Abstract
The treatment of tuberculosis has become more difficult with the worldwide spread of multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains of Mycobacterium tuberculosis. Moreover, the prevalence of human disease caused by atypical mycobacteria has also increased in the past two decades and has further complicated the problem of the treatment of mycobacterial infections. It is therefore urgent to develop new highly active molecules against these bacteria. The present study reports the isolation from a Moroccan soil of a Bacillus strain that exhibits an important antimycobacterial activity. The strain was identified as Brevibacillus laterosporus using DNA sequencing of the 16S ribosomal RNA gene. The antimycobacterial activity was assigned to a substance with a protein nature. This nature was revealed using a liquid-liquid extraction with organic solvents, precipitation with ammonium sulfate and treatment with a protease. This study suggested the identification and the characterization of this active metabolite enabling therapeutic investigations further.
Keywords: Mycobacteria, Antimycobacterial, Antibiotic, Brevibacillus
INTRODUCTION
According to the latest report published by the World Health Organization (WHO) in 2009 (27), the incidence of tuberculosis (TB) worldwide is estimated at 9.27 million cases with a mortality of 1.7 million, including 0.2 million HIV-positive cases. Developing countries are the most affected, with approximately 95% of the cases and 99% of the deaths. Indeed, the estimated incidence is the highest in sub-Saharan Africa, followed by Southeastern Asia (18). In industrialized countries, tuberculosis has again become a major threat after the emergence of drug-resistant forms in the early 1990s. However, the incidence remains low and does not exceed an average of 17 cases per 100000 inhabitants (18).
The causes of failure in the control of this disease are multi-factorial and are primarily related to the advent of acquired immunodeficiency syndrome (AIDS), the relative effectiveness of the vaccine Bacille Calmette–Guérin (BCC) (2) and the expansion of multidrug-resistant and extensively drug-resistant strains of Mycobacterium tuberculosis (6). This resistance has been favored by the length of the currently available therapeutic schemes and by the inappropriate use of antibiotics.
A multidrug-resistant strain (MDR-TB) is defined as a strain resistant to at least the two major first-line TB drugs, isoniazid (INH) and rifampicin (RIF). If a MDR-TB strain is also resistant to at least one of the three second-line injectable TB drugs (capreomycin, kanamycin or amikacin) in addition to any one of the fluoroquinolones, the strain is considered to be extensively drug-resistant (XDR-TB) (16). It is urgent to develop new highly active molecules against M. tuberculosis, including MDR and XDR strains. These molecules must act rapidly, have a long half-life for intermittent administration and be capable of sterilizing the sites where the bacteria remain persistent (10).
Telluric microorganisms are the primary source of antibiotics (11). Bacillus is a bacterial genus that is abundant in soil and contains several species that produce a large number of antibiotics with different chemical structures (29). These antibiotics are mainly active against Gram-positive bacteria, but compounds such as polymyxin, colistin and circulin inhibit the growth of Gram-negative bacteria (3), while bacillomycin, mycobacillin and fungistatin are active against yeast and fungi (12). The antibiotics produced by Bacillus mainly have a peptidic nature, and the majority of them are produced by strains of Bacillus subtilis and Bacillus brevis (13).
The aim of this study was the isolation and identification of a strain of Brevibacillus laterosporus that exhibits an important antimycobacterial activity. Furthermore, This study aimed to determine the nature of the substance responsible for this activity. To our knowledge, this is the first report on a strain of Brevibacillus laterosporus showing an antimycobacterial activity.
MATERIALS AND METHODS
Samples and microbial strains
Soil samples were collected from ten Moroccan ecosystems. Using a large sterile spatula, the first five centimeters of the surface layer of the soil were removed. Then, with a small sterile spatula, 100 to 150 g of soil were taken from the subjacent layer at 5 to 15 cm of depth and deposited on a sterile aluminum sheet. The large debris, such as stones and roots, were eliminated, and approximately 50 g of the remaining material was placed in a sterile flask and transported as quickly as possible to the laboratory.
The microbial strains used in this study were:
– Mycobacterium aurum A+: a non-pathogenic Mybacterium species with a generation time of approximately 6 h. This strain was used as a model to evaluate the effect of active substances on the growth of M. tuberculosis (4),
– Mycobacterium smegmatis mc(2)155: a non-pathogenic atypical mycobacterial strain with a generation time of approximately 3 h,
– Mycobacterium kansasii ATCC 12478: an atypical mycobacterial strain causing opportunistic infections,
– Mycobacterium bovis BCG IP: the vaccine strain,
– Mycobacterium vaccae ATCC 1548314 and
– Escherichia coli DH5α.
The mycobacteria were cultivated at 37 °C on Lowenstein Jensen medium or Sauton medium (1, 21).
The Escherichia coli strain was cultivated on Luria Bertani (LB) medium containing the following: peptone (Biokarps Diagnostics, Beauvais, France), 10 g/l; yeast extract (Biokarps Diagnostics, Beauvais, France), 5 g/l; and sodium chloride (Riedel- de Haën, Seelze, Germany), 10 g/l.
Screening for bacterial strains with antimycobacterial activity
To isolate bacterial strains with antimycobacterial activities, 4 g of each soil sample were dissolved in 36 ml of sterile saline (NaCl, 9 g/l) and shaken for 2 h at ambient temperature. The supernatant was then recovered, and various dilutions were prepared in LB medium (10–1 to 10–7). A volume of 100 μl of each dilution was plated onto LB agar previously seeded with approximately 106 CFU/ml of M. aurum A+ or M. smegmatis cultures. After incubation at 37°C for 48 h, the bacterial clones surrounded by inhibition zones were recovered. One of these clones showed strong activity and was maintained for this work.
Antimycobacterial activity assay
The isolated clone was cultivated under agitation on LB medium at 37°C for 24 h. A volume of 10 ml of the bacterial culture was centrifuged for 10 min at 6000 g. The supernatant was then recovered and sterilized by filtration. The antimycobacterial activity was evaluated using an agar well diffusion assay on plates pre-seeded with the indicator strains, M. aurum A+ or M. smegmatis (23).
The agar wells were filled with 100 µl of the prepared filtrates (20). The zones of inhibition were analyzed after 72 h of incubation at 37°C. Each test was repeated 3 times. For the negative control, an E. coli culture filtrate was used.
The antimycobacterial activity was also studied after a liquid-liquid extraction using ethyl acetate and ether solvents. The isolated clones were cultivated under agitation on LB medium at 37°C for 48 h. A volume of 100 ml of the bacterial culture was centrifuged for 10 min at 6000 g. Then, the supernatant was recovered, sterilized by filtration and added to 100 ml of organic solvent, either ethyl acetate or ether. After agitation for one hour at room temperature, the organic extract obtained was evaporated under vacuum at 37°C. The dry residue was taken up in 1 ml of sterile distilled water and filtered. The antimycobacterial activity was measured using the agar well diffusion assay on plates pre-seeded with the indicator strains, M. aurum A+ and M. smegmatis, as described above. For the negative control, an E. coli culture filtrate was used.
Kinetics of the active substance production
The bacterial growth was assessed by monitoring the optical density at 595 nm (O.D595 nm). To determine the kinetics of the production of the active substance, a colony of the tested bacterial strain was inoculated in a volume of 100 ml of LB medium and incubated at 37°C for 12 h. This culture was diluted 104 fold in a volume of 2 l of fresh LB medium and incubated at 37°C under moderate agitation. At regular time intervals, 100 ml of this culture was recovered and a liquid-liquid extraction using ether was performed as previously described. A volume of 100 μl of the organic extract was then deposited in wells on Sauton agar medium already pre-seeded with M. aurum A+ (O.D595 nm: 0.2). The diameters of inhibition were measured after 72 h of incubation at 37°C.
Identification of the antimycobacterial-producing strain
To identify the antimycobacterial-producing strain, amplification and sequencing of the 16S rRNA gene was performed. The use of 16S rRNA is a powerful tool that has been used to identify bacteria from various sources, such as environmental or clinical specimens, and to establish phylogenetic relationships between bacteria (5, 19, 25, 28). The DNA extraction from bacteria was carried out according to a standard method (17). For the PCR amplification, the universal primers fD1 (5’- AGAGTTTGATCCTGGCTCAG-3’) and rP2 (5’-TACGGCTACCTTGTTACGACTT- 3’) were used to amplify a 1.5-kb fragment of the 16S rDNA (9, 26). The PCR was performed under the following conditions: 94°C for 5 min, 35 cycles of 94°C for 45 s, 55°C for 1 min and 72°C for 2 min and finally 72°C for 10 min.
For sequencing, the PCR products were purified using a PCR product purification kit according to the manufacturer (JETquick, Genomed). The sequencing reaction mixture contained 2.0 µL of BigDye V 1.1, 2.7 µL of the PCR product and 0.5 µM of the primer, either fD1 or rP2. A final reaction volume of 10 µL was used. The amplification conditions included an initial denaturation at 96ºC (3 min) followed by 35 cycles of denaturation at 96ºC (20 s), annealing at 50ºC (5 s) and extension at 60ºC (4 min). The sequencing of the PCR products was performed on an ABI PRISM sequencing apparatus (ABI Prism 310 Genetic Analyzer, Applied Biosystems), and the data analysis was completed using sequencing analysis software.
Characterization of the protein nature of the antimycobacterial metabolite
The bacterial strain was grown in 100 ml of LB medium at 37°C until the early stationary phase. The cells were removed by centrifugation for 10 min at 6000 g and 4°C, and the supernatant was recovered and filtered using a 0.22-μm filter (Millipore, Molsheim, France).
A precipitation with 80% saturated ammonium sulfate was carried out overnight at 4°C under agitation (29). The resulting precipitate was harvested by centrifugation at 10000 g for 20 min at 4°C, resuspended in 1 ml of potassium phosphate buffer (50 mM; pH 6) and dialyzed against the same buffer at 4°C for 24 h. The buffer was renewed several times to facilitate a better dialysis. The antimycobacterial activity of the resulting precipitate was measured using the agar well diffusion assay on plates pre-seeded with the indicator strain M. aurum A+ (O.D595 nm: 0.2).
The diameters of inhibition were determined after 72 h of incubation at 37°C. This experiment was repeated twice. The control that was used corresponded to a dialyzed product obtained from an E. coli culture prepared in the same conditions as the test strain. The proteins in the resulting precipitate were quantified using the Lowry method (15) and analyzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
To establish the protein nature of the antimycobacterial metabolite, the metabolite was also tested for its sensitivity to proteinase K (29). Two controls were used: (1) the ammonium sulfate extract untreated by proteinase K and (2) proteinase K without the extract. The samples were incubated in the presence of proteinase K (1 mg/ml) at 37°C for 3 h. The antimycobacterial effect of these preparations was examined using the agar well diffusion assay against M. aurum A+ as previously described.
RESULTS AND DISCUSSION
Isolation of a bacterial strain with antimycobacterial activity
Telluric microorganisms were the principal source of antibiotics discovered in the second half of the 20th century (13). In this work, we chose particular biotopes to isolate bacteria that express antimycobacterial activity. A clone surrounded by an inhibition zone was obtained on LB-agar medium pre-seeded with M. smegmatis. Furthermore, the filtrate prepared from the isolated clone was able to inhibit the growth of M. smegmatis and M. aurum A+ (Table 1), indicating that the active substance was secreted by the clone. The filtrate was also active against M. vaccae, M. bovis BCG and M. kansasii (data not shown). In addition, the ethyl acetate and ether extracts showed a more accentuated antimycobacterial activity than that of the filtrate (Table 1), indicating that the solvents were capable of extracting and concentrating the active substance responsible for the observed biological activity.
Table 1.
Antimycobacterial activity of the bacterial isolate (measured in mm).
| Microorganism | Filtrate | Ethyl acetate extract | Ether extract |
|---|---|---|---|
| M. smegmatis | 10 ± 0.5 | 32 ± 0.0 | 33 ± 1.5 |
| M. aurum A+ | 11.5 ± 0.7 | 34.5 ± 2.12 | 35 ± 0.7 |
The data are average values of triplicates ± one standard deviation.
The production kinetics of the active substance were also studied (Fig. 1). The antimycobacterial activity was detected after six hours and increased progressively during the exponential phase of growth. The maximum activity was reached after 24 h of growth and then decreased rapidly. These results (Table 1, Fig. 1) indicate that the active substance, responsible for the observed antimycobacterial effect, is synthesized during the exponential phase and early stationary phase.
Figure 1.
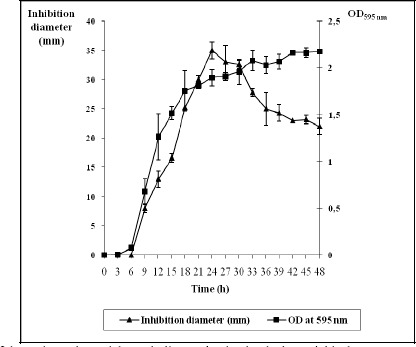
Time course of the antimycobacterial metabolite production by the bacterial isolate.
Molecular identification of the antimycobacterial-producing isolate
The lengths of the 16S rDNA sequences analyzed were 713 bp and 648 bp for the primers fD1 and rP2, respectively. Their analysis in comparison with the sequences available in Gen Bank, EMBL, DDJB and PDB databases showed that the antimycobacterial-producing strain was closely related to Brevibacillus laterosporus species with 100% similarity (accession number: DQ371289.2).
Characterization of the protein nature of the antimycobacterial metabolite
The protein extract of the Brevibacillus laterosporus strain was prepared and shown to be capable of inhibiting M. aurum A+ growth (Table 2). Moreover, this activity was totally lost in the presence of proteinase K (Table 2). The same result was also obtained after treatment of the organic extracts, obtained with ethyl acetate and ether, with proteinase K (Table 2). This result indicates that the bioactive metabolite secreted by the Brevibacillus strain is of a protein nature.
Table 2.
Characterization of the protein nature of the antimycobacterial metabolite.
| Inhibition diameters against M. aurum A+ (mm) | ||||||
|---|---|---|---|---|---|---|
| Without treatment with proteinase K | After treatment with proteinase K | Controls | ||||
| T1 | T2 | T3 | ||||
| ASP | 33 ± 0.7 | 0 | 0 | 0 | 0 | |
| EAE | 34.5 ± 1.5 | 0 | 0 | 0 | 0 | |
| EE | 24.5 ± 0.0 | 0 | 0 | 0 | 0 | |
ASP: ammonium sulfate precipitate; EAE: ethyl acetate extract; EE: ether extract; T1: ammonium sulfate precipitate of sterile LB medium; T2: ammonium sulfate precipitate of LB medium inoculated with E. coli; T3: proteinase K solution. The data are average values of duplicates ± one standard deviation.
The concentration of the protein extract was determined to be 0.0104 mg/ml. An analysis of the extract using polyacrylamide gel electrophoresis revealed the presence of nine bands (Fig. 2) with molecular weights comprised between 40 and 240 kDa. These bands were absent in the control, an E. coli protein extract. These results (Table 2, Fig. 2) indicate that the antimycobacterial activity of the bacterial isolate could be due to one protein or the synergistic action between two or more of these proteins.
Bacillus species produce approximately 167 biological compounds (12). Brevibacillus laterosporus comb. nov. (24), previously classified as Bacillus laterosporus (Laubach 1916), has demonstrated a very wide spectrum of biological activity (7). Several antibiotics produced by Brevibacillus laterosporus have been described, such as laterosporamine, laterosporin (12), tauramamide and loloatin A.
Tauramamide is a lipopeptide antibiotic active against pathogenic Enterococcus sp (8), while loloatin A was identified as a cyclic decapeptide with cyanolytic activity (14). Moreover, an antimicrobial substance produced by a Brevibacillus laterosporus strain isolated from a marine habitat was recently purified and characterized. The antimicrobial substance had high activities against Gram-positive bacteria, such as Streptococcus, Staphylococcus aureus and Clostridium; Gram-negative bacteria, such as Escherichia coli and Pseudomonas putrefaciens; and against Candida albicans (22).
As previously described, the Brevibacillus laterosporus strain isolated in this work is able to secrete an active substance of a protein nature that inhibits the growth of various mycobacteria. To our knowledge, this study is the first report describing such a substance produced by a strain of B. laterosporus. Based on this study, our goal is to test the antimycobacterial metabolite on M. tuberculosis and on mycobacteria hosted by macrophages. Moreover, the identification and the characterization of this compound would facilitate further therapeutic investigations.
REFERENCES
- 1.Allen B.W. Mycobacteria: general culture methodology and safety considerations. Methods Mol. Biol. 1998;101:15–30. doi: 10.1385/0-89603-471-2:15. [DOI] [PubMed] [Google Scholar]
- 2.Billy C., Lévy-Bruhl D. Vaccin BCG et place de l’intradermoréaction en 2006. La Revue de médecine interne. 2007;28(3):151–160. doi: 10.1016/j.revmed.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Bottone E.J., Peluso R.W. Production by Bacillus pumilus (MSH) of an antifungal compound that is active against Mucoraceae and Aspergillus species: preliminary report. J. Med. Microbiol. 2003;52:69–74. doi: 10.1099/jmm.0.04935-0. Pt 1. [DOI] [PubMed] [Google Scholar]
- 4.Chung G.A., Aktar Z., Jackson S., Duncan K. High throughput screen for detecting antimycobacterial agents. Antimicrob. Agents. Chemother. 1995;39(10):2235–2238. doi: 10.1128/aac.39.10.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarridge J.E. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 2004;17(4):840–862. doi: 10.1128/CMR.17.4.840-862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danilchanka O., Mailaender C., Niederweis M. Identification of a novel multidrug efflux pump of Mycobacterium tuberculosis. Antimicrob. Agents. Chemother. 2008;52(7):2503–2511. doi: 10.1128/AAC.00298-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Oliveira E.J., Rabinovitch L, Monnerat R.G., Passos L.K.J., Zahner V. Molecular characterization of Brevibacillus laterosporus and its potential use in biological control. Appl. Environ. Microbiol. 2004;70(11):6657–6664. doi: 10.1128/AEM.70.11.6657-6664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desjardine K., Pereira A., Wright H., Matainaho T., Kelly M., Andersen R.J. Tauramamide, a lipopeptide antibiotic produced in culture by Brevibacillus laterosporus isolated from a marine habitat: structure elucidation and synthesis. J. Nat. Prod. 2007;70(12):1850–1853. doi: 10.1021/np070209r. [DOI] [PubMed] [Google Scholar]
- 9.Drancourt M., Bollet C., Carlioz A., Martelin R., Gayral J.P., Raoult D. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J. Clin. Microbiol. 2000;38(10):3623–3630. doi: 10.1128/jcm.38.10.3623-3630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutta N.K., Dastidar S.G., Kumar A., Mazumdar K., Ray R., Chakrabarty A.N. Antimycobacterial activity of the antiinflammatory agent diclofenac sodium, and its synergism with streptomycin. Braz. J. Microbiol. 2004;35:316–323. [Google Scholar]
- 11.Gillespie D.E., Brady S.F., Bettermann A.D., Cianciotto N.P., Liles M.R., Rondon M.R., Clardy J., Goodman R.M., Handelsman J. Isolation of antibiotics turbomycin A and B from a metagenomic library of soil microbial DNA. App. Environ. Microbiol. 2002;68(9):4301–4306. doi: 10.1128/AEM.68.9.4301-4306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz E., Demain A.L. The peptide antibiotics of Bacillus: chemistry, biogenesis and possible functions. Bacteriol. Rev. 1977;41(2):449–474. doi: 10.1128/br.41.2.449-474.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleinkauf H., von Döhren H. Nonribosomal biosynthesis of peptide antibiotics. Euro. J. Biochem. 1990;192(1):1–15. doi: 10.1111/j.1432-1033.1990.tb19188.x. [DOI] [PubMed] [Google Scholar]
- 14.Krachkovskiĭ S.A., Sobol' A.G., Ovchinnikova T.V., Tagaev A.A., Iakimenko Z.A., Azizbekian R.R., Kuznetsova N.I., Shamshina T.N., Arsen'ev A.S. Isolation, biological properties, and spatial structure of an antibiotic loloatin A. Bioorg. Khim. 2002;28(4):298–302. doi: 10.1023/a:1019531505769. [DOI] [PubMed] [Google Scholar]
- 15.Lowry O.H., Rosebrough N.J, Farr A. L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 16.Marigot-Outtandy D., Perronne C. Les nouveaux antituberculeux. Réanimation. 2009;18(4):334–342. [Google Scholar]
- 17.Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J. Mol. Biol. 1961;3(2):208–218. [Google Scholar]
- 18.Martinez V., Gicquel B. Techniques diagnostiques de la tuberculose et des autres mycobactérioses. Arch. Pediatr. 2005;12:96–101. doi: 10.1016/s0929-693x(05)80023-5. [DOI] [PubMed] [Google Scholar]
- 19.Mignard S., Flandrois J.P. 16S rRNA sequencing in routine bacterial identification: a 30-month experiment. J. Microbiol. Methods. 2006;67(3):574–581. doi: 10.1016/j.mimet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Motta A.S., Cladera-Olivera F., Brandelli A. Screening for antimicrobial activity among bacteria isolated from the Amazon Basin. Braz. J. Microbiol. 2004;35:307–310. [Google Scholar]
- 21.Papa F., Rivière M., Fournié J.J., Puzo G., David H. Specificity of a Mycobacterium kansasii phenolic glycolipid (mycoside A) immunoserum. J. Clin. Microbiol. 1987;25(12):2270–2273. doi: 10.1128/jcm.25.12.2270-2273.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren Z.Z., Zheng Y., Sun M., Liu J.Z., Wang Y.J. Purification and properties of an antimicrobial substance from marine Brevibacillus laterosporus Lh-1. Wei. Sheng. Wu. Xue. Bao. 2007;47(6):997–1001. [PubMed] [Google Scholar]
- 23.Sánchez J.G. B., Kouznetsov V. V. Antimycobacterial susceptibility testing methods for natural products research. Braz. J. Microbiol. 2010;41(2):270–277. doi: 10.1590/S1517-83822010000200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shida O., Takagi H., Kadowaki K., Komagata K. Proposal for two new genera, Brevibacillus gen. nov. and Aneurinibacillus gen. nov. Int. J. Syst. Bacteriol. 1996;46(4):939–946. doi: 10.1099/00207713-46-4-939. [DOI] [PubMed] [Google Scholar]
- 25.Wang W., Sun M. Phylogenetic relationships between Bacillus species and related genera inferred from 16s rDNA sequences. Braz. J. Microbiol. 2009;40:505–521. doi: 10.1590/S1517-838220090003000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisberg W.G., Barns S.M., Pelletier D.A., Lane D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991;173(2):679–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO Report . 2009. [Accessed August 21, 2010]. Global tuberculosis control - epidemiology, strategy, financing. WHO/HTM/TB/2009.411. Available at http://www.who.int/tb/publications/global_report/2009/en/ [Google Scholar]
- 28.Woo P.C., Lau S.K., Teng J.L., Tse H., Yuen K.Y. Then and now: use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin. Microbiol. Infect. 2008;14(10):908–934. doi: 10.1111/j.1469-0691.2008.02070.x. [DOI] [PubMed] [Google Scholar]
- 29.Wu S., Jia S., Sun D., Chen M., Chen X., Zhong J., Huan L. Purification and characterization of tow novel antimicrobial peptides Subpeptin JM4-A and Subpeptin JM4-B produced by Bacillus subtilus JM4, Curr. Microbiol. 2005;51(5):292–296. doi: 10.1007/s00284-005-0004-3. [DOI] [PubMed] [Google Scholar]


