Abstract
Agro-industrial wastes such as sugarcane bagasse, wheat bran, rice bran, corn cob and wheat straw are cheapest and abundantly available natural carbon sources. The present study was aimed to production of amylase and xylanase simultaneously using agro-industrial waste as the sole carbon source. Seven thermophilic strains of actinomycete were isolated from the mushroom compost. Among of these, strain designated MSC702 having high potential to utilize agro-industrial wastes for the production of amylase and xylanase. Strain MSC702 was identified as novel species of Streptomyces through morphological characterization and 16S rRNA gene sequence. Enzyme production was determined using 1% (w/v) of various agro-industrial waste in production medium containing (g/100mL): K2HPO4 (0.1), (NH4)2SO4 (0.1), NaCl (0.1), MgSO4 (0.1) at pH 7.0 after incubation of 48 h at 50°C. The amylase activity (373.89 IU/mL) and xylanase activity (30.15 IU/mL) was maximum in rice bran. The decreasing order of amylase and xylanase activity in different type of agro-industrial wastes were found rice bran (RB) > corn cob (CC) > wheat bran (WB) > wheat straw (WS) > sugarcane bagasse (SB) and rice bran (RB) > wheat bran (WB) > wheat straw (WS) > sugarcane bagasse (SB) > corn cob (CC), respectively. Mixed effect of different agro-industrial wastes was examined in different ratios. Enzyme yield of amylase and xylanase was ~1.3 and ~2.0 fold higher with RB: WB in 1:2 ratio.
Keywords: Agro-industrial wastes, thermophilic actinomycetes, Streptomyces, amylase, xylanase
INTRODUCTION
The continued development of biosustainable and renewable resource technology is of great importance with respect to environmental concerns. The bioconversion of lignocellulosics, natural and anthropogenic, in the production of biofuels is an extremely important part of renewable resource technology. A large amount of agro-industrial waste generated every year in all over the world contained high lignocellulosics and starch content. Lignocellulose is the major component of biomass, comprising around half of the plant matter produced by photosynthesis (also called photomass) and representing the most abundant renewable organic resource in soil (34). It consists of three types of polymers, cellulose, hemicellulose and lignin that are strongly intermeshed and chemically bonded by non-covalent forces and by covalent cross-linkages (28).
The microorganisms degrade lignocellulosic and starchy materials due to their highly efficient enzymatic system. The two types of extracellular enzymatic systems are present in microorganisms; the hydrolytic system (responsible for polysaccharide degradation) and ligninolytic system (degrades lignin and opens phenyl rings) (34). Thermophilic actinomycetes are well known components of the microbiota of composts and play an important role in habitats where decomposition of organic matter such as lignocellulose, starch and chitin, takes place at elevated temperatures and under aerobic conditions. They are aerobic, Gram-positive with high G + C in DNA (10).
Amylases and xylanases are hydrolytic enzymes those facilitate the complete hydrolysis of starch and xylan respectively. Xylan is the major structural component of plant cell walls and the most renewable hemicellulose, composed of 1, 4- linked β-D-xylopyranosyl residues. A great deal of attention has been received due to the potential application of amylases in sugar, textile, paper, brewing, distilling industries and pharmaceuticals (2, 13, 27) while of xylanases in the food, feed, pulp and paper industries (5, 7, 16, 24, 43). The advantages of using thermostable enzymes in industrial processes include the decreased risk of contamination, cost of external cooling and increased diffusion rate with resistant to denaturing agents, solvents and proteolytic enzymes (6, 20).
In order to convert biomass to ethanol, the efficient utilization of both starch-derived and hemicellulose-derived carbohydrates is essential. At present, the dominant cost element in fermentative production of fuel ethanol is starch, though some fermentable starch and sucrose are easily obtainable from agricultural by-products such as sugarcane bagasse, wheat bran, rice bran, corn cob and wheat straw (12, 19, 36). Expansion of this fermentation process towards utilization of low-value substrates such as agro-industrial wastes offers a great potential for reducing the production cost and increasing the use of ethanol as a fuel additive. The conventional method of starch and hemicellulose hydrolysis using acid has been replaced by processes using saccharifying enzymes of microbial origin (39).
The selection of a particular strain, however, remains a tedious task, particularly when commercially significant enzyme yields are required. Agro-industrial wastes are generally considered the best substrates for the solid state fermentation (SSF) processes (17) but in present study we utilized these in submerged fermentation (SmF). Since thermostability is a feature of most of the enzymes sold in bulk for industrial application, thermophilic microorganisms are of particular interest for the production of thermostable amylases and xylanases. This study was focused on the isolation and selection of particular strain having affinity to produce industrially valuable thermostable extracellular enzymes (amylase and xylanase) from the low cost carbon substrates to reduce the cost of enzyme production and make a better alternative for utilization of crop biomass (largest renewable reservoir of potentially fermentable carbohydrates) generated every year in million tones as “waste”.
MATERIALS AND METHODS
All the media ingredients and chemicals were of analytical grade, procured from E-Merck, Hi-Media and Qualigen Chemicals, India, Ltd. All agro-industrial wastes (sugarcane bagasse, wheat bran, rice bran, corn cob and wheat straw) used as sole carbon source, were obtained from the local market, Kanpur, India. All the substrates prior to the use for enzyme production were oven dried at 65°C. Sugarcane bagasse, corn cob and wheat straw were powdered in a grinder to get 4–5 mm particle size while wheat bran and rice bran were used at mesh size 2–4 mm.
Sampling site and collection of samples
Our sampling site Kanpur is situated in India within Uttar Pradesh province in between 26° 28′ North latitude and 80° 21′ East longitude with an elevation of 125.6 m. Twelve mushroom compost samples were collected from Mushroom Research and Development Centre, Department of Plant Pathology, Chandra Shekhar Azad University of Agriculture and Technology, Kanpur, India. These samples were collected from four different zones (outermost, under depth 25 cm, under depth 50 cm, centre) covering whole diameter of the heap of mushroom compost, in three visits.
Samples of mushroom compost were collected by inserting a polyvinyl corer (10 cm diameter) (previously sterilized with alcohol) into the heap of mushroom compost. The central portion of the top 2 cm sediment sample has been taken out with the help of a sterile spatula. These samples have been transferred to a sterile polythene bags and kept at 4°C until the experiments were carried out.
Isolation and maintenance of isolates
Serial dilution method (42) was applied for isolation of thermophilic actinomycetes. They were isolated on M medium agar (25) containing (g/100mL) soluble starch 1.0, dipotassium hydrogen phosphate (K2HPO4) 0.1, ammonium sulphate {(NH4)2SO4} 0.1, sodium chloride (NaCl) 0.1, magnesium sulphate (MgSO4. 7H2O) 0.1, pH 7.0 at 45°C. M medium was modified with 1% (v/v) trace metal salt solution (40). All isolated strains of thermophilic actinomycetes were maintained on modified M medium agar slants at 4°C.
Selection of working strain
Selection of working strain was done on the basis of their enzyme (amylase and xylanase) productivity on agro-industrial waste (wheat straw). Seven isolated strains of thermophilic actinomycetes were inoculated in M broth medium (25) contained 1% (w/v) wheat straw (WS). Highest enzyme (amylase and xylanase) producing strain was selected for further study.
To enhance the production of both enzymes, different other types of agro-industrial wastes (sugarcane bagasse (SB), wheat bran (WB), rice bran (RB) and corn cob (CC)), its combinations (1:1 ratio) and ten combinations (0.5:0.5; 1:1; 1:2; 1:3; 1:4; 1:5; 2:1; 3:1; 4:1 and 5:1) of the rice bran and wheat bran were analyzed.
Inoculum preparation
The inoculum was prepared by adding 10 ml sterile distilled water into the 2–3 days old culture slant and a cell suspension was made with the help of a sterile loop. 1 ml of the cell suspension (1.9–2.2 × 108 CFU/ml) was used in 100 ml medium (250 mL Erlenmeyer) as the inoculum for subsequent fermentation. The number of viable spores in the inoculum was determined by the pour plate count technique.
Enzyme production in submerged fermentation (SmF)
Amylase and xylanase production in SmF was carried out using the basal medium containing (g/100mL) dipotassium hydrogen phosphate (K2HPO4) 0.1; ammonium sulphate {(NH4)2SO4} 0.1; sodium chloride (NaCl) 0.1; magnesium sulfate (MgSO4.7H2O) 0.1, pH 7.0. Initially all the tested agro-industrial wastes were used at 1% (w/v) in basal medium. Production medium (100 mL) was placed in Erlenmeyer flask (250 mL) and autoclaved at 121°C (15 lbs pressure) for 20 min and cooled. After sterilization, production medium was inoculated with 1% inoculum and incubated at 50°C for 48 h. The fermented biomass were filtered with Whatman filter papers 1 (qualitative circles, 125 mm diameter) and centrifuged at 5000 g for 20 min at 4°C, and the clear cell-free supernatant was used as a source of enzymes.
Analytical procedure
Amylase and xylanase activity was estimated by measuring the reducing sugar released during hydrolysis of 1.0% (w/v) starch and oat-spelt xylan, respectively in 0.1 M phosphate buffer (pH 7.0) by enzyme (cell free supernatant) incubated at 50°C for 10 minutes using 3, 5-dinitrosalicylic acid method (21). Absorbance at 550 nm and 540 nm respectively was recorded by using UV-visible spectrophotometer (UV-1700 Pharmaspec Shimadzu) and activity was calculated from a standard curve using maltose and xylose as the standard. One unit (U) of enzyme activity was defined as the amount of enzyme required for the liberation of 1 μmol of reducing sugar equivalent to maltose or xylose respectively per minute under assay condition. All experiments were carried out in triplicate and average values were given in presented data.
Sequencing of 16S rRNA and analysis of sequence data
The chromosomal DNA of strain MSC702 was isolated according to Rainey et al. (32). The 16S rRNA gene was amplified with primers 8–27f (5'-AGAGTTTGATCCTGGCTCAG-3') and 1500r (5'-AGAAAGGAGGTGATCCAGCCA-3'). The amplified DNA fragment was separated on 1% agarose gel, eluted from the gel and purified using a QIAquick gel extraction kit (Qiagen). The purified PCR product was sequenced with four forward and three reverse primers, namely 8–27f, 357f (5'-CTCCTACGGGAGGCA GCAG-3'), 704f (5'-TAGCGGTGAAATGCGTAGA-3'), 1114f (5'-GCAACGAGCGCAACC-3'), 685r (5'-TCTACGCATTTCAC CGCTAC-3'), 1110r (5'-GGGTTGCGCTCGTTG-3') and 1500r (Escherichia coli numbering system). The 16S rRNA gene sequence was determined by the dideoxy chain-termination method with the Big-Dye terminator kit using an ABI 310 Genetic Analyzer (Applied Biosystems). The 16S rRNA gene sequence of strain MSC702 generated in this work (1474 bases) was aligned with 16S rRNA gene sequences of other members of the genus Streptomyces. A sequence similarity search was done using GenBank BLASTN (1).
RESULTS AND DISCUSSION
Thermophilic microorganisms have been proven as a potential source of bioactive compounds and richest source of secondary metabolites. They are the most economically and biotechnologically valuable microorganisms. However, the research on thermophilic actinomycetes from Indian peninsula is very scanty. In the present study we have demonstrated that our isolated strains of thermophilic actinomycetes having potential to produce extracellular enzymes (amylase and xylanase) simultaneously using agro-industrial waste as sole carbon source. The increasing expansion of agro-industrial activity has led to the accumulation of a large quantity of lignocellulosic residues all over the world.
Isolation and screening of thermophilic actinomycetes
In the course of isolation and systematic screening of thermophilic actinomycetes, we have isolated seven thermophilic strains of actinomycetes. 10–6 dilution was found to be most suitable for isolation of thermophilic actinomycetes from mushroom compost. Morphological and colony characteristics of isolates were described in Table 1. Screening of isolates for utilization of agro-industrial wastes and their simultaneous production of amylase and xylanase was done on wheat straw as sole carbon source. Out of seven isolates four isolates (MSC702, MSC703, MSC704 and MSC707) have potential to produce amylase and xylanase enzymes simultaneously on agro-industrial waste (wheat straw) (Table 1). Isolate designated MSC702 having highest potential to produce amylase (234.54 IU/ml) and xylanase (28.40 IU/ml) on wheat straw. Strain MSC702 seems to be a novel species of Streptomyces through morphological characterization and 16S rRNA gene sequence.
Table 1.
Colony characteristics of isolates and their enzyme (amylase and xylanase) productivity.
| Colony characteristics | Enzyme productivity | |||||
|---|---|---|---|---|---|---|
| Isolates | Aerial mycelia | Substrate mycelia | Colony form (Growth, Colony type, Edge, Elevation, Texture) | Soluble pigment | Amylase activity (IU/mL) | Xylanase activity (IU/mL) |
| MSC701 | White | Colorless | Abundant, Irregular, Undulate, Flat, Pasty | None | 194.82 | - |
| MSC702 | White | Colorless | Abundant, Circular, Entire, Raised, Dry Powdery | None | 234.54 | 28.40 |
| MSC703 | White | Colorless | Circular, Entire, Raised, Granular | None | 203.29 | 16.28 |
| MSC704 | Steel grey | Cream | Circular, Entire, Umbonate, Pasty | None | 186.41 | 26.98 |
| MSC705 | White | Colorless | Punctiform circular, Entire, Raised, Dry Powdery | None | - | - |
| MSC706 | White | Colorless | Circular, Entire, Convex, Dry Powdery | None | 129.76 | - |
| MSC707 | White to grey | Colorless to black | Circular, Entire, Raised, Dry Powdery | None | 197.55 | - |
Characterization of the strain
An almost complete 16S rRNA gene sequence (1474 nucleotides) for strain MSC702 was determined in this study. This sequence was subjected to similarity searches against public databases to infer a possible relationship of strain MSC702. This analysis revealed that strain MSC702 was a member of the genus Streptomyces. The comparative analysis of 16S rRNA gene sequence yielded following closest fits: 98.28% (25 nucleotides differences out of 1492) S. thermocoprophilus B19T (18), 98.13% with S. mexicanus CH-M-1035T (27 nucleeotides differences out of 1450) (29), 98.08% with S. laurentii LMG 19959T (28 nucleotides differences out of 1460) (41), 97.94% with S. coeruleorubidus NBRC 12761T (30 nucleotides differences out of 1459) (31), 97.89% with S. curacoi NRRL B-2901 (31 nucleotides differences out of 1471) (Cataldi 1963; have no publication) and 97.88% with S. thermoviolaceus subsp. apingens DSM 41392T (31 nucleotides differences out of 1462) (14).
It is apparent from the genotypic data that strain MSC702 forms a distinct centre of taxonomic variation within the genus Streptomyces. Further chemotaxonomical and biochemical studies will be carried out for this microorganism be recognized as a novel species of Streptomyces.
Streptomyces sp. MSC702 was deposited in the Microbial Type Culture Collection and Gene Bank (MTCC), Chandigarh, India with accession number MTCC 10772.
Production of amylase and xylanase by Streptomyces sp. MSC702
To check the agro-industrial wastes suitability for amylase and xylanase production by Streptomyces sp. MSC702, fermentation was done with the addition of 1 % (w/v) sugarcane bagasse (SB), wheat bran (WB), rice bran (RB) and corn cob (CC) separately in the production medium by replacing wheat straw (WS). The order of substrate suitability was RB > CC > WB > WS > SB and RB > WB > WS > SB > CC for amylase and xylanase production respectively (Table 2). Table 2 also describes the starch and hemicelluloses content in agro-industrial wastes. Rice bran was found most suitable for amylase (373.89 IU/mL) and xylanase (30.15 IU/mL) production by Streptomyces sp. MSC702. Present study on utilization of agro-industrial wastes for the production of amylase and xylanase by Streptomyces sp. MSC702 showed that production of amylase is directly related to the raw starch content in agro-industrial wastes while high hemicellolose content in agro-industrial wastes have no effect on xylanase production. This may be due to the preferred utilization of starch for their metabolic activities by Streptomyces sp. MSC702 over hemicellulose. Stutzenberger (38) reported production of extracellular enzymes (amylase and xylanase) on sugarcane bagasse as sole carbon source by Thermomonospora curvata. Recently, both the enzymes were attempted separately by the workers. Kammoun et al. (15) reported amylase (148.0 U/mL) production on gruel (wheat grinding by-product) based medium by Aspergillus oryzae CBS 819.72 in SmF. Dey et al. (8) used wheat bran as media component for the production of amylase (76.4 U/ml) by Bacillus circulans GRS 313 in SmF. Shatta et al. (35) utilized many agro-industrial wastes (wheat bran, rice bran, yellow maize meal, dry yeast, brewery yeast and corn steep liquor) in basal medium as sole carbon source for the production of amylase by Streptomyces aureofaciens 77 in SmF. Dhillon et al. (9) found that agro-industrial wastes (bagasse, rice straw, oat husk, rice husk and wheat straw) were the best inducers for the xylanase production by Bacillus circulans AB 16 in SmF. They reported highest xylanase activity (20.6 IU/mL) on rice straw.
Table 2.
Starch and hemicellulose contents of utilized agro-industrial wastes and amylase and xylanase activity by Streptomyces sp. MSC702 in presence of agro-industrial waste in basal medium.
| Agro-industrial wastes | Starch (%) | Hemicellulose (%) | Amylase activity (IU/mL) | Xylanase activity (IU/mL) |
|---|---|---|---|---|
| Sugar cane bagasse | - | 16.35 ± 0.49 | 201.14 | 9.32 |
| Wheat bran | 13.39 | 7.55 ± 0.49 | 261.48 | 29.91 |
| Rice bran | 10.0 | 9.60 ± 1.98 | 373.89 | 30.15 |
| Corn cob | - | 16.20 ± 0.57 | 368.87 | 6.23 |
| *Wheat straw | - | 29.0 ± 3.0 | 234.54 | 28.40 |
To enhance the production of amylase and xylanase by Streptomyces sp. MSC702 different combinations (1:1 w/v) of other agro-industrial wastes with rice bran were analyzed in production medium (Fig. 1). In the present study, out of the different combinations (RB:SB; RB:WB; RB:CC and RB:WS) tested, amylase and xylanase activity reached up to 398.10 IU/mL and 53.15 IU/mL respectively in rice bran and wheat bran containing medium. Ten different combinations (0.5:0.5; 1:1; 1:2; 1:3; 1:4; 1:5; 2:1; 3:1; 4:1 and 5:1) of rice bran and wheat bran were analyzed to maximize amylase and xylanase production (Fig. 2). The highest amylase (549.11 IU/mL) and xylanase (63.91 IU/mL) activity was found at the level from the cultivation of mixed substrates of rice bran: wheat bran in 1:2 ratio. Mixed agro-industrial wastes (wheat bran: rice husk; wheat bran: wheat straw) were also utilized by many workers for the production of amylases and xylanases (3, 4) by different microorganisms in SSF.
Figure 1.
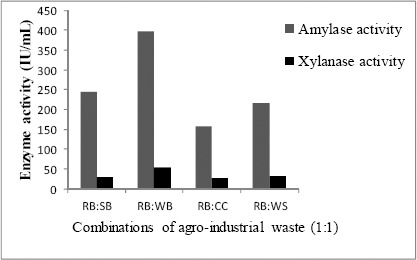
Effect of mixed agro-industrial wastes (% w/v) on the production of amylase and xylanase by Streptomyces sp. MSC702.
Figure 2.
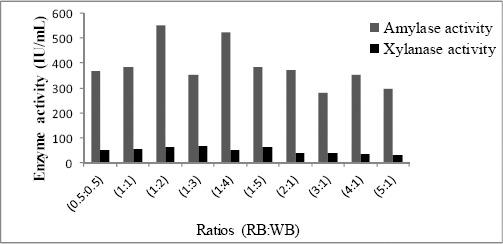
Effect of different ratios (% w/v) of rice bran and wheat bran on the production of amylase and xylanase by Streptomyces sp. MSC702.
There are very few reports for the production of extracellular enzymes on mixed agro-industrial wastes in SmF. Mostly, mixed agro-industrial wastes are employed in SSF processes. Rather than applying SmF or SSF, it’s important to utilize the agro-industrial wastes for production of industrially valuable products for making low cost processes and point of environmental concern. Various waste and underutilized lignocellulosic agricultural residues can serve as low-cost feed stock for production of industrially important extracellular enzymes (amylase and xylanase).
CONCLUSION
Lignocellulosic residues from wood, grass, agricultural, forestry wastes and municipal solid wastes are particularly abundant in nature and have a potential for bioconversion into many useful biological and chemical products. The results showed the potential use of cheaply available raw substrates (agro-industrial wastes) for single step production of extracellular enzymes (amylase and xylanase) by a newly isolated strain of thermophilic actinomycete, Streptomyces sp. MSC702 in SmF. The mixture of rice bran and wheat bran (1:2) was found as the best substrate and support in SmF in laboratory level fermentations. The enzyme activity was reached up to 549.11 IU/mL (~1.3-fold increase) and 63.91 IU/mL (~2.0-fold increase) for amylase and xylanase, respectively, on the mixture of rice bran and wheat bran (1:2) as sole carbon source. The use of this microorganism, Streptomyces sp. MSC702 in low cost bioremediation processes might be attractive for given their highly efficient starch and lignocellulose hydrolysis enzyme machinery.
ACKNOWLEDGEMENTS
Renu Singh thanks the University Grant Commission, Selection and Award Bureau, New Delhi, Government of India for providing ‘Rajiv Gandhi National Fellowship’ to carry out this work.
REFERENCES
- 1.Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aygan A., Arikan B., Korkmaz H., Dincer S., Colak O. Highly thermostable and alkaline α-amylase from a halotolerant-alkaliphilic Bacillus sp. AB68. Braj. J. Microbiol. 2008;39(3):547–553. doi: 10.1590/S1517-838220080003000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azin M., Moravej R., Zareh D. Production of xylanase by Trichoderma longibrachiatum on a mixture of wheat bran and wheat straw: Optimization of culture condition by Taguchi method. Enzyme Microb. Tech. 2007;40:801–805. [Google Scholar]
- 4.Baysal Z., Uyar F., Aytekin C. Solid state fermentation for production of α-amylase by a thermotolerant Bacillus subtilis from hot-spring water. Process Biochem. 2003;38:1665–1668. [Google Scholar]
- 5.Beg Q.A., Kapoor M., Mahajan G., Hoondal S. Microbial xylanases and their industrial applications: a review. Appl. Microbiol. Biotechnol. 2001;56:326–338. doi: 10.1007/s002530100704. [DOI] [PubMed] [Google Scholar]
- 6.Bragger J.M., Daniel R.M., Coolbear T., Morgan H.W. Very stable enzymes from extremely thermophilic archebacteria and eubacteria. Appl. Microbiol. Biotechnol. 1989;31:556–561. [Google Scholar]
- 7.Damaso M.C.T., Andrade C.M.M.C., Jr N.P. Production and properties of the cellulase-free xylanase from Thermomyces lanuginosus IOC-4145. Braj. J. Microbiol. 2002;33:333–338. [Google Scholar]
- 8.Dey G., Mitra A., Banerjee R., Maiti B.R. Enhanced production of amylase by optimization of nutritional constituents using response surface methodology. Biochem. Eng. J. 2001;7:227–231. [Google Scholar]
- 9.Dhillon A., Gupta J.K., Jauhari B.M., Khanna S. A cellulase-poor, thermostable, alkalitolerant xylanase produced by Bacillus circulans AB 16 grown on rice straw and its application in biobleaching of eucalyptus pulp. Bioresource Technol. 2000;73:273–277. [Google Scholar]
- 10.Edwards C. Isolation properties and potential applications of thermophilic actinomycetes. Appl. Biochem. Biotechnol. 1993;42:161–178. [Google Scholar]
- 11.Fang H.Y., Chang S.M., Hsieh M.C., Fang T.J. Production, optimization growth conditions and properties of the xylanase from Aspergillus carneus M34. J. Mol. Catal. B: Enzymatic. 2007;49:36–42. [Google Scholar]
- 12.Goyal M., Kalra K.L., Sareen V.K., Soni G. Xylanase production with xylan rich lignocellulosic wastes by a local soil isolate of Trichoderma viride. Braj. J. Microbiol. 2008;39(3):535–541. doi: 10.1590/S1517-838220080003000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta R., Gigras P., Mohapatra H., Goswami V.K., Chauhan B. Microbial α-amylases: a biotechnological perspective. Process Biochem. 2003;38:1599–1616. [Google Scholar]
- 14.Henssen A. Morphology and system of thermophilic actinomycetes. Arch. Mikrobiol. 1957;26(4):373–414. [PubMed] [Google Scholar]
- 15.Kammoun R., Naili B., Bejar S. Application of a statistical design to the optimization of parameters and culture medium for α-amylase production by Aspergillus oryzae CBS 819.72 grown on gruel (wheat grinding by-product) Bioresource Technol. 2008;99:5602–5609. doi: 10.1016/j.biortech.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 16.Kapoor M., Beg Q.K., Bhushan B., Singh K., Dadhich K.S., Hoondal G.S. Application of an alkaline and thermostable polygalacturonase from Bacillus sp. MG-cp-2 in degumming of ramie (Boehmeria nivea) and sunn hemp (Crotalaria juncea) bast fibers. Process Biochem. 2001;36:803–807. [Google Scholar]
- 17.Kapoor V., Singh R., Banerjee R., Kumar V. Application of response surface methodology (RSM) for optimization of physico-chemical parameters for the production of endoglucanase by Trichoderma ressei Rut C-30 using agro-residues. Dyn. Biochem. Process Biotech. Mol. Biol. 2011;5(2):35–40. [Google Scholar]
- 18.Kim B., Al-Tai A.M., Kim S.B., Somasundaram P., Goodfellow M. Streptomyces thermocoprophilus sp nov, a cellulase-free endo-xylanase-producing streptomycete. Int. J. Syst. Evol. Microbiol. 2000;50:505–509. doi: 10.1099/00207713-50-2-505. [DOI] [PubMed] [Google Scholar]
- 19.Lee J. Biological conversion of lignocellulosic biomass to ethanol. J. Biotechnol. 1997;56:1–24. doi: 10.1016/s0168-1656(97)00073-4. [DOI] [PubMed] [Google Scholar]
- 20.Lin L.L., Chyau C.C., Hsu W.H. Production and properties of a raw starch-degrading amylase from the thermophilic and alkalophilic Bacillus sp. TS-23. Biotechnol. Appl. Biochem. 1998;28:61–68. [PubMed] [Google Scholar]
- 21.Miller L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428. [Google Scholar]
- 22.McKendry P. Energy production from biomass: overview of biomass. Bioresource Technol. 2002;83:37–43. doi: 10.1016/s0960-8524(01)00118-3. [DOI] [PubMed] [Google Scholar]
- 23.Mihara S., Inaba Y., Tachibana K., Endo T., Yasui E. Process for complete separation of constituents of rice-bran and the like. 1974 United State Patent, 3852504. [Google Scholar]
- 24.Niehaus F., Bertoldo C., Kahler M., Antranikian G. Extremophiles as a source of novel enzymes for industrial applications. Appl. Microbiol. Biotechnol. 1999;51:711–729. doi: 10.1007/s002530051456. [DOI] [PubMed] [Google Scholar]
- 25.Obi S.K.C., Odibo F.J.C. Partial purification and characterization of a thermostable actinomycete β-amylase. Appl. Environ. Microbiol. 1984;47(3):571–575. doi: 10.1128/aem.47.3.571-575.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osborne T.B., Mendel L.B. The nutritive value of the wheat kernel and its milling products. J. Biol. Chem. 1919;37:557–601. [Google Scholar]
- 27.Pandey A., Nigam P., Soccol C.R., Soccol V.T., Singh D., Mohan R. Advances in microbial amylases. Biotechnol. Appl. Biochem. 2000;31:135–152. doi: 10.1042/ba19990073. [DOI] [PubMed] [Google Scholar]
- 28.Perez J., Munoz-Dorado J., De-la-Rubia T., Martinez T. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int. Microbiol. 2002;5:53–63. doi: 10.1007/s10123-002-0062-3. [DOI] [PubMed] [Google Scholar]
- 29.Petrosyan P., Varela M.G., Madrigal A.L., Huitron C., Flores M.E. Streptomyces mexicanus sp. nov., a xylanolytic micro-organism isolated from soil. Int. J. Syst. Evol. Microbiol. 2003;53:269–273. doi: 10.1099/ijs.0.02251-0. [DOI] [PubMed] [Google Scholar]
- 30.Prassad S., Singh A., Joshi H.C. Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour. Conserv. Recycling. 2007;50:1–39. [Google Scholar]
- 31.Pridham T.G., Hesseltin C.W., Benedict R.G. A guide for the classification of streptomycetes according to selected groups. Placement of strains in morphological sections. Appl. Microbiol. 1958;6:52–79. doi: 10.1128/am.6.1.52-79.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rainey F.A., Ward-Rainey N., Kroppenstedt R.M., Stackebrandt E. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Bacteriol. 1996;46:1088–1092. doi: 10.1099/00207713-46-4-1088. [DOI] [PubMed] [Google Scholar]
- 33.Rowell M.R. Washington, DC: American Chemical Society; 1992. Opportunities for lignocellulosic materials and composites. Emerging technologies for material and chemicals from biomass: Proceedings of symposium; pp. 26–31. [Google Scholar]
- 34.Sanchez C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009;27:185–194. doi: 10.1016/j.biotechadv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Shatta A.M., El-hamahmy A.F., Ahmed F.A., Ibrahim M.M.K., Arafa M.A.I. The influence of certain nutritional and environmental factors on the production of amylase enzyme by Streptomyces aureofaciens 77. J. Islamic Acad. Sci. 1990;3(2):134–138. [Google Scholar]
- 36.Singh R., Kapoor V., Kumar V. Production of thermostable, Ca+2-independent, maltose producing α-amylase by Streptomyces sp. MSC702 (MTCC 10772) in submerged fermentation using agro-residues as sole carbon source. Ann. Microbiol. 2011 DOI: 10.1007/s13213-011-0340-4. [Google Scholar]
- 37.Singh R., Kapoor V., Kumar V. Influence of carbon and nitrogen sources on the α-amylase production by a newly isolated thermophilic Streptomyces sp. MSC702 (MTCC 10772) Asian J. Biothechnol. 2011;3(6):540–553. [Google Scholar]
- 38.Stutzenberger F. Extracellular enzyme production grown on by Thermomonospora curvata grown on bagasse. J. Ind. Microbiol. 1994;13:35–42. [Google Scholar]
- 39.Sukumaran R.K., Singhania R.R., Mathew G.M., Pandey A. Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renewable Energy. 2009;34:421–424. [Google Scholar]
- 40.Techapun C., Sinsuwongwat S., Poosaran N., Watanabe M., Sasaki K. Thermostable and alkaline - tolerant cellulase - free xylanase produced by thermotolerant Streptomyces sp. Ab106. J. Biosci. Bioeng. 2002;93(4):431–433. doi: 10.1016/s1389-1723(02)80080-9. [DOI] [PubMed] [Google Scholar]
- 41.Trejo W.H., Dean L.D., Pluscec J., Meyers E., Brown W.E. Streptomyces laurentii, a new species producing thiostrepton. The J. Antibiot. 1977;30(8):639–643. doi: 10.7164/antibiotics.30.639. [DOI] [PubMed] [Google Scholar]
- 42.Waksman S.A. Principles of Soil Microbiology. London: Bailliere Tindall and Cox; 1927. [Google Scholar]
- 43.Wong K.K.Y., Saddler J.N. Trichoderma xylanases, their properties and application. Crit. Rev. Biotechnol. 1992;12:413–435. [Google Scholar]


