Abstract
The adhesion of the solids presents in food can difficult the process of surface cleaning and promotes the bacterial adhesion process and can trigger health problems. In our study, we used UHT whole milk, chocolate based milk and infant formula to evaluate the adhesion of Enterobacter sakazakii on stainless steel coupons, and we determine the work of adhesion by measuring the contact angle as well as measured the interfacial tension of the samples. In addition we evaluated the hydrophobicity of stainless steel after pre-conditioning with milk samples mentioned. E. sakazakii was able to adhere to stainless steel in large numbers in the presence of dairy products. The chocolate based milk obtained the lower contact angle with stainless steel surface, higher interfacial tension and consequently higher adhesion work. It was verified a tendency of decreasing the interfacial tension as a function of the increasing of protein content. The preconditioning of the stainless steel coupons with milk samples changed the hydrophobic characteristics of the surfaces and became them hydrophilic. Therefore, variations in the composition of the milk products affect parameters important that can influence the procedure of hygiene in surface used in food industry.
Keywords: Enterobacter sakazakii, hydrophobicity, adhesion work, interfacial tension, dairy products
INTRODUCTION
According to public health organizations, each year, millions of illnesses in the USA and throughout the world can be traced to foodborne pathogens. While the food supply in the USA is one of the safest in the world, the Centers for Disease Control and Prevention (5,6) estimates that 76 million people get sick, more than 300,000 are hospitalized, and 5,000 die each year due to foodborne illness (14).
Adhesion of microorganisms to food processing equipment surfaces and the problems it causes are a matter of concern to the food industry. Biofilms have the potential to act as a chronic source of microbial contamination which may compromise food quality and represent a significant health hazard. The adherence process and biofilm formation begins with surface conditioning by the presence of food residues, and microorganisms have access to the conditioned surfaces. Therefore, if the food processing surfaces do not have good conditions hygienic-sanitary, the evolution of the adhesion process is favored (2).
The increased resistance of these sessile organisms by disinfectants and sanitizing agents often exacerbates the problems caused by microbial fouling and can contribute to the inefficacy of cleaning in place systems (4).
Enterobacter sakazakii is an emergent pathogenic bacterium, recently classified by the International Commission on Microbiological Specifications for Foods (ICMSF) as “severe risk for restricted population, representing threat of death or chronic sequels of long duration”. The most vehicle common of the infection has been powdered infant formulas, used in hospitals and maternities for the preparation of baby’s bottles. E. sakazakii can produce structures called capsules and the material of the capsules is very firm and adhered gum, which facilitates the formation of biofilm and makes cleaning and disinfection more difficult (16). Iversen et al. (11) showed that an encapsulated strain was able to form dense biofilm on latex, silicone, polycarbonate and stainless steel.
The molecules present in milk products interact with the surface molecules and the strength of the attraction depends on nature of both chemical species. The contact angle measurement formed by the solid-liquid interface and the liquid vapor interface provides the intensity of the work adhesion. If there is a strong attraction, the drop tends to spread to a greater degree and forms a film. For a drop with a weak attraction, the liquid tend to bead-up into a sphere and minimizes its contact with the surface (13). The work of the adhesion of the solids presents in food can difficult the process of surface cleaning and promotes the bacterial adhesion process.
Surface tension, also often known as interfacial tension is also an important property of a liquid. In a simple way, it is the force acting on the surface of a liquid, tending to minimize the surface area (3). Surface tension of whole milk is approximately 44 mN/m, while the surface tension of water is about 72 mN/m (7).
Milk products are not homogenous substances. They are composed of various dissolved and suspended solids. These solids have molecular attractions for the solid surface. The nature and quantity of the dissolved and suspended solids, temperature, method of treatment, chemical composition, time and other factors will influence the surface tension of the liquid fraction of the milk products (8).
The milk compounds can influence the value of surface tension such as the surfactants agents present: proteins and free fatty acids that provoke decrease on milk surface tension. On the other hand, the soluble constituents: lactose and most salts are believed to increase the surface tension of the milk. In addition, the increasing in the fat content seems to have an interesting effect on the surface tension.
This study aimed to measure and compare the work of adhesion of different milk products on stainless steel surface using contact angle and surface tension. In addition, we also estimated the number of the adhered cells of E. sakazakii in conditioned stainless steel surfaces.
MATERIALS AND METHODS
Food sample preparation
E. sakazakii ATCC 51329 was grown three times in Tryptic Soy Broth (DIFCO (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and incubated at 37 °C for 24 h.
UHT milk, chocolate based milk and infant formulation (NAN COMFOR 1® – Nestlé) were purchased from local supermarket. They were stored in a refrigerator at 4 ± 1 °C. Four sets of each milk sample were prepared. One set was used to inoculate with suspensions of E. sakazakii and to promote the attachment of cells on stainless steel coupons; another one was used to measure the contact angle of stainless steel with milk samples. The third one was used for the measurement of interfacial tension of the milk samples and the latter for the pre-condition of the stainless steel surface to determinate the hydrophobicity.
Attachment of cells
Surfaces of coupons (10 mm × 10 mm × 0.5 mm) of stainless steel AISI 304, #4 were first cleaned by washing with liquid neutral detergent and water, followed by rinsing with distilled water and then immersion in 70% ethyl alcohol for 1 h to remove fat. Subsequently, they were rinsed with distilled water, air-dried, and sterilized at 121°C for 15 min (15). The cleaned and sanitized coupons were placed in 250 mL flasks containing 100 mL of whole milk, chocolate based milk or infant formulation, which had previously been inoculated with suspensions of E. sakazakii (ATCC 51329). The initial number of cells was around 103 CFU.mL-1, and the flasks were incubated at 30 °C for 24 h.
Enumeration of adhered cells: After the time of incubation coupons with adhered cells were immersed in 10 mL of 0.1 % peptone water to remove planktonic cells. The coupons were then immersed in 10 mL of the same solution and swirled with a vortex mixer for 1 min to release sessile cells. Appropriate dilutions, in 900 µL of diluents contained in eppendorfs, were prepared and transferred 0,1mL to Petri dishes containing PCA (Merck, São Paulo, Brazil) by spread plate, which were incubated statically at 30 °C for 24 h. The number of CFU.cm-2 was determinate by the following equation (15).
| (1) |
where VD is the volume used for rinsing (mL); VA is the volume used for the aliquot plated (mL); M is the average colony number after incubation on plate (CFU); D is the decimal dilution; A is the area of the test coupon (cm2).
Work of adhesion
The work of adhesion (Wa) in mN/m units was calculated using a combination of the Young and Dupré Equations (9). This was accomplished by substituting Equation (2) into Equation (3). The work of adhesion allowed to estimate the influence of the liquid portion of the samples to the force required to separate the milk products from the stainless steel surfaces.
| (2) |
| (3) |
and this yields:
| (4) |
where γL is the liquid-liquid energy (surface tension) and θ is the contact angle at the solid-liquid interface. The data for the surface tension were obtained from the pendant drop experiments. The data for the solid-liquid interface were obtained from the contact angle measurements.
Determination of the interfacial tension (γL): The interfacial tension for the UHT whole milk, chocolate based milk and infant formulation was determined by the Pedant Drop Method (12) using a goniometer (Kruss, Germany).
Measurement of contact angle: The contact angle between UHT whole milk, chocolate based milk, infant formulation and the stainless steel surface was determinate using the Goniometer (Kruss Germany) equipment at room temperature. The angle contact and interfacial tension values were applied on Equation 2 e 3 to calculate the work of the adhesion.
Pre-conditioning of the surface
The stainless steel coupons were immersed in UHT whole milk, chocolate based milk, infant formulation, ultrapure water (control) for 24 h at 25 °C. After this period, they were dried at room temperature and used to measurement the hydrophobicity. The objective of such conditioning was left the surface with food waste.
The free energy of interaction (hydrophobicity) of the coupons pre-conditioned with milk samples was determined using the contact angle between the stainless steel surfaces and water (Mili-Q), formamide (LGC Bio, Brazil) and α-bromonaphtalene (MERCK, Brazil). Measurements of the contact angle (°) of one 2.0 µl drop were taken each second, for 30 s for the three liquids at room temperature.
Determination of the total interfacial tension (γstot):
The total interfacial tension was determined by the sum of the apolar and polar components of interfacial tension (Equation 5).
| (5) |
where is the total interfacial tension of the liquid; γLWis the interfacial tension of the interactions of the Lifshitz-van der Waals forces; γ+ is the interfacial tension of the electron acceptor component of the acid-base component; γ− is the interfacial tension of electron donor component of the acid-base component, θ is the contact angle and s e l indicate surface and liquid respectively (20).
The three components of the interfacial tension of the surfaces were determined from the contact angles obtained from three liquids with different polarities, whose interfacial tensions are known, as shown in Table 1.
Table 1.
Components of the interfacial tensions of the substances.
| Substances | Interfacial tension ( mJ/m2) | |||
|---|---|---|---|---|
| γLW | γ+ | γ− | ||
| α-bromonaphtalene at 25°C | 44.4 | 44.4 | 0.0 | 0.0 |
| Water at 25°C | 72.8 | 21.8 | 25.5 | 25.5 |
| Formamide at 25°C | 58.0 | 39.0 | 2.28 | 39.6 |
The interfacial tension is the resultant of the sum of the two components ( and ) that calculated from equations of the van Oss (1994).
where is the interfacial tension of the interactions of the Lifshitz-van der Waals forces; is the polar component of the Lewis acid-base interaction.
Free Energy of Interaction (ΔGswsTOT ): The total free energy of interaction (ΔGswstot) among molecules of the surface(s) immersed in water (w) was determined by the sum of the apolar and polar free energy of interaction, ΔGswsLW and ΔGswsAB, respectively (21).
| (6) |
Analysis of results
The experiment was analyzed by a completely randomized design with three repetitions. A significance level of 5 % was used for Tukey’s test, using Statistical Analysis System (SAS) version 9.1.
RESULTS AND DISCUSSION
The number of cells adhered in stainless steel after incubation with UHT whole milk, chocolate based milk, infant formulation and nutrient broth differed (p≥0.05) being higher for UHT whole milk, chocolate based milk and infant formulation than nutrient broth (Table 2). Such results demonstrate that E. sakazakii was able to adhere to stainless steel in large numbers in the presence of dairy products, which is cause for concern because of the severity of this microorganism. The number of cells adhered to the stainless steel coupons after incubation with UHT whole milk and chocolate based milk was almost two log cycles higher than that occurred when coupons were incubated with nutrient broth.
Table 2.
Number of Enterobacter sakazakii (CFU.cm-2) cells adhered on stainless steel, after incubation with different milk samples and nutrient broth.
| Products | Enterobacter sakazakii adhered cells in stainless steel (log CFU cm-2) |
|---|---|
| UHT whole Milk | 7.14 a |
| Infant formulation | 7.04 b |
| Chocolate based Milk | 6.74 c |
| Nutrient broth (control) | 5.17d |
The averages followed by the same letters, in the columns, did not differ between them (P≥0.05), by Tukey test.
It is important to emphasize that studies have been directed toward the understanding and control of events leading to the microbial colonization of surfaces. The adhesion of bacteria to a surface depends on various microbiological, physical, and chemical factors of the cell surface and physicochemical properties related to the food processing surface (10).
Table 3 presents the average for the work of adhesion values between the UHT whole milk, chocolate based milk, infant milk and stainless steel which is the surface usually used in food processing industry. In addition, this table also presents the values obtained for contact angles and interfacial tension of these milk samples. This study demonstrated that the chocolate based milk sample showed the strongest adhesion force on the stainless steel. The higher the work of adhesion the higher the difficult to clean the surface and consequently, higher the possibility to start a microbial adhesion process on this surface.
Table 3.
Interfacial tension, contact angle with stainless steel and work of the adhesion of the milk samples
| Samples | Interfacial tension (mN/m) | Contact angle (°) with stainless steel | Work of the adhesion (mN/m) |
|---|---|---|---|
| UHT whole milk | 46.92 a | 55.12 a | 73.70 a |
| Chocolate based milk | 47.30 a | 49.18 b | 78.20 b |
| Infant formulation | 39.53 b | 52.50 a | 63.60 c |
The averages followed by the same letters, in the columns, did not differ between them (P≥0.05), by Tukey test.
In regard to the contact angle measurements we observed that the contact angle of the UHT whole milk sample and infant formulation did not varied (p≥0.05). The chocolate based milk obtained the lower contact angle and consequently higher work of adhesion.
The three samples differ between themselves in relation to their composition, for example, in fat, protein, sugar contents and other minor constituents. These differences can explain the results obtained in this experiment. The lower contact angle between chocolate based milk and stainless steel can be explained by the high level of milk and whey powder; which are rich in surface active compounds, such as phospholipids and whey proteins. Surface active substances are amphiphilic molecules that are able to diminish interfacial tension between liquid-surface and liquid-air; increasing the interactions among stainless steel molecules and chocolate based milk and of the latter and air molecules, as illustrates Figure 1.
Figure 1.
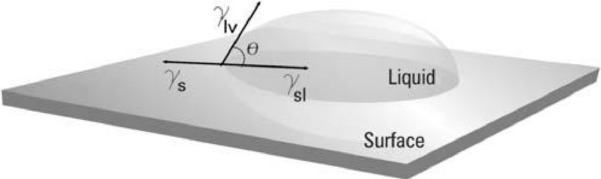
Contact angle between a liquid drop and a smooth surface and horizontal showing the interfacial tensions of solid surface, liquid in equilibrium with the vapor, surface, and liquid, respectively.
Adhikari et al. (1) stated that the lower the contact angle of a liquid on a solid surface, the stronger the attraction and greater the forces required to separate these surfaces.
In the dairy industry, the work of adhesion of milk constituents on surfaces processing can influence the hygienic procedure. Another important factor is the surface conditioning, which can be a contamination source to the food.
If there is the presence of the fissures and grooves in the stainless steel surface, the higher interaction between liquid and surface promotes the entrance of the food residues, such as protein, fat, sugars in the flaws of the surface that difficult the efficiency of cleaning process. In this flaw can accumulate bacteria that in turn can initiate an adhesion process (2).
Handojo et al. (9) studied the work of adhesion of milk products (whole, 2% reduced fat, skim and chocolate based milk) on glass surfaces and analyzed before and after manual washing. The whole and chocolate based milk had the lowest contact angle (21°) with stainless steel surface. The authors also calculated the work of adhesion and these results showed that the chocolate based milk had the strongest and the 2 % reduced fat milk had the weakest adhesion force. They concluded that the surface became more difficult to clean when there are the presence of the whole and chocolate based milk residues.
In the literature, it is observed that the interfacial tension at room temperature range from 44.0 mN/m to 49.2 mN/m for whole and skim milk, respectively. The surface tension of milk products can be affected by its different surface-active components such as proteins, minerals, phospholipids and fats (13). The results obtained in the present work agree with the literature values where was verified a tendency of the decrease in interfacial tension as a function of the increasing of protein content (Table 3). The UHT whole milk, chocolate based milk and infant formulation had 3.0 % (m/m), 2.0 % (m/m) and 11.0 % (m/m) of the protein, respectively. So the product infant formulation with more protein content presented the lowest interfacial tension (39.53 mN/m),
The factors that are known to affect surface tension forces are intermolecular forces where stronger intermolecular forces result in higher values of surface tension, for example, hydrogen bonding, in which liquids with hydrogen bonds have higher values of surface tension; temperature, due to result in lower values of surface tension when the temperature increase.
According to Table 4, it can be stated that the preconditioning of the stainless steel coupons with milk samples changed the hydrophobic characteristics of the surfaces became them hydrophilic. This alteration is provoked by constituents in product, mainly the amphiphilic substances, which have a polar part situated in liquid present in the interface solid surface /air. Such surface modifications imply many alterations in the behavior of the surface in relation to bacterial adhesion. The hydrophobicity property may be the primary driving force for the adhesion of most pathogens (19). The microorganisms have many different ways of using the hydrophobic effect in order to adhere to substrata (8). However, it is well known that bacteria change their surface composition in response to the environment. Therefore, cell surface hydrophobicity is not necessarily constant for bacteria, and there is no clear trend in cell adhesion based solely on hydrophobicity effects (17).
Table 4.
Total energy of interaction (hydrophobicity) of coupons immersed in different milk samples and water.
| Treatments | ΔGTOT (mJ/m2) |
|---|---|
| Coupons immersed in water | −46,842a |
| Coupons immersed in UHT whole milk | 8,653 b |
| Coupons immersed in infant formulation | 13,524c |
| Coupons immersed in chocolate based milk | 57,480 d |
The averages followed by the same letters, in the column, did not differ between them (P≥0.05), by Tukey test.
All aspects of the biology of bacteria, the cell wall and surface properties of bacteria play important roles in bacterial adhesion and in the formation of biofilms. For both gram-positive and gram-negative bacteria is essentially the presence of biomolecules in the cell wall that determine the surface properties of the bacteria and thus the interaction of the bacterium with the environment (18).
Researchers have shown that the physical and chemical properties of the cell surface and food contact surfaces contribute to the adherence process. These properties include hydrophobicity, electrical charge, and roughness. The adhesion process also depends on the bacterial species and strains since they have different physicochemical characteristics. Some parameters in the general environmental, such as temperature, time of exposure, bacterial concentration, electrolyte concentrations, pH value, and the associated flow conditions, can affect the bacterial adhesion process.
CONCLUSION
The significant variation in the composition of different samples of milk affects the contact angle between dairy products and stainless steel, work of adhesion and tension interfacial. The chocolate based milk obtained the lower contact angle and higher work of adhesion. In the dairy industry, the work of adhesion of milk constituents on surfaces processing can influence the hygienic procedure. Interfacial tension is a property affected by the amount of surface-active agents like fat and proteins presents in products and in this work the protein content had higher influence in the surface tension.
REFERENCES
- 1.Adhikari B., Howes T., Bhandari B.R., Truong V. Stickiness in foods: a review of mechanisms and test methods. Int. J. Food Prop. 2001;4:1–33. [Google Scholar]
- 2.Andrade N.J. São Paulo: Varela; 2007. Higiene na indústria de alimentos. [Google Scholar]
- 3.Atkins P.W. Physical Chemistry. New York, USA: Oxford University Press; 1994. [Google Scholar]
- 4.Barnes L.M., Lo M.F., Adams M.R., Chamberlain A.H.L. Effect of Milk Proteins on Adhesion of Bacteria to Stainless Steel Surfaces. Appl. Environ. Microb. 1999;65(10):4543–4548. doi: 10.1128/aem.65.10.4543-4548.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Vol. 52. MMWR: Morbidity and Mortality Weekly Report; 2003. [Accessed 3 September 2010]. Multistate outbreak of Salmonella serotype Typhimurium infections associated with drinking unpasteurized milk–Illinois, Indiana, Ohio, and Tennessee,2002–2003. pp. 613–615. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5226a3.htm. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . Vol. 53. MMWR: Morbidity and Mortality Weekly Report; 2004. [Accessed 3 September 2010]. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food–selected sites, United States, 2003; pp. 338–343. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5316a2.htm. [PubMed] [Google Scholar]
- 7.Chandan R. Dairy Based Ingredients: Practical Guides for the Food Industry. Minessota, USA: Eagen Press Handbook Series; 1997. [Google Scholar]
- 8.Doyle R.J. Contribution of the hydrophobic effect to microbial infection. Microbes Infect. 2000;2:391–400. doi: 10.1016/s1286-4579(00)00328-2. [DOI] [PubMed] [Google Scholar]
- 9.Handojo A., Zhai Y., Frankel G., Pascall M.A. Measurement of adhesion strengths between various milk products on glass surfaces using contact angle measurement and atomic force microscopy. J. Food Eng. 2009;92:305–311. [Google Scholar]
- 10.Hilbert L.R., Bagge-Ravn D., Kold J., Gram L. Influence of surface roughness of stainless steel on microbial adhesion and corrosion resistance. Int. Biodeter. Biodegr. 2003;52:175–185. [Google Scholar]
- 11.Iversen C., Lane M., Forsythe S. The growth profile, thermotolerance and biofilm formation of Enterobacter sakazakii in infant formula milk. Lett. Appl. Microbiol. 2004;38(5):378–382. doi: 10.1111/j.1472-765X.2004.01507.x. [DOI] [PubMed] [Google Scholar]
- 12.Man k. F. Surface tension measurements of liquid metals by the quasi containerless pendant drop method. Int J Thermophys. 2000;21:793–804. [Google Scholar]
- 13.Michalski M.C., Briard V. Fat.ralated surface tension and wetting properties of milk. Milchwissenschaft. 2003;58(1/2):26–29. [Google Scholar]
- 14.Oliver S.P., Jayarao B.M., Almeida R.A. Foodborne pathogens in milk and the dairy farm environment: food safety and public health implications. Foodborne Pathog. Dis. 2005;2:115–129. doi: 10.1089/fpd.2005.2.115. [DOI] [PubMed] [Google Scholar]
- 15.Parizzi S.Q.F., Andrade N.J., Soares N.F.F., Silva C.A.S., Monteiro E.A.M. Bacterial adherence to different inert surfaces evaluated by epifluorescence microscopy and plate count method. Braz. Arch. Biol Techn. 2004;47(1):77–83. [Google Scholar]
- 16.Santos R.F.S. Ocorrência de Enterobacter sakazakii em fórmulas infantis para lactentes em hospitais e maternidades da região de Campinas/SP. Campinas, SP, Brasil: 2006. p. 118. M.Sc. Dissertation. Ciência de Alimentos, Universidade Estadual de Campinas.UNICAMP. [Google Scholar]
- 17.Sorongon M.L., Bloodgood R.A., Burchard R.P. Hydrophobicity, adhesion, and surface-exposed proteins of gliding bacteria. Appl. Environ. Microbiol. 1991;57:3193–3199. doi: 10.1128/aem.57.11.3193-3199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ubbink J., Schar-Zammaretti P. Colloidal properties and specific interactions of bacterial surfaces. Curr. Opin. Colloid In. 2007;12:263–270. [Google Scholar]
- 19.van Loosdrecht M., Norde W., Lyklema L., Zehnder J. Hydrophobic and electrostatic parameters in bacterial adhesion. Aquat. Sci. 1990;51:103–114. [Google Scholar]
- 20.van Oss C.J. Interfacial Forces in Aqueous Media. New York: Marcel Dekker, Inc; 1994. [Google Scholar]
- 21.van Oss C.J., GIESE R.F., COSTANZO P.M. DLVO and non-DLVO interactions in hectorite. Clays Clay Minerals. 1990;38:151–159. [Google Scholar]


