Abstract
The media claims for the consumption of natural resource-based food have gradually increased in both developing and developed countries. The interest in the safety of these products is partially due to the possible presence of toxigenic fungi acting as mycotoxin producers, such as aflatoxins produced during the secondary metabolism of Aspergillus flavus, A. parasiticus and A. nomius. Aflatoxins, mainly aflatoxin B1, are directly associated with liver cancer in human beings. This paper is aimed at evaluating the presence of aflatoxin B1 in a few vegetable drugs, dried plant extracts and industrialized products traded in 2010 in the city of Belo Horizonte, State of Minas Gerais, Brazil. The method used for the quantification of aflatoxin B1 was based on extraction through acetone:water (85:15), immunoaffinity column purification followed by separation and detection in high efficiency liquid chromatography. Under the conditions of analysis, the Limits of Detection and Quantification were 0.6 µg kg-1 and 1.0 µg kg-1 respectively. The complete sets of analyses were carried out in duplicate. Aflatoxin B1 was noticed in a single sample (< 1.0 µg kg-1). The results revealed low aflatoxin B1 contamination in the products under analysis. However, it is required to establish a broad monitoring program in order to obtain additional data and check up on the actual extension of contamination.
Keywords: medicinal plants, fungi, mycotoxin, high-performance liquid chromatography (HPLC)
INTRODUCTION
The use of plants for medicinal purposes for the treatment, cure and prevention of diseases is one of the earliest known medical practices in History. At present, a significant amount of medicinal plant commercialization is carried out in drugstores and natural product stores, where vegetable preparations are marketed under industrialized labeling (16).
The intake of certain plants deemed as medicinal ones in the form of teas has always been meaningful in Brazil, mainly in the lower economic level populations, owing to low costs and the popular belief ascribed to their effects (30). In industrialized countries, it is believed that 30% to 50% of the populations make regular use of medicinal plants and/or vitamin and mineral supplements (29).
The increase in the consumption of natural products has become a public health issue. Practices of cultivation, harvest, storage and distribution make natural products subject to a great variety of contamination. Within such a context, the microbiological risk of medicinal plants may vary according to the different stages presented by the production line. The practices of field cultivation and harvest in association with the absence of an effective sanitary control stand for a potential risk for this type of product (18, 21, 31).
The interest in the safety of these products is greatly due to the possible presence of pathogenic bacteria and toxigenic fungi that produce mycotoxins such as aflatoxins B1, B2, G1 and G2. Aflatoxin B1 (AFB1) is the most common and most toxic one produced mainly by filamentous fungi as Aspergillus flavus, A. parasiticus and A. nomius. The toxic effects of the aflatoxins include immunosuppressive, mutagenic, teratogenic, and hepatocarcinogenic activity. The most potent hepatocarcinogen agent described in mammals is AFB1 , which is classified by the International Agency for Research on Cancer as Group 1 (probable carcinogen). (2, 7, 14, 19, 22).
The occurrence of toxigenic fungi in medicinal plants in Brazil has already been verified by several authors. Bugno, Almodovar, Pereira, Pinto, Sabino (2006) evaluated 91 samples of medicinal plants, composed by 65 different species marketed in São Paulo. Aspergillus flavus was the dominant and often isolated species (58 isolates/23.39%). Among these, 16/27.6% were able to produce aflatoxin B1 or B1 and B2. Aquino, Gonçalez, Rossi, Campos Nogueira, Reis, Corrêa (2010) analyzed 80 samples, including 20 samples of each one of the four tested plants: Boldo (Peumus boldus), green tea (Camellia sinensis), Espinheira-Santa (Maytenus ilicifolia), and Senna (Cassia angustifolia). Except for three samples of P. boldus e two samples of C. sinensis, all the samples presented fungal contamination, with 75% above the limit established by the World Health Organization for the Total Fungal Count: 103 UFC/g (32). Prado, Andrade, Oliveira, Leal, Oliveira, Batista (2009) identified 8 A. flavus isolates in chamomile (Matricaria recutita) sold in Belo Horizonte, two of which were aflatoxin producers (B1 and B2). The same research reported the presence of Aspergillus ostianus, ochratoxin A-producer in artichoke (Cynara scolymus). The total fungal count reached values over 105 UFC/g in these plants. In Argentina, Rizzo, Vedoya, Maurutto, Haidukowski, Varsavsky (2004) detected 52% of the genus Aspergillus in 152 medicinal plant samples, corresponding to 56 species. A. flavus and A. parasiticus were the prevalent species, 50% among the 40 aflatoxin-producer isolates. In Croatia, Malaysia and Nigeria, the widely prevailing fungi in researched medicinal plants belonged to the genera Penicillium and Aspergillus, which are potential producers of mycotoxins (10, 12, 23). In South Africa, 15 out of 16 samples of traditional medicinal plants were contaminated by several fungi species. A. niger was the most common isolated contaminant (50% of the samples), followed by Fusarium (6/16) and Penicillium (5/16). Approximately 60% of the samples were co-contaminated by Alternaria and Rhizopus spp (17). Roy, Sinha, Chourasia (1988) isolated 15 different vegetable drugs in India, in addition to 158 A. flavus isolates, 49 of which being aflatoxin B1-producers.
In Brazil, the National Health Surveillance Agency (ANVISA) issued Regulation no. 10 (March 9, 2010), which brings in norms about microbiological contaminants for vegetable drugs, medicinal plants and their parts, which will undergo a heat extraction process (infusion and decoction), and plants that will not be submitted to an extraction process (ground sample). The norm sets forth the absence of aflatoxins (6).
Due to the absence of data about the occurrence of aflatoxins in natural products in Brazil, the aim of this paper was to evaluate the presence of aflatoxins B1 in some vegetable drugs, dried plant extracts used in the preparation of phytotherapy drugs and industrialized products commercialized in 2010 in Belo Horizonte.
MATERIALS AND METHODS
Samples
A number of 37 samples were purchased in natural-product stores in the city of Belo Horizonte in 2010, including: (1) 8 dried extracts of each of the species quoted below, which are used for the preparation of phytotherapeutic medications 1 sample belonging to each species; (2) Green Tea (Camellia sinensis): 5 samples presented as leaves and 4 industrialized products; (3) Espinheira-Santa leaves (Maytenus ilicifolia Martius – 2 samples); (4) Valerian Root (Valeriana officinalis L. – 3 samples); (5) Horse Chestnut seed (Aesculus hippocastanum L. – 2 samples); (6) Cascara Sagrada bark (Rhamnus purshiana D. C. – 2 samples); (7) Senna leaflets (Cassia angustifolia Vahl – 2 samples); (8) Passion Fruit leaves – Passiflora sp Sims – 2 samples) and (9) 7 samples of Guarana powder (Paulinia cupana H. B. K).
Chemicals
Aflatoxin B1 was purchased from Sigma Chemicals Co. St. Louis, MO. It was diluted in benzene:acetonitrile, chromatographic grade. Benzene came from Tedia (Fairfield, OH, USA) and acetonitrile from Merck (Darmstadt, Germany). Acetone, HPLC grade, employed for the extraction of aflatoxin B1 came from Merck (Darmstadt, Germany). The methanol, HPLC grade, used for the preparation of the mobile phase and elution of aflatoxin B1 in the immunoaffinity column came from Carlo Erba (Rodano, Milan, Italy). The water used in the analytical process was obtained through a Milli-Q purification and filtration system with an 18 MΩ cm-1 resistivity (Millipore, Bedford, MA, USA). The present study used EASI-EXTRACT Aflatoxin immunoaffinity columns, Product code RP71/70N, R-Biopharm Rhône, Glasgow, Scotland. Column storage took place at a temperature ranging from 2 and 8° C and they were used at room temperature. The entire glassware used for aflatoxin determination was decontaminated by Alkaline Extran MA 01, 7555 (Merck, Darmstadt, Germany) at 20%, (pH > 12), remaining in contact for 24 hours and further washing with distilled water.
Standard Aflatoxin B1 (AFB1) Solution
The stock standard solution of AFB1 (8,2105 µg mL-1) was prepared by dissolving the solid standard in benzene:acetonitrile (98:2, v/v). The precise concentration was measured in Shimadzu UV-1601 PC spectrophotometer, Shimadzu Scientific Instruments, Japan, as described by AOAC (4). An intermediate standard solution from the stock solution was prepared in benzene:acetonitrile (98:2, v/v) in a concentration of 9.855 ng mL-1. This solution was utilized for the elaboration of a calibration curve in the range 0.1–9.8 ng/mL. All the solutions were packed in amber vials at -18° C.
Extraction and clean-up procedures for high-performance liquid chromatography (HPLC) analysis
Samples were analyzed using a validated method by reversed-phase HPLC separation and fluorescence detection after post-column derivatization (3). A ground sample (10 g) was blended with 100 mL extraction solvent: acetone:water (85:15, v/v) for 30 min. Then the mixture was filtered through Whatman no. 1 filter paper. After filtration, the extract (5 mL) was diluted with water (75 mL). The immunoaffinity column was connected to the vacuum manifold, and the reservoir was attached to the immunoaffinity column. A number of 40 mL of diluted sample extract were added to the reservoir and passed through the immunoaffinity column at a flow rate of ca. 3 mL/min (ca. 1 drop/s; gravity). Do not exceed a flow rate of 5 mL/min. The column was washed twice with 10 mL water at a flow rate of maximum 5 mL/min and dried by applying little vacuum for 5–10 s. Finally, aflatoxin B1 was eluted with 0.5 mL methanol and passed through by gravity. The eluate was collected in a vial. After 1 minute, a second portion of 0.5 mL methanol was applied. After 1 minute, a second portion of 0.5 mL methanol was applied. Most of the applied elution solvent was collected by pressing air or vacuum through. The extract was evaporated to dryness under a nitrogen stream at ca. 50 °C and reconstituted with 250 µL with methanol:water (2:3), v/v. Aflatoxins are subject to light degradation, thus it was necessary to protect the work from light by using amber vials. As a result, the method was found to be fit-for-purpose for the determination of AFB1 in medical herbs at levels of 1.0 µg kg-1 and above.
Determination of AFB1 by HPLC method
The presence of AFB1 was detected by HPLC after post-column derivatization with the electrochemical generation of bromine (KOBRA cell – Rhone diagnostic technologies, UK) with a current of 100 µA and a fluorescence detector (Shimadzu LC-10 AD Model; 360 nm excitation wavelength; 435 mm emission wavelength; with Shim-Pack CLC – ODS column, 5 µm, 4.6 × 250 mm, preceded by a guard column Shim – Pack G – ODS, 5 µm, 4 × 10 mm). The mobile phase was deionized water-acetonitrile-methanol (60:20:20, v/v/v) with the addition of 350 µL of 4M HNO3 and 120 mg of KBr at a flow rate of 1 mL/min. The injection volume was 50 µL. The quantification of AFB1 was performed by measuring their peak areas at AFB1 retention time (23.4 min.) and comparing it with the calibration curve. (25).
The performance of the method, aflatoxin B1 recovery and effectiveness of the cleanup procedure, was evaluated by the samples of medical herbs spiked with AFB1, in duplicate, at level of 2.96 ng/g.
RESULTS AND DISCUSSION
The linearity was evaluated within the range under study and calculated from the linear regression equation and determined by the least squares method. The linear correlation coefficient (r2) was used as the indicator of the straight line as a mathematical model. The values were always over 0.99 as recommended by Green (11). Figures 1, 2 and 3 show an aflatoxin B1 calibration curve, the chromatogram of a mixture of aflatoxins standards, and the extract of a horse chestnut seed sample contaminated by 2.96 ng/g of aflatoxin B1, after post-column derivatization with the electrochemical generation of bromine.
Figure 1.
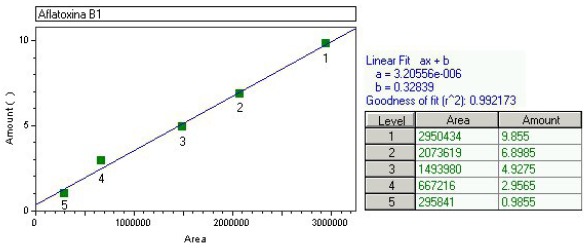
Standard curve used for the quantification of aflatoxin B1 with the area obtained in the readings, the concentration of aflatoxin B1 in ng/mL, the linear fit equation and the r2 value.
Figure 2.
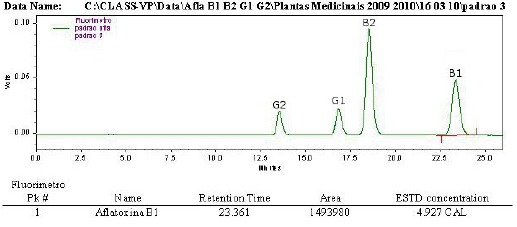
HPLC chromatogram with fluorescence detection post-column derivatization (electrochemically) – Kobra cell of a mixture of aflatoxin standards; Shim-pack CLC-ODS column and water:metahanol:acetonitrile, 60:20:20,v/v/v as mobile phase. Aflatoxin B1 (4.93 ng/mL).
Figure 3.
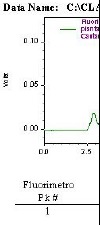
HPLC chromat ogram with fluoresce ence detection of an extract of horse chestnut seed. Aflatoxin B1-contaminated sample (2.96 ng g-1); immunoaffinity column purification; HPLC using a post-column derivatization (electrochemically) – Kobra cell; Shim-pack CLC-ODS column and water:metahanol:acetonitrile, 60:20:20,v/v/v as mobile phase.
Despite the existence of several methods described towards the determination of aflatoxins in medicinal plants, none of such methods can be applied to all types of samples or all aflatoxins (15). The difficulty stems from the chemical complexity of the compounds of every different medicinal plant. Results of recovery experiments and coefficient of variation for aflatoxin B1 are showed in Table 1. The recovery values obtained for aflatoxin B1 revealed that the methodology in use and the analytical conditions developed in the laboratory are in compliance with the provisions set forth by law no. 401/2006 (02/23/2006) in the European Union (8). All recovery values are within the range of 70 to 110%, which is required when the aflatoxin concentration is in the range of 1–10 µg kg-1. In relation to the variation coefficient values, all stayed below 20% as recommended by Horwitz and Albert (1982), pointing to a good precision of the applied methodology.
Table 1.
Recovery in medical herbs spiked with AFB1 standard (2.96 ng/g)
| Sample | Amount recovered (ng/g) | Recovery mean (%) | Coefficient of variation (%) |
|---|---|---|---|
| Senna | 2.56 | ||
| (Cassia angustifolia Vahl) | 2.99 | 94 | 10.9 |
| Espinheira-Santa | 3.38 | ||
| (Maytenus ilicifolia Martius) | 2.89 | 106 | 11 |
| Passion Fruit | 2.53 | ||
| (Passiflora sp Sims) | 3.13 | 96 | 14.9 |
| Guarana Powder | 2.40 | ||
| (Paullinia cupana H.B.K) | 2.69 | 86 | 8.1 |
| Valerian Root | 3.04 | ||
| (Valeriana officinalis Linné) | 3.04 | 103 | 0 |
| Cascara Sagrada | 2.57 | ||
| (Rhamnus purshiana D.C.) | 2.04 | 78 | 16.2 |
| Horse Chestnut Seed | 3.10 | ||
| (Aesculus hippocastanum L) | 3.02 | 104 | 1.2 |
| Green Tea | 2.99 | ||
| (Camellia sinensis) | 2.36 | 91 | 17 |
As far as the remaining aflatoxins are concerned (data shown in Table 1), the recovery values for aflatoxin G2 were below 60% in all tested plants. Recovery values of 56% were obtained for aflatoxin B2 in green tea and 53% in guarana powder. The others presented values between 83% and 103%. Recovery values for aflatoxin G1 were between 95% and 103%, except for horse chestnut seed, Espinheira-Santa and guarana, which presented values over 115%. Low recovery, mainly in relation to aflatoxin G2 might be due to little mycotoxin affinity towards the antibody, which depends on the extracts used or the insufficient quantity of antibody bound to the gel of the immunoaffinity column (1).
The presence of aflatoxin B1 was noticed in just a single sample of green tea (Camellia sinensis) at a concentration below the Limit of Quantification (1.0 ng/g). A possible explanation for the absence of aflatoxins would be the lack of toxigenic fungi in the samples or the environmental conditions during harvest and storage. It is well reported in literature that temperature and water activity are the main factors that influence fungal invasion and the production of aflatoxins in stored products (9). Kulshrestha, Gupta, Shukla, Kundu, Bhatnagar, Katiyar (2008) evaluated that medicinal plants with water activity below 0.81 under temperatures of 25 ± 2° C, 30 ± 2° C and 40 ± 2° C and water activity over 0.81 and temperature below 10 ± 2° C did not present aflatoxins, even in the presence of Aspergillus flavus, an aflatoxin producer.
Similar results were found by Romagnoli, Menna, Gruppioni, Bergamini (2007) in Italy as 48 infusions and medicinal plants were analyzed. None of the samples presented detectable levels of aflatoxins for an analytical methodology with a limit of detection and quantification of 0.5 and 1.5 ng/g of aflatoxin B1, respectively. Ali, Hashim, Saad, Safan, Nakajima, Yoshizawa (2005) evaluated 23 traditional medicinal plants from Malaysia and Indonesia. They observed aflatoxin B1 in 16 samples with an average 0.26 ng/g. In Thailand, Tassaneeyakul, Razzazi-Fazeli, Porasuphatana, Bohm (2004) detected aflatoxins in 5 out of the 28 analyzed samples within a range of 1.7 to 14.3 ng/g. Unlike the quoted authors, Selim, Popendorf, Ibramim, Sharkawy, Kashory (1996) observed high contamination of aflatoxin B1 in 9 samples in Egypt, after having analyzed 31 medicinal plants within a range of 24 to 105 ng/g and an average 49 ng/g.
In Brazil, a small number of papers evaluated the quantification of aflatoxins in medicinal plants. Aquino, Gonçalez, Rossi, Campos Nogueira, Reis, Corrêa (2010) evaluated boldo (Peumus boldus), green tea (Camellia sinensis), Espinheira-Santa (Maytenus ilicifolia), and senna (Cassia angustifolia), when aflatoxins were not detected through the use of VICAM immunoaffinity columns (AflaTest kit), a monoclonal antibody-based affinity chromatography system and posterior confirmation by thin-layer chromatography. Braga, Medeiros, Oliveira, Macedo (2005) analyzed different samples of M. illicifolia (undisclosed figures) sold in stores and drugstores in the city of João Pessoa, State of Paraíba, when aflatoxins were not detected either. In this case, the method in use involved immunoaffinity column purification and high efficiency liquid chromatography, excluding the derivatization stage.
Owing to the relevance of human exposure to aflatoxin B1 for public health, the lack of information and the Brazilian climatic conditions, which favor fungal production as well as mycotoxin production, a constant supervision of the quality of medicinal plants and phytotherapeutic products is required to guarantee good health conditions for the users of such products.
ACKNOWLEDGEMENTS
The authors are grateful to the Research Foundation of the State of Minas Gerais (Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG) for the financial support received.
REFERENCES
- 1.Ali N., Hashim N.H., Saad B., Safan K., Nakajima M., Yoshizawa T. Evaluation of a method to determine the natural occurrence of aflatoxins in commercial traditional herbal medicines from Malaysia and Indonesia. Food Chem. Toxicol. 2005;43:1763–1772. doi: 10.1016/j.fct.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Aquino S., Gonçalez E., Rossi M.H., Campos Nogueira J.H., Reis T.A., Corrêa B. Evaluation of fungal burden and aflatoxin presence in packed medicinal plants treated by gamma radiation. J. Food Prot. 2010;73:932–937. doi: 10.4315/0362-028x-73.5.932. [DOI] [PubMed] [Google Scholar]
- 3.Arranz E., Egmond H.V., Stzoo E., Kroegner K., Legarda T.M., Burdanpal P., Reif K., Stroka J. Determination of aflatoxin B1 in medical herbs: interlaboratory study. J. AOAC Int. 2006;89:595–605. [PubMed] [Google Scholar]
- 4.Association of Official Analytical Chemists (AOAC) Official Methods of the AOAC International. 16. Gaithersburg: AOAC International; 1997. [Google Scholar]
- 5.Braga S.M.L.F.M., Medeiros F.D., Oliveira E.J., Macedo R.O. Development and validation of a method for the quantitative determination of aflatoxin contaminants in Maytenus ilicifolia by HPLC with fluorescence detection. Phytoch. Anal. 2005;16:267–271. doi: 10.1002/pca.837. [DOI] [PubMed] [Google Scholar]
- 6.Brasil. Agência Nacional de Vigilância Sanitária. Resolução n°. 10, de 9 de março de 2010. Diário Oficial {da} República Federativa do Brasil. Brasília; 2010. Dispõe sobre limites para contaminantes microbiológicos em drogas vegetais. 10 mar. [Google Scholar]
- 7.Bugno A., Almodovar A.A.B., Pereira T.C., Pinto T.J.A., Sabino M. Occurrence of toxigenic fungi in herbal drugs. Braz. J. Microbiol. 2006;37:47–51. [Google Scholar]
- 8.Commission regulation (EC) No. 401/2006 of 23 february. Laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Official Journal of the European Communitties. 2006:L70–12. [Google Scholar]
- 9.Council for Agricultural Science ad Technology—CAST. Mycotoxins: economics and health riscks. 2003. Ames, Iowa: Council for Agricultural Science and Tecnology; p. 139. Task Force Report. [Google Scholar]
- 10.Efuntoye M.O. Fungi associated with herbal drug plants during storage. Mycopath. 1996;136:115–118. doi: 10.1007/BF00437505. [DOI] [PubMed] [Google Scholar]
- 11.Green J.M. A pratical guide to analytical method validation. Anal. Chem. 1996;68:305–309. [Google Scholar]
- 12.Halt M. Moulds and mycotoxins in herb tea and medicinal plants. European J. Epid. 1998;14:269–274. doi: 10.1023/a:1007498613538. [DOI] [PubMed] [Google Scholar]
- 13.Horwitz W., Albert R. The reliability of aflatoxin assays. Assoc. Food Drug Off. Quart. Bull. 1982;46:14–24. [Google Scholar]
- 14.International Agency for Research on Cancer. Some naturally occurring substances: food itens and constituents, heterocyclic aromatic amines and mycotoxins. IARC monographs on the evaluation of carcinogenic risks to humans. IARC Sci. Publ. 1993;56:19–23. [Google Scholar]
- 15.Ip S.P., Che C.T. Determination of aflatoxins in Chinese medicinal herbs by high-performance liquid chromatography using immunoaffinity column cleanup. Improvement of recovery. J Chrom. 2006;1135:241–244. doi: 10.1016/j.chroma.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Junior V.F.V., Pinto A.C., Maciel M.A.M. Plantas medicinais: Cura segura? Quim. Nova. 2005;28:519–528. [Google Scholar]
- 17.Katerere D.R., Stockenstrom S., Thembo K.M., Shephard G.S., Vismer H.F. A preliminary survey of mycological and fumonisin and aflatoxin contamination of African traditional herbal medicines sold in South Africa. Hum. Exper. Toxic. 2008;27:793–798. doi: 10.1177/0960327108099535. [DOI] [PubMed] [Google Scholar]
- 18.Kneifel W., Czech E., Knopp B. Microbial contamination of medicinal plants—a review. Planta Med. 2002;68:5–15. doi: 10.1055/s-2002-20060. [DOI] [PubMed] [Google Scholar]
- 19.Kosalec I., Cvek J., Tomic S. Contaminants of medicinal herbs and herbal products. Arh Hig Rada Toksikol. 2009;60:485–501. doi: 10.2478/10004-1254-60-2009-2005. [DOI] [PubMed] [Google Scholar]
- 20.Kulshrestha R., Gupta C.P., Shukla G., Kundu M.C., Bhatnagar S.P., Katiyar C.K. The effect of water activity and storage temperature on the growth of Aspergillus flavus in Medicinal herbs. Planta Med. 2008;14:1308–1315. doi: 10.1055/s-2008-1074561. [DOI] [PubMed] [Google Scholar]
- 21.Martins H.M., Martins M.L., Dias M.I., Bernardo F. Evaluation of microbiological quality of medicinal plants used in natural infusions. Int. J. Food Microbiol. 2001;68:149–153. doi: 10.1016/s0168-1605(01)00480-9. [DOI] [PubMed] [Google Scholar]
- 22.Prado G., Andrade M.C., Oliveira M.S., Leal A.S., Oliveira B.R., Batista L.R. Efeito da irradiação gama na microbiota fúngica de plantas medicinais. Ciên. Agrotec. 2009;33:1372–1378. [Google Scholar]
- 23.Razak M.F.A., Aidon K.E., Candlish A.G.G. Mixed herbs drugs: Inhibitory effect on growth of the endogenous mycoflora and aflatoxin production. Mycopath. 2009;167:273–286. doi: 10.1007/s11046-008-9167-3. [DOI] [PubMed] [Google Scholar]
- 24.Rizzo I., Vedoya G., Maurutto S., Haidukowski M., Varsavsky E. Assessment of toxigenic fungi on Argentinean medicinal herbs. Microbiol. Res. 2004;159:113–120. doi: 10.1016/j.micres.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Romagnoli B., Menna V., Gruppioni N., Bergamini C. Aflatoxins in spices, aromatic herbs, herb-teas and medicinal plants marketed in Italy. (2007) Food Control. 2007;18:697–701. [Google Scholar]
- 26.Roy A.K., Sinha K.K., Chourasia H.K. Aflatoxin contamination of some common drug plants. Appl. Environ. Microbio. 1988;54:842–843. doi: 10.1128/aem.54.3.842-843.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selim M.I., Popendorf W., Ibramim M.S., Sharkawy S.E., Kashory S. Aflatoxin BR1 Rcommon Egyptian foods. J AOAC Int. 1996;79:1124–1129. [PubMed] [Google Scholar]
- 28.Tassaneeyakul W., Razzazi-Fazeli E., Porasuphatana S., Bohm J. Contamination of aflatoxins in herbal medicinal products in Thailand. Mycopath. 2004;158:239–244. doi: 10.1023/b:myco.0000041892.26907.b4. [DOI] [PubMed] [Google Scholar]
- 29.Trucksess M.W., Scott P.M. Mycotoxins in botanicals and dried fruits: a review. Food Addit. Contam. 2008;25:181–192. doi: 10.1080/02652030701567459. [DOI] [PubMed] [Google Scholar]
- 30.Vieira I.F.R., Leal A.S., Krambrock K., Tambourg E.B. Identificação de plantas medicinais irradiadas através da ressonância paramagnética eletrônica. Braz. J. Food Technol. 2007;10:63–69. [Google Scholar]
- 31.Weaver C.M., Trucksess M.W. Determination of aflatoxins in botanical roots by a modification of AOAC Official MethodSM 991.31: Single-Laboratpry validation. J AOAC int. 2010;93:184–189. [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Geneva: World Health Organization; 1998. Quality control methods for medicinal plant materials. [Google Scholar]


