in tray type solid state fermentors. Effect on yields and productivities. Bioproc. Eng. 8(1–2), 67–72. [Google Scholar]
- 15.Grohmann, K.; Baldwin, E.A. (1992). Hydrolysis of orange peel with pectinase and cellulase enzymes. Biotechnol. Lett. 14, 1169–1174. [Google Scholar]
- 16.Grohmann, K.; Cameron, R.G.; Buslig, B.S. (1995). Fractionation and pretreatment of orange peel by dilute acid hydrolysis. Bioresour. Technol. 54, 129–141. [Google Scholar]
- 17.Hang, Y.D.; Luh, B.S.; Woodams, E.E. (1987). Microbial production of citric acid by solid state fermentation of kiwifruit peel. J. Food Sci. 52, 226–227. [Google Scholar]
- 18.Hang, Y.D.; Woodams, E.E. (1986). Solid-state fermentation of apple pomace for citric acid production, MIRCEN J. Appl. Microbiol. Biotechnol. 2(2), 283–287. [Google Scholar]
- 19.Hang, Y.D.; Woodams, E.E. (1986). Utilization of grape pomace for citric acid production by solid-state fermentation. Am. J. Enol. Vitic. 37(2), 141–2. [Google Scholar]
- 20.Hang, Y.D.; Woodams, E.E. (1989). A process for leaching citric acid from apple pomace fermented with Aspergillus niger in solid-state culture. MIRCEN J. Appl. Microbiol. Biotechnol. 5(3), 379–82. [Google Scholar]
- 21.Harvey, E.M.; Rygg, G.L.J. (1936). Physiological changes in the rind of California oranges during growth and storage. J. Agric. Food Chem. 52, 723–46. [Google Scholar]
- 22.Imandi, S.B.; Bandaru, V.V.R.; Somalanka, S.R.; Bandaru, S.R.; Garapati, H.R. (2008). Application of statistical experimental designs for the optimization of medium constituents for the production of citric acid from pineapple waste. Bioresour. Technol. 99(10), 4445–4450. [DOI] [PubMed] [Google Scholar]
- 23.Ingram, L.O.; Buttke, T.M. (1984). Effects of alcohols on microorganisms. In: Advances in Microbial Physiology 25, Academic Press, London, UK, pp. 253–300. [DOI] [PubMed] [Google Scholar]
- 24.Kang, S.K.; Park, H.H.; Lee, J.H.; Lee, Y.S.; Kwon, I.B.; Sung, N.K. (1989). Citric acid fermentation from mandarin orange peel by Aspergillus niger, Sanop Misaengmul Hakhoechi 17, 510–518. [Google Scholar]
- 25.Kapoor, K.K.; Chaudhary, K.; Tauro, P. (1982). Prescott and Dunn’s Industrial Microbiology, 4th edn. G. Reed (Ed), AVI Publishing Co, Wesrport, CT. [Google Scholar]
- 26.Kesterson, J.W.; Braddock, R.J. (1976). By-products and specialty products of Florida citrus, Bull. Agric. Experiment State (Florida), pp. 7841–119.
- 27.Khare, S.K.; Jha, K.; Gandhi, A.P. (1996). Citric acid production from okara (soy-residue) by solid-state fermentation. Bioresour. Technol. 54(3), 323–5. [Google Scholar]
- 28.Kubicek, C.P. (2001). Organic acids, In: C.R. Ratledge, B. Kristiansen (eds), Basic Biotech. Cambridge University Press, Cambridge, pp. 305–324. [Google Scholar]
- 29.Kumar, A.; Jain, V.K. (2008). Solid state fermentation studies of citric acid production, Afri. J. Biotechnology 7(5), 644–650. [Google Scholar]
- 30.Kumar, D.; Jain, V.K.; Shanker, G.; Srivastava, A. (2003). Citric acid production by solid state fermentation using sugarcane bagasse. Process Biochem. 38(12), 1731–1738. [Google Scholar]
- 31.Lotfy, W.A.; Ghanem, K.M.; El-Helow, E.R. (2007). Citric acid production by a novel Aspergillus niger isolate: I. Mutagenesis and cost reduction studies. Bioresour. Technol. 98, 3464–3469. [DOI] [PubMed] [Google Scholar]
- 32.Lu, M.; Brooks, J.D.; Maddox, I.S. (1997). Citric acid production by solid-state fermentation in a packed-bed reactor using Aspergillus niger. Enzyme Microb. Tech. 21, 392–397. [Google Scholar]
- 33.Ma, E.; Cervera, Q.; Mejía Sánchez, G.M. (1993). Integrated utilization of orange peel. Bioresour. Technol. 44, 61–63. [Google Scholar]
- 34.Maldonado, M.C.; Strasser De Saad, A.M. (1998). Production of pectinesterase and polygalacturonase by Aspergillus niger in submerged and solid state systems. J. Ind. Microbiol. Biotechnol. 20(1), 34–38. [DOI] [PubMed] [Google Scholar]
- 35.Murado, M.A., González, M.P.; Torrado, A.; Pastrana, L.P. (1997). Amylase production by solid state culture of Aspergillus oryzae on polyurethane foams. Some mechanistic approaches from an empirical model. Process Biochem. 32(1), 35–42. [Google Scholar]
- 36.Narayanamurthy, G.; Ramachandra, Y.L., Rai, S.P.; Ganapathy, P.S.S.; Kavitha, B.T.; Manohara, Y.N. (2008). Comparative studies on submerged, liquid surface and solid state fermentation for citric acid production by Aspergillus niger RCNM17. Asian J. Microbiol. Biotechnol. Environ. Sci. 10, 361–364. [Google Scholar]
- 37.Navaratnam, P.; Arasaratnam, V.; Balasubramaniam, K. (1998). Channelling of glucose by methanol for citric acid production from Aspergillus niger. World J. Microbiol. Biotechnol. 14(4), 559–563. [Google Scholar]
- 38.Prado, F.C.; Vandenberghe, L.P.S.; Woiciechowski, A.L.; Rodrigues-Leon, J.A.; Soccol, C.R. (2005). Citric acid production by solid – state fermentation on a semi-pilot scale using different percentages of treated cassava bagasse. Braz. J. Chem. Eng. 22(4), 547–555. [Google Scholar]
- 39.Raimbault, M.; Alazard, D. (1980). Culture method to study fungal growth in solid state fermentation. Eur. J. Appl. Microbiol. Biotechnol. 9, 199–209. [Google Scholar]
- 40.Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche. C. (2006). Gluconic Acid: Properties, Applications and Microbial Production. Food Technol. Biotechnol. 44(2), 185–195. [Google Scholar]
- 41.Ramana Murthy, M.V.; Karanth, N.G.; Raghava Rao, K.S.M.S. (1993). Biochemical engineering aspects of solid-state fermentation. Adv. Appl. Microbiol 38, 99–147. [Google Scholar]
- 42.Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguez, J.M. (2008). Submerged citric acid fermentation on orange peel autohydrolysate. J. Agric. Food Chem. 56, 2380–2387. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigues, C.; Porto de Souza Vandenberghe, L.; Teodoro, J.; Pandey, A.; Zoclo, C.R. (2009). Improvement on citric acid production in solid-state fermentation by Aspergillus niger LPB BC mutant using citric pulp. Appl. Biochem. Biotechnol. 158(1), 72–87. [DOI] [PubMed] [Google Scholar]
- 44.Roukas, T. (1998). Citric acid production from carob pod by solid-state fermentation. Enzyme Microb. Tech. 24 (1/2), 54–59. [Google Scholar]
- 45.Roukas, T. (2000). Citric and gluconic acid production from fig by Aspergillus niger using solid-state fermentation. J. Ind. Microbiol. Biot. 25(6), 298–304. [DOI] [PubMed] [Google Scholar]
- 46.Selvi, V.; Kanna, K.S.; Banerjee, R.; Singh, G.; Ram, L.C. (2006). Citric acid production from sugarcane bagasse through solid state fermentation by mutants of Aspergillus niger Asian J. Microbiol. Biotechnol. Environ. Sci. 8, 791–794. [Google Scholar]
- 47.Sinclair, W.B.; Crandall, P.R. (1953). Polyuronide fraction and soluble and insoluble carbohydrates of orange peel. Bot. Gaz. 115, 162–73. [Google Scholar]
- 48.Shojaosadati, S.A.; Babaeipour, V. (2002). Citric acid production from apple pomace in multi-layer packed bed solid-state bioreactor. Process Biochem. 37(8), 909–914. [Google Scholar]
- 49.Soccol, C.R.; Vandenberghe, L.P.S.; Rodrigues, C.; Pandey, A. (2006). New Perspectives for Citric Acid Production and Application. Food Technol. Biotechnol. 44, 141–149. [Google Scholar]
- 50.Solís-Pereira, S.; Favela-Torres, E.; Viniegra-González, G.; Gutierrez-Rojas, M. (1993). Effects of different carbon sources on the synthesis of pectinase by Aspergillus niger in submerged and solid state fermentations. Appl. Microbiol. Biotechnol. 39(1), 36–41. [Google Scholar]
- 51.Strickland, J.D.H.; Parsons, T.R. (1968). A Practical Handbook of Seawater Analysis. In: Fisheries Research Board of Canada, Queen's Printer, Ottawa, Ont., Canada, pp. 167–311. [Google Scholar]
- 52.Tran, C.T.; Sly, L.I.; Mitchell, D.A. (1998). Selection of a strain of Aspergillus for the production of citric acid from pineapple waste in solid-state fermentation. World J. Microb. Biot. 14(3), 399–404. [Google Scholar]
- 53.Tran, C.T.; Mitchell, D.A. (1995). Pineapple waste – a novel substrate for citric acid production by solid – state fermentation. Biotechnol. Lett. 17(10), 1107–10. [Google Scholar]
- 54.Tsay, S.S.; To, K.Y. (1987). Citric acid production using immobilized conidia of Aspergillus niger TMB 2022. Biotechnol. Bioeng. 19, 297–304. [DOI] [PubMed] [Google Scholar]
- 55.Vandenberghe, L.P.S.; Soccol, C.R.; Prado, F.C.; Pandey, A. (2004). Comparison of citric acid production by solid-state fermentation in flask, column, tray, and drum bioreactors. Appl. Biochem. Biotech. 118(1–3), 293–303. [DOI] [PubMed] [Google Scholar]
- 56.Wang, J.; Liu, P. (1996). Comparison of citric acid production by Aspergillus niger immobilized in gels and cryogels of polyacrylamide. J. Ind. Microbiol. 16, 351–353. [Google Scholar]
- 57.Wong, C.M.; Wong, K.H.; Chen, X.D. (2008). Glucose oxidase: natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 78(6), 927–938. [DOI] [PubMed] [Google Scholar]
- 58.Xie, G.; West. T.P. (2009). Citric acid production by Aspergillus niger ATCC 9142 from a treated ethanol fermentation co-product using solid-state fermentation. Lett. Appl. Microbiol. 48, 639–644. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, Q. (1988). Utilization of citrus wastes in production of citric acid. Shipin Kexue 104, 21–24. [Google Scholar]


