Abstract
The bacterial cell wall consists of peptidoglycan (PG), a sturdy mesh of glycan strands cross-linked by short peptides. This rigid structure constrains cell shape and size, yet is sufficiently dynamic to accommodate insertion of newly synthesized PG, which was long hypothesized, and recently demonstrated, to require cleavage of the covalent peptide crosslinks that couple previously inserted material. Here, we identify several genes in Vibrio cholerae that collectively are required for growth – particularly elongation - of this pathogen. V. cholerae encodes three putative periplasmic proteins, here denoted ShyA, ShyB, and ShyC, that contain both PG-binding and M23 family peptidase domains. While none is essential individually, the absence of both ShyA and ShyC results in synthetic lethality, while the absence of ShyA and ShyB causes a significant growth deficiency. ShyA is a D,D-endopeptidase able to cleave most peptide chain crosslinks in V. cholerae’s PG. PG from a shyA mutant has decreased average chain length, suggesting that ShyA may promote removal of short PG strands. Unexpectedly, ShyA has little activity against muropeptides containing pentapeptides, which typically characterize newly synthesized material. ShyA’s substrate-dependent activity may contribute to selection of cleavage sites in PG, whose implications for the process of side-wall growth are discussed.
Keywords: peptidoglycan, endopeptidase, elongation, M23, Vibrio cholerae
Introduction
All free-living bacteria are surrounded by a cell wall that is essential for maintaining cell shape and structural integrity in the face of dynamic osmotic conditions (Cabeen & Jacobs-Wagner, 2005). The principal component of the cell wall is peptidoglycan (PG), an intricate mesh composed of poly-[N-acetylglucosamine N-acetylmuramic acid] (GM) strands crosslinked via short peptide side chains (e. g. L-Ala-D-Glu-Diaminopimelic acid (DAP)-D-Ala) extending from the muramic acid subunits (Holtje, 1998, Typas et al., 2011). Remarkably, covalent bonds in the net-like PG sacculus are routinely broken to enable its expansion (Vollmer, 2012), yet it is able to withstand intracellular pressures of 29 (Escherichia coli) to 190 (Bacillus subtilis) kPa (Koch & Pinette, 1987, Whatmore & Reed, 1990, Deng et al., 2011). PG’s importance for cell survival becomes apparent when essential parts of its assembly machinery are blocked with antibiotics, which often induce rapid cell death and lysis (Tomasz, 1979, Tomasz, 1986, Chung et al., 2009). Not surprisingly, drugs targeting cell wall synthesis, such as beta lactams, carbapenems and vancomycin, are among the most widely-used agents to treat bacterial infections (Schneider & Sahl, 2010).
PG synthesis starts in the cytoplasm, where an assembly line of essential enzymes synthesizes GM-pentapeptide molecules that are transported into the periplasm for incorporation into the cell wall. Assembly of new PG from these precursors is largely mediated by two classes of synthetic reactions. Transglycosylation (TG) reactions polymerize GM subunits into longer glycan chains, while transpeptidation (TP) reactions generate crosslinks between peptide side chains. Both classes of reactions can be catalyzed by penicillin binding proteins (PBPs), although enzymes that do not bind penicillin have also been identified (Sauvage et al., 2008). Most Gram-negative bacteria, including V. cholerae, form crosslinks by bonding the carboxy group of the D-Ala at position 4 on one muropeptide (the donor residue) to the amino group at the D-center of DAP in position 3 of an adjacent muropeptide (the acceptor residue). Such (D,D) crossbridges are produced by D,D-transpeptidases, so named for the chirality of the crosslinked amino acids (Glauner et al., 1988, Cava et al., 2010). In (D,D) crossbridges, donor residues are always components of tetrapeptides (due to the cleavage of the terminal (5th position) D-Ala providing the energy for the crosslinking reaction), while acceptor residues are penta-, tetra-, or tripeptides (tetrapeptides are most common), depending upon whether processing of the acceptor peptide has occurred. In addition, some bacterial species can form crosslinks in which the L-carboxyl group of DAP in one muropeptide is bonded to the D-amino group of DAP in a neighboring muropeptide. Donor residues are always components of tripeptides, due to the cleavage of a tetrapeptide providing the energy for this type of crosslinking reaction. This alternate reaction (approximately 1–3 % of total crosslinkage in V. cholerae (Cava et al., 2010)) is performed by L,D-transpeptidases (Mainardi et al., 2005, Magnet et al., 2008, Cava et al., 2010).
In bacterial species that incorporate new PG constituents along their side- walls, it has long been assumed that cleavage of the PG mesh is necessary for its expansion (Weidel & Pelzer, 1964, Koch & Doyle, 1985, Koch, 1985, Holtje, 1998). In particular, it has been presumed that adjacent polysaccharide chains – which are thought to run perpendicular to the long axis of rod shaped cells (Gan et al., 2008) - must be uncoupled, via cleavage of the crosslinks that connect them, to allow for cell elongation. However, only recently have PG endopeptidases critical for cell elongation been identified. As with many other aspects of PG synthesis, functional redundancy has been detected among the enzymes that catalyze this essential process. In E. coli, where at least seven proteins are known to have PG endopeptidase activity (Korat et al., 1991, Romeis & Holtje, 1994, Uehara et al., 2009, van Heijenoort, 2011), three D,D-endopeptidases were recently reported to be “redundantly essential”. In the absence of all three, incorporation of new PG precursors into the cell wall was markedly reduced, and cells lyse (Singh et al., 2012). Similarly, deletion or mutational inactivation of the active sites of two Bacillus subtilis endopeptidases was found to result in synthetic lethality (Bisicchia et al., 2007, Hashimoto et al., 2012), although these enzymes, unlike those of E. coli, catalyze cleavage of D,L bonds. It is presumed that the activity of such PG hydrolases must be carefully regulated, so that cleavage does not outpace incorporation of new material, and overall PG integrity is maintained. This has been elegantly demonstrated for amidases involved in cell division, whose activities are post-translationally tightly controlled in space and time, often by accessory proteins (Morlot et al., 2010, Uehara et al., 2010, Uehara & Bernhardt, 2011, Peters et al., 2011). However, very little is known about the regulation or specificity of hydrolases involved in cell elongation and how such processes control PG architecture and cell growth.
Here, we show that the previously uncharacterized putative peptidase Vca0079 (re-named ShyA for side-wall hydrolase A), is a D,D-endopeptidase. ShyA is essential for growth of V. cholerae lacking an additional putative peptidase, ShyC, and depletion of ShyA in a shyC mutant dramatically impairs cell elongation. Purified ShyA specifically cleaves D,D crosslinked muropeptides, and has a marked preference for dimeric tetrapeptide-tetrapeptide (D44) substrates; ShyA was less active on dimeric tetrapeptide-pentapeptide (D45) substrates. This endopeptidase also preferentially releases short glycan chains from purified sacculi, and on average V. cholerae lacking ShyA contain shorter glycan chains and increased levels of PG crosslinks. Collectively, our in vitro and in vivo analyses suggest that ShyA’s cleavage of PG crosslinks is a key step in V. cholerae cell elongation and that its activity is at least in part dependent on substrate cues. These findings have important implications for the mechanism of V. cholerae growth.
Results
ShyA and ShyC are conditionally essential and promote cell elongation
A screen for loci that were predicted to be involved in PG metabolism led to the identification of vca0079 (renamed shyA for sidewall hydrolase A), a locus that is predicted to encode a protein containing both a putative PG binding domain (OapA domain, (Weiser et al., 1995)) as well as an M23 metallopeptidase domain. Analysis of the genome of the canonical El Tor O1 V. cholerae strain N16961 revealed that this organism encodes two additional proteins that are also predicted to contain both these domains (Fig. S1,S2). The presence of a putative PG-binding domain in these proteins suggested that they might function as PG endopeptidases. To explore their importance in V. cholerae, we generated strains with individual in-frame deletions of each of the three genes: shyA, vc0503 (now shyB), and vc0630 (shyC). In LB medium, the growth of all three strains was indistinguishable from that of the wild type, clearly indicating that none of these genes are essential for growth (Fig. 1A); however, deletion of shyA resulted in a modest reduction of the growth rate of cells grown in minimal media (Fig. 1B).
Fig. 1. Growth phenotypes of V. cholerae strains with deletions in orfs containing OapA and M23 peptidase domains.
Cells were grown in 200 µL in (A) LB medium or (B) and (C) M9 MM 0.2 % glucose, monitoring OD600. Error bars represent standard deviation of three independent experiments. (D) ShyA depletion in the ΔshyC background. TD541 (ΔshyA ΔshyC PIPTG:shyA) and TD543 (TD541 ΔshyB) were grown in liquid medium in the presence of IPTG, washed, and resuspended in fresh medium containing glucose (T0). Error bars show the standard deviations for three independent experiments.
To explore whether functional redundancy among these three proteins might mitigate the impact of individual mutations, we attempted to generate and characterize strains with pairs of mutations. We found that the ΔshyA ΔshyB and ΔshyB ΔshyC double mutants were viable and had no obvious growth or shape defects during growth in LB (Fig 1A). However, the ΔshyA ΔshyB mutant exhibited a marked growth defect that surpassed that of the ΔshyA mutant in minimal medium (Fig.1C), suggesting that the products of these two putative endopeptidase genes have redundant functions in some conditions.
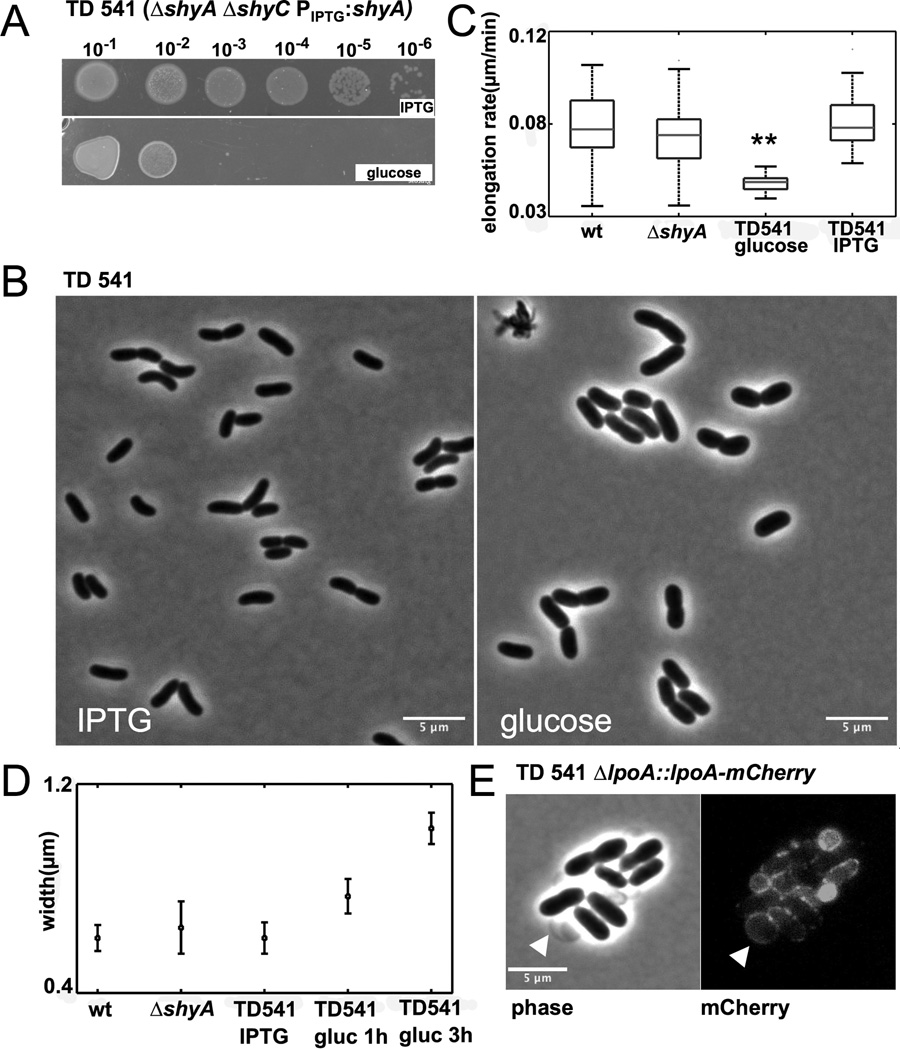
Notably, we were unable to make a ΔshyA ΔshyC mutant, suggesting that simultaneous deletion of these genes results in synthetic lethality. To further investigate this possibility, we created a ΔshyA ΔshyC strain with an IPTG-inducible shyA inserted in a neutral locus (strain TD541). As expected, growth in liquid medium and formation of colonies by this strain was dependent on IPTG (Fig. 1D, 2A), confirming that shyA and shyC mutations confer synthetic lethality. After depletion of ShyA in liquid cultures, cells stopped growing and became wider, but did not lyse (Fig. 1D, 2B,D). No difference was observed between ShyA depletion in a ΔshyC mutant and ShyA depletion in a ΔshyB ΔshyC double mutant (Fig. 1D).
Fig. 2. shyA and shyC are synthetically lethal.
(A) TD541 (ΔshyA ΔshyC PIPTG:shyA) was grown to stationary phase in LB medium containing IPTG and spotted on LB agar plates containing either IPTG or glucose. (B) ShyA depletion in liquid culture. TD541 was grown in medium containing IPTG, washed twice in LB medium with glucose and resuspended in an equal volume LB/glucose. Cells were imaged just before addition of glucose and three hours after and analyzed using MicrobeTracker. (C) Average elongation rates of around 100 cells of wild type, TD536 (ΔshyA) and TD541 before and after ShyA depletion. Cells were grown on agarose pads with 10 % LB and glucose or IPTG and imaged every minute. Elongation rate is the slope of length increase over time. Asterisks indicate statistical significance (t-test, p<0.01) (D) Average cell widths in liquid cultures of wild type, TD536 and TD541 before and after ShyA depletion. (E) Vesicles emanating from TD541 cells grown on agarose pads contain outer membrane. A functional mCherry fusion of the outer membrane protein LpoA (Vc0581) was expressed from its native promoter and imaged 120 min after plating on an agarose pad containing glucose.
Time-lapse microscopy on agarose pads revealed that TD541’s cell elongation rate decreased by nearly 50% after shyA expression was inhibited by glucose (Fig. 2C). However, as in liquid cultures (Fig. 2D), the width of ShyA-depleted cells increased on agarose pads (Fig. S3 A,B), resulting in an almost twofold increase in cell volume (Fig. S3C). In contrast, the width of wt cells and TD541 expressing ShyA remained relatively constant. As ShyA depletion progressed, cell shape on agarose pads also became increasingly irregular, with vesicles protruding from the cells (Fig. 2E and Fig. S3A, arrowheads). These vesicles appear to contain outer membrane, since they included the outer membrane-linked lipoprotein LpoA (Fig. 2E). These data suggest that the absence of both ShyA and ShyC impairs cell elongation but not outer membrane biogenesis, and thus suggest that cell-wall synthesis and outer membrane synthesis are not inextricably coupled.
ShyA is localized to the lateral wall and ShyC to the lateral wall and septum
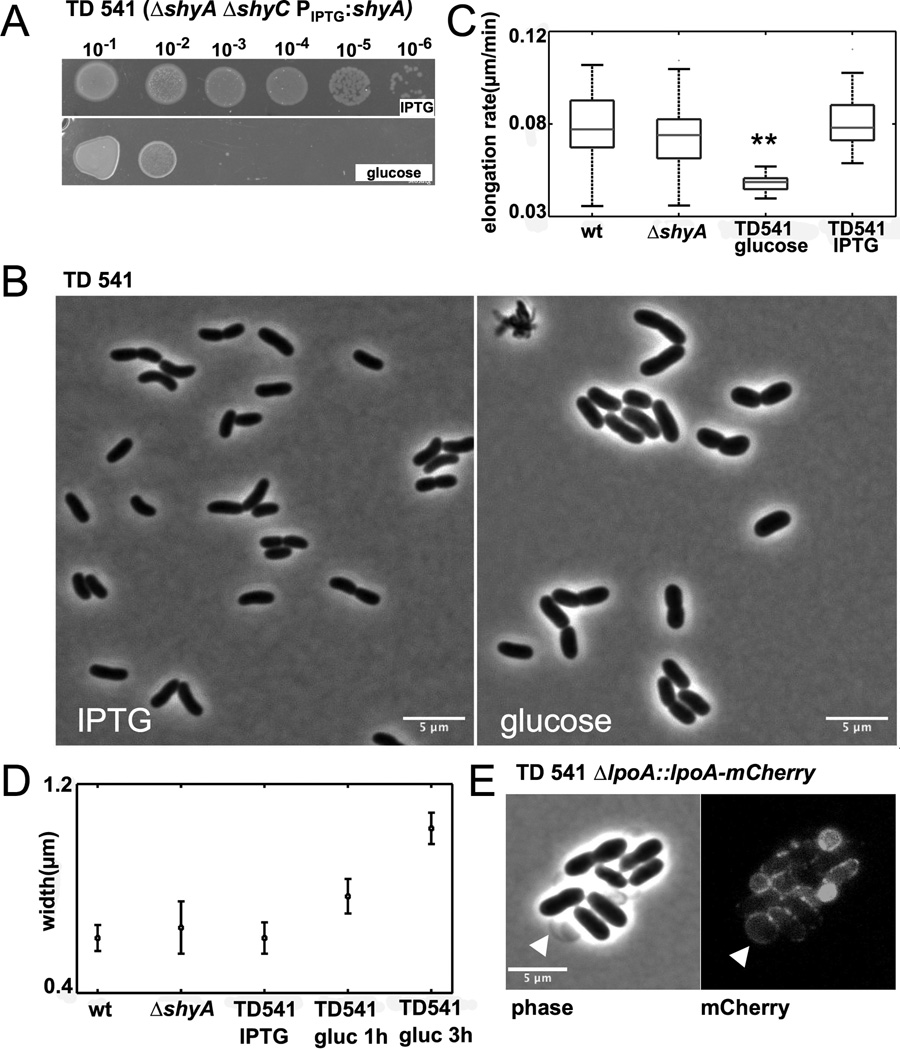
We constructed mCherry fusions to the C-termini of ShyA and ShyC to investigate the subcellular localization of these proteins. Both ShyA-mCherry and ShyC-mCherry were stable (Fig. S4) and restored growth of a ΔshyA ΔshyC mutant and were thus functional (data not shown). Using a plasmolysis assay (Lewenza et al., 2006), we found that ShyA is a soluble periplasmic protein, while ShyC does not appear to be free within the periplasm (Fig. 3A); both localizations are consistent with bioinformatic predictions, since ShyA contains a putative signal sequence, while ShyC is predicted to be tethered to the inner membrane (Fig. S1). In the absence of an osmotic challenge, ShyA uniformly localized to the periphery of the cell with no distinct pattern (Fig. 3B). Notably, ShyA was never observed at the division site, which is consistent with the idea that its principal role is in cell elongation rather than cell division. In contrast, ShyC displayed localization at the midcell in a subset of cells (Fig. 3C) in addition to its distribution at the cell periphery. ShyC’s pattern of localization suggests it may contribute to re-modeling of septal PG for cell division, in addition to contributing to bacterial elongation.
Fig. 3. ShyA and ShyC have distinct localization patterns.
V. cholerae cells expressing mCherry fusions were grown overnight in M9 MM containing 0.2 % glucose and 20 µM IPTG. Cells were then diluted 1:100 into fresh medium containing 100 µM IPTG and grown for 2 h, applied to a 0.8 % agarose pad containing M9 MM glucose and imaged with epifluorescence (500 ms exposure). (A) Cells imaged after 15 min exposure to 15 % sucrose (see experimental procedures for details). Arrowheads point to enlarged periplasmic pockets that result from withdrawal of the inner membrane. (B) and (C) Phase contrast and fluorescence images of V. cholerae carrying (B) pshyA-mCherry and (C) pshyC mCherry.
ShyA is a D,D-endopeptidase
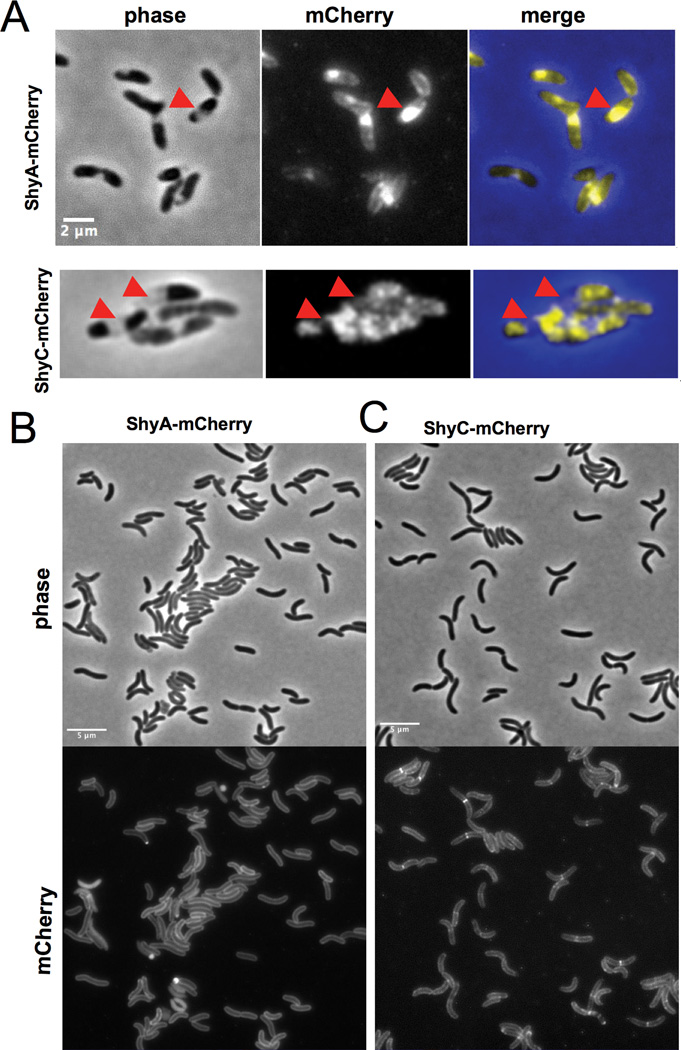
We purified His-tagged ShyA and ShyC without their signal/transmembrane sequences (ShyA[36–430]-His6 and ShyC[34–433]-His6) to test whether these proteins display PG endopeptidase activity as predicted. We also engineered and purified ShyA containing an H375A substitution within its signature M23 peptidase active site (His-Lys-His) to use as negative control. The activity of these proteins was tested using V. cholerae sacculi labeled with remazol-blue as a substrate (Zhou et al., 1988). Wild type ShyA, but not the active site mutant, released dye from sacculi (which are insoluble, and can be pelleted by ultracentrifugation) into the supernatant fraction of the reaction, thereby confirming that ShyA has PG hydrolase activity (Fig. 4A,B). HPLC-based analyses of material released from unlabelled sacculi confirmed this conclusion, and revealed that digestion of sacculi by ShyA proceeds rapidly (Fig. S5). ShyA appears to be a Zn-metalloprotease, as solubilization (i.e., cleavage) of remazol-blue labeled PG was blocked by addition of 1 mM EDTA to the reaction, and the effect of EDTA could be reversed by the addition of 1 mM ZnCl2. Excess zinc (>2 mM) inhibited activity, consistent with observations in other zinc-dependent metallopeptidases (Gomez-Ortiz et al., 1997), (Fig. 4C). Purified His-tagged ShyC had no activity against purified sacculi (data not shown), and consequently it was not further investigated in biochemical assays.
Fig. 4. ShyA has PG hydrolase activity in vitro.
(A) Remazol-Blue (RBB) stained sacculi were incubated with 5 µM of purified ShyA-His, ShyAH375A-His, or lysozyme (a positive control) for 3 h at 37˙C. Undigested material was removed by ultracentrifugation, then OD585 of the supernatant was measured. Error bars represent standard deviations of 3 independent experiments. (B) Representative picture of supernatants from mock or ShyA-digested RBB-stained sacculi after ultracentifugation. (C) RBB stained sacculi were treated as described in (A) with the additions indicated on the x-axis. Error bars represent standard deviations of two independent experiments.
To further characterize the activity of ShyA, we used High Performance Liquid Chromatography followed by Mass Spectrometry (HPLC/MS) to analyze the PG fragments released from purified V. cholerae sacculi by digestion with ShyA. The solubilized fraction consisted of polymeric GM dissacharides bearing non-crosslinked stem tetrapeptides (Fig. 5A, B, S6). The absence of cross-linked stem peptides in this material suggests that ShyA cleaves the D,D bond between DAP and D-Ala that constitutes most of the cross-links between stem peptides; thus, ShyA appears to be a D,D-endopeptidase.
Fig. 5. ShyA is a D,D-endopeptidase.
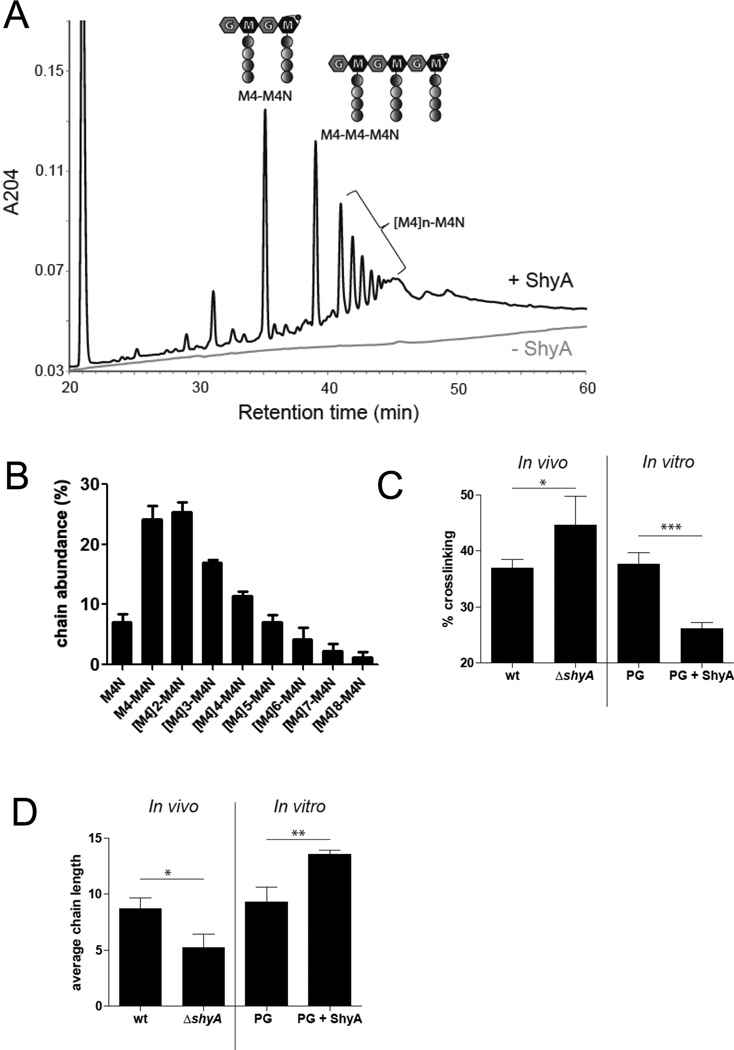
(A) Representative HPLC chromatograms of the supernatant of undigested sacculi (lower line) and sacculi digested with ShyA (peaks). PG was digested with 5 µM ShyA for 3 h at 37°C. (B) Relative abundance of PG-chains released by ShyA. Sacculi were digested with ShyA as in (A) and PG chains were quantified using HPLC (see experimental procedures for details).
(C) Quantification of PG crosslinks in sacculi from wild type (wt) and ΔshyA cells (In vivo) as well as of wild type PG partially digested (0.1 µM for 1 h at ambient temperature) with ShyA (PG+ ShyA) or undigested (PG) (in vitro). P values (wt vs ΔshyA): 0.0447; (PG vs PG + ShyA): 0.0010.
(D) Average chain length of the wt and ΔshyA mutant as well as PG partially digested with ShyA as in (B). Chain length was estimated based on the relative abundance of anhydromuropeptides. P values (wt vs ΔshyA): 0.0181; (PG vs PG + ShyA): 0.0061. (B–D) correspond to three independent experiments each, error bars represent standard deviation.
To further test this hypothesis, we analyzed the abundance of crosslinks in sacculi partially digested with ShyA. We observed a dramatic reduction of crosslinks relative to an undigested control, a result strongly in support of ShyA’s function as a D,D-endopeptidase (Fig. 5C, Fig. S7). Comparative analysis of sacculi from wild type and ΔshyA cells were also consistent with this conclusion, as cross-links were more abundant in sacculi derived from the ΔshyA mutant.
Importantly, analyses of PG from wild type and ΔshyA cells suggest that ShyA activity may result in the preferential release of short glycan chains from the PG mesh in vivo. The average chain length of PG from wild type cells (based on quantification of anhydro residues) was ~8, whereas PG from the shyA mutant averaged ~4 disaccharides (Fig. 5D, Fig. S7), which is consistent with more frequent retention of short chains within the mutant PG. Additionally, following partial digestion of purified sacculi with ShyA, the residual undigested material contained longer glycan chains on average than untreated PG (Fig. 5D, in vitro). Short chains were also abundant in the material released from digested PG (Fig. 5A, B), although it should be noted that GM polymers longer than 11–12 disaccharides could not be quantified with the specific HPLC columns used (see experimental procedures for details), and that these data therefore cannot be used to calculate the absolute frequency of short chains or the average length of GM chains released by ShyA. Although all our data suggest that ShyA-mediated PG cleavage may predominantly release short PG polymers, there is no evidence that ShyA exhibits length-dependent substrate specificity. Instead, release of short chains may simply require fewer cleavage events and less enzyme processivity, and hence occur more frequently than release of longer chains. Interestingly, ShyA’s activity in vivo does not have a dramatic effect upon the total cellular PG levels: PG abundance in wild type and shyA cells did not differ significantly (data not shown).
ShyA preferentially cleaves crosslinks between tetrapeptides
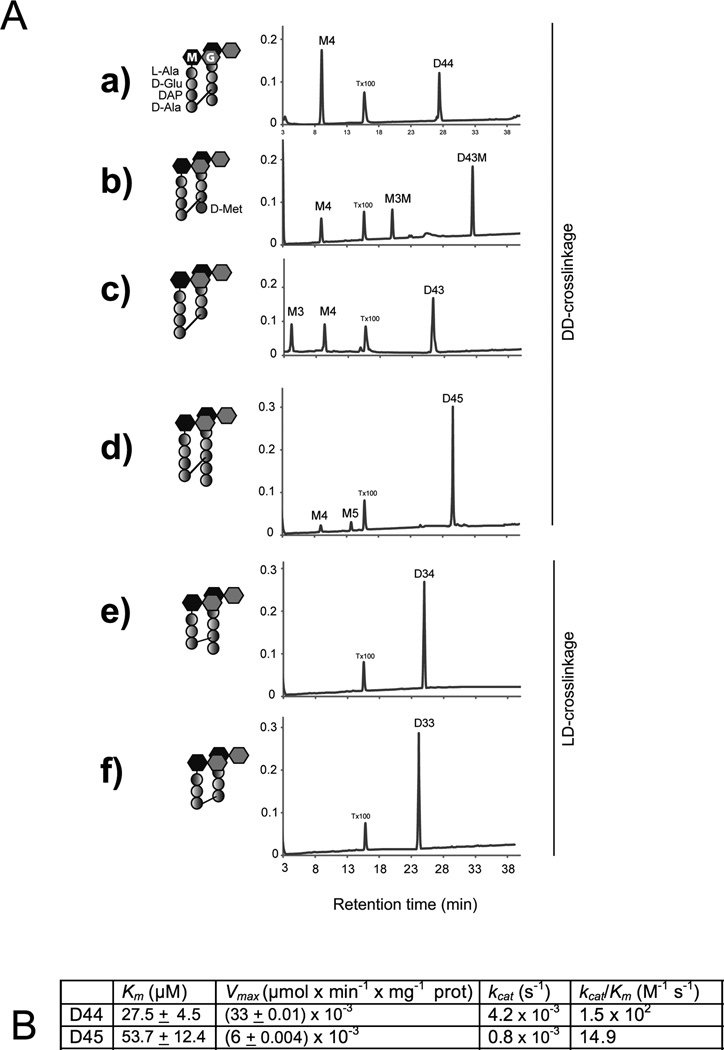
To more precisely define ShyA’s activity, we assessed its capacity to cleave a variety of purified muropeptide substrates: the DD-crosslinked dimers D44, D43, D43M and D45; and the LD-crosslinked dimers D33 and D34. ShyA converted more than 50% of D44 into its monomeric constituent (M4), consistent with its role as a D,D-endopeptidase (Fig. 6A). Similarly, ShyA cleaved more than 50% of D43M, which contains D-Met instead of D-Ala in the fourth position of the acceptor moiety, suggesting that ShyA activity does not depend on the identity of the D-amino acid at peptide position 4. ShyA also converted more than 50% of D43 to product, but it cleaved less than 10% of D45, although the crosslink in D45 connects the same amino acids that are linked in D44, D43 and D43M (Fig. 6A). Thus, although ShyA has activity on D,D crosslinks in several peptide contexts, the enzyme’s endopeptidase activity appears reduced when the acceptor stem is a penta rather than a tetrapeptide. Comparative steady-state kinetic analyses of ShyA’s catalytic efficiency were consistent with this idea: the enzyme’s kcat/km was 10-fold higher for D44 than for D45 (Fig. 6B).
Fig. 6. ShyA substrate preferences.
(A) HPLC analysis of ShyA endopeptidase activities on muropeptides The indicated DD-crosslinked dimers (D44, D43, D43M, D45) and LD-crosslinked dimer muropeptide substrates (D33 and D34) were incubated with 7.8 µM ShyA-His for 3h at 37˙C before HPLC analysis. ShyA endopeptidase activity was assayed by monitoring the appearance of the monomeric compounds M4 (GM-L-Ala-D-Glu-DAP-D-Ala); M3 (GlcNAc-MurNAc-L-Ala-D-Glu-DAP), M5 (GM-L-Ala-D-Glu-DAP-D-Ala-D-Ala) and M3M (GM-L-Ala-D-Glu-DAP-D-Met). Assays were carried out in duplicates and representative HPLC chromatograms are shown. X-axis = A204 (B) Kinetics of ShyA-His D,D-endopeptidase activities. D,D-endopeptidase activity in vitro on D44 and D45 muropeptide substrates was measured as described in experimental procedures. All kinetic constants were calculated using data obtained with 7.8 µM ShyA with various amounts (1–300 µM) of the dimers bis-dissacharide tetratetrapeptide (D44) and bis-dissacharide tetrapentapeptide (D45). The enzymatic reactions were analyzed by HPLC assay as described in experimental procedures. All kinetic constants must be considered apparent values because of the impossibility of calculating initial enzyme velocities by HPLC. Values are means ± standard deviations of experimental triplicates.
A small subset of peptide crosslinks in PG consist of an L,D bond that connects two DAP moieties, rather than the previously analyzed D,D bonds that link D-Ala and DAP. Notably, no cleavage of such bonds by ShyA was detected, either using D34 or D33 substrates (Fig. 6A). The precise roles for L,D crosslinks, which are generated by L,D transpeptidases (Mainardi et al., 2005, Magnet et al., 2008, Cava et al., 2010) are not clear; consequently, the physiological significance of the resistance of such bonds to ShyA digestion is not yet known.
Discussion
The factors and mechanisms that enable and control elongation of the PG mesh that surrounds rod-shaped bacteria remain poorly understood. PG elongation is thought to require cleavage of the stem peptide linkages between glycan strands. Here, we identified three V. cholerae loci, designated shyA, shyB and shyC, predicted to encode periplasmic PG-binding peptidases, candidate elongation-associated endopeptidases. The products of these loci appear to function at least in part in a redundant fashion, as double mutations were required to elicit significant growth defects. Notably, shyA and shyC were synthetically lethal, and depletion of ShyA in a ΔshyC background led to a marked elongation defect. Purified ShyA was highly active against purified cell wall and released non-crosslinked muropeptides from sacculi, suggesting that this enzyme is a D,D-endopeptidase that cleaves the D,D bond between D-Ala and DAP that constitutes most of the cross-links in V. cholerae PG. Additional biochemical studies using purified muropeptides as substrates confirmed that ShyA is a D,D-endopeptidase and revealed that it has significantly lower activity against crosslinks in a D45 substrate than against substrates with shorter acceptor chains (e.g., D44, D43M, and D43). To our knowledge, comparable substrate specificity has not previously been reported for elongation-associated endopeptidases.
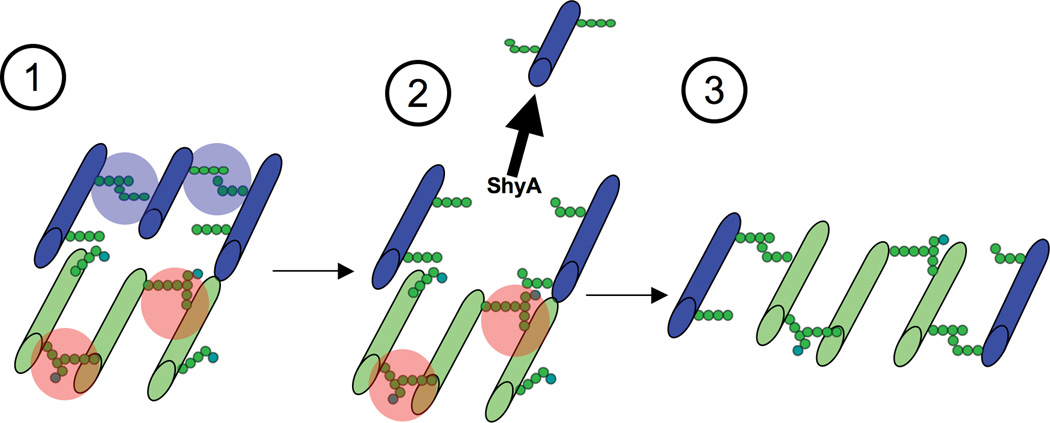
ShyA’s substrate specificity (in particular, its reduced capacity to digest D45 dimers relative to D44 dimers) may enable fine tuning of PG processing and thereby facilitate PG expansion and cell growth. In vivo, pentapeptide sidechains are rare (approx. 0.9 % of PG in exponentially growing V. cholerae (Cava et al., 2010)) and are likely most abundant in newly synthesized/inserted PG, since such strands have not yet been subject to modification by carboxypeptidases ((Glauner et al., 1988, Ghosh et al., 2008, Potluri et al., 2010, Chowdhury et al., 2010)). In E. coli, pentapeptides are rapidly converted to tetrapeptides by enzymes such as PBP5 (Ferreira et al., 1988, Hesek et al., 2004, Chowdhury et al., 2010), which is also present at sites of active PG synthesis (Potluri et al., 2010). Thus, ShyA’s substrate preference may be a means to avoid immediate removal of new PG strands following their incorporation into the cell wall (Fig. 7).
Fig. 7. Model of ShyA-mediated sacculus enlargement.
(1) New PG (colored green) is synthesized and contains ShyA-resistant 4/5 peptide bonds (shaded red, the fifth D-Ala is colored light blue). Old PG (colored blue) contains mostly ShyA-sensitive 4/4 crosslinks (shaded blue). We follow the “three for one” model proposed by Höltje (Holtje, 1998) and thus assume that three new strands are synthesized for incorporation. (2) ShyA cleaves and removes an old PG strand without affecting the bonds in new PG. Newly synthesized PG might either be already connected to the old material (as originally proposed in the three for one model) or it might be crosslinked concomitant with ShyA action. (3) Three nascent strands replace one old strand, enlarging the PG mesh.
A high degree of PG turnover has been reported during normal bacterial growth (Park & Uehara, 2008), indicating that insertion of new cell wall material is constantly accompanied by removal of old material. Our analyses of average PG chain length suggest that when ShyA uncouples fragments of PG from an intact network, likely freeing it for cellular recycling, short strands of PG are preferentially released. Short strands are the product of processing, likely by lytic transglycosylases (Holtje et al., 1975, Scheurwater et al., 2008, van Heijenoort, 2011) and therefore represent ‘mature’ PG. Thus, our analyses of chain length, like those of substrate specificity, suggest that ShyA-mediated cleavage is more influential in mature PG than in newly synthesized material. Interestingly, we also found that ShyA activity was more efficient on purified sacculi than pure substrate, even after extensively optimizing conditions for the latter reactions. This suggests that the macromolecular context is important for ShyA activity.
Very little is known about the substrate specificity of most PG hydrolases. However, reduced cleavage of D45 substrates is not characteristic of all D,D- endopeptidases. For example, the E. coli penicillin binding protein AmpH (which has not been shown to be required for elongation) has D,D, endopeptidase activity against D45 as well as D44 muropeptides; it instead is less active on D44N and D45N dimers containing an anhydro residue (Gonzalez-Leiza et al., 2011). Additional experiments are warranted to assess whether substrate level regulation occurs for other PG endopeptidases and whether avoidance of nascent PG is a defining feature of growth-mediating hydrolases.
D,D-endopeptidase activity only affects sacculus growth
Our observations suggest that sacculus growth in V. cholerae is not intimately coordinated with other processes required for expansion of the cell envelope. Even in the absence of ShyA and ShyC, cells continued to increase in width and started shedding vesicles containing outer membrane. Both observations suggest that V. cholerae lacks negative feedback between cell wall stress and macromolecule synthesis; even though ShyAC-depleted cells are unable to expand their sacculus, they continue increasing turgor pressure (causing an increase in cell width), and synthesizing outer membrane.
Endopeptidase activity is essential for cell growth in diverse organisms
Our findings fit well with recently published observations of E. coli and B. subtilis (Singh et al., 2012, Hashimoto et al., 2012), and provide further support for the long-standing hypothesis that endopeptidases are required for cell growth. All elongation-specific endopeptidases identified so far belong to either the M23 or P60 domain family of peptidases. Both classes are present in gram-positive and gram-negative bacteria as well as Archaea, suggesting well-conserved housekeeping functions for these enzymes. Two P60-domain hydrolases are required for sacculus growth in B. subtilis, while in E. coli this process is mediated by proteins containing either an M23 or P60 domain. In contrast, our results suggest that PG peptide crosslinks in V. cholerae are largely substrates for endopeptidases containing an M23 protease domain, as the absence or depletion of such proteins impairs cell growth and/or survival even when V. cholerae’s lone P60 domain protein, NlpC, is intact. However, since purified ShyC was inactive we cannot yet rule out the possibility that NlpC also plays an important role in V. cholerae, either as an activator of ShyC or as a target of ShyC-dependent activation. Studies by Uehara et al. (Uehara et al., 2010) have revealed that some M23-domain proteins contribute to PG metabolism by activating other enzymes (in this case, amidases) rather than through intrinsic catalytic activity.
D,D-endopeptidases as antibiotic targets
Simultaneous deletion of two M23 domain metallopeptidases, ShyA and ShyC, was sufficient to cause growth arrest and morphological defects in V. cholerae. A different combination of M23 domain peptidases (ShyA and ShyB) caused a severe growth defect at least in minimal medium. Since proteases have proven to be excellent drug targets (Raju et al., 2012), screens to identify compounds that inactivate M23 active sites could be a promising avenue for antibiotic discovery. The high level of conservation of predicted M23 domain-containing proteins in bacteria (Vollmer et al., 2008, Wyckoff et al., 2012) supports the general importance of these enzymes and suggests the possibility for generating new broad-spectrum agents. However, it remains to be seen whether most organisms, like V. cholerae, require at least one M23 peptidase for growth, or whether other classes of endopeptidases are sufficient, as in E. coli, where P60 family endopeptidases can support growth.
Experimental procedures
Media and growth conditions
Cells were grown at 37°C in LB medium or M9 minimal medium containing streptomycin (200 µg ml−1). Unless otherwise indicated, growth curves were conducted in 200 µL volume in a Bioscreen C OD reader (Growth Curves America).
Strain and plasmid construction
All strains are derivatives of Vibrio cholerae El Tor N16961 (wt). All cloning steps were conducted using isothermal assembly (Gibson et al., 2009). Oligos are summarized in table S1. Gene knockouts were constructed using the suicide vector pCVD442 (Donnenberg & Kaper, 1991). 500 to 800 bp of flanking sequences were cloned into this vector digested with XbaI in E. coli DH5α λpir, then transformed into the donor strain SM10 λpir and mated overnight at 37 °C with V. cholerae by mixing equal volumes (1 mL) of exponential cultures, concentrating into 100 µL, and spot-plating. Successful first crossover recipients were selected on LB agar plates containing 200 µg/ml streptomycin and 50 µg/ml carbenicillin, restreaked to single colony and then plated on salt-free LB agar containing 10 % sucrose and streptomcyin. Colonies were tested by PCR for successful knockout and by plating on carbenicillin for loss of pCVD442. The following primers were used to construct knockout plasmids: shyA (TDP498/499 + TDP 500/501), shyB (TDP685/686 + TDP687/688), shyC (TDP689/690 + TDP691/692).
For depletion of ShyA, the vca0079 ORF was amplified using primers TDP 529/530 and cloned into SmaI – digested pHL100 (a derivative of pWSK129 (Wang & Kushner, 1991) carrying the lac promoter and lacIq). The lacIq PLac-shyA region was then amplified using primers TDP674/675 carrying 35bp homology regions to pJL1((Butterton et al., 1995) a suicide vector with a multiple cloning site within lacZ, for chromosomal insertion into this neutral locus) and cloned into StuI-digested pJL1. Successful clones were transformed into SM10 λpir. This strain was then mated with strain TD538 (ΔshyC), then shyA was deleted in this strain as described above, creating strain TD541. Deleting shyB in this strain then resulted in strain TD543.
For outer membrane marking of strain TD541, lpoA was amplified from the chromosome using primers TDP236/237 carrying a 35 bp overhang homologous to the 5’ region of the lpoA orf and a 3’ overhang containing 35 bp homologous to the gene encoding mCherry. mCherry was amplified using primers TDP238/239 carrying 3’ overhangs with the 3’ flanking region of lpoA. Finally, flanking homology regions of lpoA (~500 bp on each side) were amplified using primers TDP 304/306 and TDP305/307 that carry overhangs homologous to pCVD442 digested with XbaI. The fragments were cloned into pCVD442 and SM10 carrying the resulting plasmid mated with TD541 as described above, creating strain TD542.
Site-directed mutagenesis was performed as described in the QuikChange mutagenesis kit (Stratagene) on pET28BshyA-his using primers TDP595/596. For construction of mCherry-tagged endopeptidases, genes were amplified using primers TDP518/529 (shyA) or TDP723/753 (shyC) containing 3’ overhangs homologous to pHL100 and 5’ overhangs homologous to mCherry. mCherry was amplified using primers TDP238/239, and cloned into SmaI-digested pHL100 together with the respective endopeptidase.
Plasmolysis assay
The plasmolysis assay for membrane binding was conducted as described (Lewenza et al., 2006). Cells were grown to OD600 = 0.5, pelleted and resuspended at 20× concentration in 20 mM Tris, 15 % sucrose and 0.03 % sodium azide, incubated for 15 min at room temperature and then imaged.
Protein purification and Western blot analysis
E. coli strain RosettaGami (Invitrogen) carrying pET28b with the respective N-terminally 6×His-tagged ORFs was grown overnight in LB medium containing 0.4 % glucose and 12 µg ml−1 tetracycline, 50 µg ml−1 streptomycin, 20 µg ml−1 chloramphenicol, 50 µg ml−1 kanamycin. Cells were diluted 1:1000 in fresh medium of the same composition and grown at 37°C to OD ~0.6, then left at 4°C for 20 min, and induced with 1 mM IPTG overnight at room temperature. Cells were then harvested by centrifugation (6000 × g, 20 min), washed 1× in PBS, resuspended in Buffer T (20 mM Tris, 150 mM NaCl, 10 % glycerol, 10 mM β-mercaptoethanol (BME)+ complete protease inhibitor (Roche)) and stored at −80 °C.
After thawing, 0.1 % reduced Triton x-100 (Sigma) was added and cells were disrupted by passing them 3 × through a French press. The lysate was centrifuged for 1 h at 25,000 rpm, then NaCl was adjusted to 300 mM, Imidazole added to 30 mM and the cell lysate mixed with 1 ml Ni NTA resin (Qiagen) equilibrated in buffer T. The mixture was rotated at 4°C for 2 h, then the resin was washed with 10 × 5 ml buffer W (buffer T + 500 mM NaCl) in Qiagen chromatography columns and eluted with 1 mL aliquots of buffer W containing a gradient of 60 – 300 mM Imidazole. Fractions were visualized by SDS page followed by Coomassie stain. Fractions containing the protein of interest were pooled, dialyzed 2× against Buffer T adjusted to 300 mM NaCl and kept at −80°C.
Western blotting was conducted using standard techniques with Living Colors monoclonal anti mCherry (Clontech) and detection by secondary antibody coupled with horseradish peroxidase.
Remazol blue assay of hydrolase activity
Murein sacculi from stationary phase V. cholerae were prepared as described (Cava et al., 2010) and stained with remazol brilliant blue (RBB) as described (Zhou et al., 1988). In short, purified sacculi were added to a basic (0.25 M NaOH) solution of remazol blue, incubated overnight and washed with water by repeated ultracentrifugation until the supernatant stayed clear. Stained sacculi were stored in 10 % glycerol at −20˙C.
Hydrolase assays were conducted in 200 µL of Pulldown buffer (Moll et al., 2010) (20 mM Tris/maleate, 30 mM Nacl, 10 mM MgCl, 1 mM DTT, 0.1 % Triton X-100) containing 50 µL of RBB stained sacculi (stock concentration 4 µg ml−1). Proteins were added at 5 µM and incubated at 37 °C for 3 h. Reactions were then stopped by the addition of 1 % SDS and centrifuged 80.000 rpm for 15 min in a Beckman Coulter ultracentifuge using rotor TLA110. Supernatants were removed and A585 measured in 96well plates using a Synergy HT (Bio-Tek) microplate reader.
Microscopy and image analysis
For depletion of ShyA, TD541 was grown to OD = 0.3 in LB containing 100 µM IPTG, then washed twice in LB containing 0.2 % glucose, left standing in the same medium for 30 min and then analyzed by phase microscopy. Time lapses were recorded on 0.8 % agarose pads containing 10 % LB at 37 °C (using an objective heating element). Images were analyzed using MicrobeTracker (Sliusarenko et al., 2011) and MATLAB (Mathworks), with which statistical analyses were also conducted. Elongation rate was calculated by re-arranging frame-by-frame (1 min apart) cell length into a length × time array and using the MATLAB diff(x) function (x2-x1,x3-x2…Xn-(Xn-1)) divided by diff(y), where x=length and y=time.
HPLC analysis of PG and muropeptide identification
Peptidoglycan from stationary phase V. cholerae cells was purified by the boiling SDS extraction method and muramidase digestion treatment (Cellosyl; Hoechst AG, Frankfurt, Germany)) as previously described (Cava et al., 2010). Solubilized muropeptide mixtures were then either directly injected into the HPLC system (native or non-reduced samples) or subjected to BH4Na reduction. Muropeptides were analyzed using a binary-pump Waters HPLC system (Waters Corporation, Milford, USA) fitted with a reverse phase RP18 Aeris peptide column (250 × 4.6 mm; 3.6 µm particle size) (Phenomenex, USA) and a dual wavelength absorbance detector. Elution was monitored at 204 nm. Elution conditions were: flow rate 1 ml min−1; temperature 35°C; 3 min isocratic elution in 50 mM sodium phosphate, pH 4.35 followed by a 57 min linear gradient to 75 mM, sodium phosphate, pH 4.95 in 15% (v/v) methanol and 10 min isocratic elution under the gradient final conditions. Muropeptides of interest were collected following HPLC separation; vacuum dried, and subjected to MALDI-mass spectrometry and electrospray ionization MS/MS as described (Cava et al., 2010).
Average PG chain length was calculated by dividing the molar amount of anhydro-muropeptide (=chain termini) by total molar amount of muropeptides in muramidase-digested PG, as described by (Glauner et al. 1988). Degree of crosslinking was calculated by calculating the molar ratio of dimers and trimers to total muropeptides.
Quantification of muropeptides
Individual muropeptides were quantified from their integrated areas using samples of known concentration as standards. Concentration of the standard muropeptides was determined as described by Work (Work, 1957). The extent of ShyA-dependent solubilization of muropeptides was analyzed over time (2, 5, 30, 60, 180 and 960 min) and compared to full solubilization of muropeptides by overnight muramidase-digestion. V. cholerae sacculi (125 µg) were digested with 7.8 µM ShyA at 37°C in buffer ShyA (20 mM Tris/maleate pH 5.2, 30 mM Nacl, 10 mM MgCl2, 1 mM DTT, 0.1 % Triton X-100). Reactions were terminated by inactivation at 100°C for 10 min and centrifuged at 14000 × g for 30 min to discard insoluble non-digested sacculi and coagulated ShyA. Next, soluble PG released by ShyA was further treated with muramidase overnight at 37°C in the same ShyA buffer prior to injection into the HPLC. Determination of the extent of ShyA-dependent PG solubilization was performed by comparing the sum of all integration areas in ShyA vs. muramidase treated samples.
HPLC assay of ShyA DD-peptidase activities
All enzymatic reactions were analyzed in triplicate. Hydrolase assays were conducted in 200 µL of ShyA buffer containing 10 µg of pure muropeptide. ShyA was added at 5 µM and incubated at 37°C for 3 h, unless indicated otherwise in figure legend. The D,D-endopeptidase activity of ShyA was determined with the dimeric compounds bis-disaccharide tetrapentapeptide (D45) and disaccharide tetra-tetrapeptide (D44), as well as its D-Met-containing derivative D43M, as substrates. To investigate the LD-endopeptidase activity, D34 and D33 muropeptides were used as substrates.
To study the activity of His-tagged ShyA on macromolecular PG, 200 µg of undigested V. cholerae sacculi were digested with ShyA as described above. All enzymatic reactions were terminated by boiling the samples for 5 min and centrifuged at 14,000 rpm for 15 min to discard precipitated protein. The supernatants (reaction products) were injected into the HPLC whereas the remaining insoluble PG was digested with muramidase and further processed for HPLC analysis as described above. Enzymatic activities were estimated from the variation in the abundance of presumed substrate and product muropeptides relative to an undigested control sample. Abundance of individual PG chains was calculated by integrating the area under the curve for each chain with the sum of all detectable peaks.
Kinetic parameters
Kinetic parameters were calculated by measuring production rates for different substrate concentrations (1–300 µM) of natural substrates D44 and D45. The activities for each substrate concentration were assessed in triplicate. All mean activities were plotted and adjusted to a Michaelis–Menten model using Excel to determine the apparent Km, Vmax, and kcat by non-linear regression (De Levie, 2001). kcat was determined as Vmax/[E0], where [E0] = nmol of protein/ml (His-tagged ShyA)
Supplementary Material
Acknowledgements
We thank members of the Waldor lab,Tom Bernhardt and Miguel Angel de Pedro for helpful discussions. Research in the Waldor lab is supported by Howard Hughes Medical Institutes (HHMI) and NIH grant R37 AI - 42347. Research in the Cava lab is supported by the Ministry of Education and Science, Spain (RYC-2010-06241), University Autonoma of Madrid (UAM-38) and by the Knut and Alice Wallenberg Foundation (KAW), TD holds a post-doctoral fellowship DO 1684/1-1 from the Deutsche Forschungsgemeinschaft (DFG).
References
- Bisicchia P, Noone D, Lioliou E, Howell A, Quigley S, Jensen T, Jarmer H, Devine KM. The essential YycFG two-component system controls cell wall metabolism in Bacillus subtilis . Mol Microbiol. 2007;65:180–200. doi: 10.1111/j.1365-2958.2007.05782.x. [DOI] [PubMed] [Google Scholar]
- Butterton JR, Beattie DT, Gardel CL, Carroll PA, Hyman T, Killeen KP, Mekalanos JJ, Calderwood SB. Heterologous antigen expression in Vibrio cholerae vector strains. Infect Immun. 1995;63:2689–2696. doi: 10.1128/iai.63.7.2689-2696.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeen MT, Jacobs-Wagner C. Bacterial cell shape. Nat Rev Microbiol. 2005;3:601–610. doi: 10.1038/nrmicro1205. [DOI] [PubMed] [Google Scholar]
- Cava F, de Pedro MA, Lam H, Davis BM, Waldor MK. Distinct pathways for modification of the bacterial cell wall by non-canonical D-amino acids. EMBO J. 2010;30:3442–3453. doi: 10.1038/emboj.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury C, Nayak TR, Young KD, Ghosh AS. A weak DD-carboxypeptidase activity explains the inability of PBP 6 to substitute for PBP 5 in maintaining normal cell shape in Escherichia coli . FEMS Microbiol Lett. 2010;303:76–83. doi: 10.1111/j.1574-6968.2009.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HS, Yao Z, Goehring NW, Kishony R, Beckwith J, Kahne D. Rapid beta-lactam-induced lysis requires successful assembly of the cell division machinery. Proc Natl Acad Sci U S A. 2009;106:21872–21877. doi: 10.1073/pnas.0911674106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Levie R. How to use Excel in Analytical Chemistry and in General Scientific Data Analysis. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- Deng Y, Sun M, Shaevitz JW. Direct measurement of cell wall stress stiffening and turgor pressure in live bacterial cells. Phys Rev Lett. 2011;107:158101. doi: 10.1103/PhysRevLett.107.158101. [DOI] [PubMed] [Google Scholar]
- Donnenberg MS, Kaper JB. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LC, Schwarz U, Keck W, Charlier P, Dideberg O, Ghuysen JM. Properties and crystallization of a genetically engineered, water-soluble derivative of penicillin-binding protein 5 of Escherichia coli K12. Eur J Biochem. 1988;171:11–16. doi: 10.1111/j.1432-1033.1988.tb13751.x. [DOI] [PubMed] [Google Scholar]
- Gan L, Chen S, Jensen GJ. Molecular organization of Gram-negative peptidoglycan. Proc Natl Acad Sci U S A. 2008;105:18953–18957. doi: 10.1073/pnas.0808035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AS, Chowdhury C, Nelson DE. Physiological functions of D-alanine carboxypeptidases in Escherichia coli . Trends Microbiol. 2008;16:309–317. doi: 10.1016/j.tim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Glauner B, Holtje JV, Schwarz U. The composition of the murein of Escherichia coli. J Biol Chem. 1988;263:10088–10095. [PubMed] [Google Scholar]
- Gomez-Ortiz M, Gomis-Ruth FX, Huber R, Aviles FX. Inhibition of carboxypeptidase A by excess zinc: analysis of the structural determinants by X-ray crystallography. FEBS Lett. 1997;400:336–340. doi: 10.1016/s0014-5793(96)01412-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Leiza SM, de Pedro MA, Ayala JA. AmpH, a bifunctional DD-endopeptidase and DD-carboxypeptidase of Escherichia coli . J Bacteriol. 2011;193:6887–6894. doi: 10.1128/JB.05764-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Ooiwa S, Sekiguchi J. Synthetic lethality of the lytE cwlO genotype in Bacillus subtilis is caused by lack of D,L-endopeptidase activity at the lateral cell wall. J Bacteriol. 2012;194:796–803. doi: 10.1128/JB.05569-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesek D, Suvorov M, Morio K, Lee M, Brown S, Vakulenko SB, Mobashery S. Synthetic peptidoglycan substrates for penicillin-binding protein 5 of Gram-negative bacteria. J Org Chem. 2004;69:778–784. doi: 10.1021/jo035397e. [DOI] [PubMed] [Google Scholar]
- Holtje JV. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli . Microbiol Mol Biol Rev. 1998;62:181–203. doi: 10.1128/mmbr.62.1.181-203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtje JV, Mirelman D, Sharon N, Schwarz U. Novel type of murein transglycosylase in Escherichia coli . J Bacteriol. 1975;124:1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AL. How bacteria grow and divide in spite of internal hydrostatic pressure. Can J Microbiol. 1985;31:1071–1084. doi: 10.1139/m85-204. [DOI] [PubMed] [Google Scholar]
- Koch AL, Doyle RJ. Inside-to-outside growth and turnover of the wall of gram-positive rods. J Theor Biol. 1985;117:137–157. doi: 10.1016/s0022-5193(85)80169-7. [DOI] [PubMed] [Google Scholar]
- Koch AL, Pinette MF. Nephelometric determination of turgor pressure in growing gram-negative bacteria. J Bacteriol. 1987;169:3654–3663. doi: 10.1128/jb.169.8.3654-3663.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korat B, Mottl H, Keck W. Penicillin-binding protein 4 of Escherichia coli: molecular cloning of the dacB gene, controlled overexpression, and alterations in murein composition. Mol Microbiol. 1991;5:675–684. doi: 10.1111/j.1365-2958.1991.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Lewenza S, Vidal-Ingigliardi D, Pugsley AP. Direct visualization of red fluorescent lipoproteins indicates conservation of the membrane sorting rules in the family Enterobacteriaceae. J Bacteriol. 2006;188:3516–3524. doi: 10.1128/JB.188.10.3516-3524.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnet S, Dubost L, Marie A, Arthur M, Gutmann L. Identification of the L,D-transpeptidases for peptidoglycan cross-linking in Escherichia coli . J Bacteriol. 2008;190:4782–4785. doi: 10.1128/JB.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainardi JL, Fourgeaud M, Hugonnet JE, Dubost L, Brouard JP, Ouazzani J, Rice LB, Gutmann L, Arthur M. A novel peptidoglycan cross-linking enzyme for a beta-lactam-resistant transpeptidation pathway. J Biol Chem. 2005;280:38146–38152. doi: 10.1074/jbc.M507384200. [DOI] [PubMed] [Google Scholar]
- Moll A, Schlimpert S, Briegel A, Jensen GJ, Thanbichler M. DipM, a new factor required for peptidoglycan remodelling during cell division in Caulobacter crescentus . Mol Microbiol. 2010;77:90–107. doi: 10.1111/j.1365-2958.2010.07224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlot C, Uehara T, Marquis KA, Bernhardt TG, Rudner DZ. A highly coordinated cell wall degradation machine governs spore morphogenesis in Bacillus subtilis . Genes Dev. 2010;24:411–422. doi: 10.1101/gad.1878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JT, Uehara T. How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan) Microbiol Mol Biol Rev. 2008;72:211–227. doi: 10.1128/MMBR.00027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters NT, Dinh T, Bernhardt TG. A fail-safe mechanism in the septal ring assembly pathway generated by the sequential recruitment of cell separation amidases and their activators. J Bacteriol. 2011;193:4973–4983. doi: 10.1128/JB.00316-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potluri L, Karczmarek A, Verheul J, Piette A, Wilkin JM, Werth N, Banzhaf M, Vollmer W, Young KD, Nguyen-Disteche M, den Blaauwen T. Septal and lateral wall localization of PBP5, the major D,D-carboxypeptidase of Escherichia coli, requires substrate recognition and membrane attachment. Mol Microbiol. 2010;77:300–323. doi: 10.1111/j.1365-2958.2010.07205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju RM, Goldberg AL, Rubin EJ. Bacterial proteolytic complexes as therapeutic targets. Nat Rev Drug Discov. 2012;11:777–789. doi: 10.1038/nrd3846. [DOI] [PubMed] [Google Scholar]
- Romeis T, Holtje JV. Penicillin-binding protein 7/8 of Escherichia coli is a DD-endopeptidase. Eur J Biochem. 1994;224:597–604. doi: 10.1111/j.1432-1033.1994.00597.x. [DOI] [PubMed] [Google Scholar]
- Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- Scheurwater E, Reid CW, Clarke AJ. Lytic transglycosylases: bacterial space-making autolysins. Int J Biochem Cell Biol. 2008;40:586–591. doi: 10.1016/j.biocel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Schneider T, Sahl HG. An oldie but a goodie - cell wall biosynthesis as antibiotic target pathway. Int J Med Microbiol. 2010;300:161–169. doi: 10.1016/j.ijmm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Simossis VA, Heringa J. PRALINE: a multiple sequence alignment toolbox that integrates homology-extended and secondary structure information. Nucleic Acids Res. 2005;33:W289–W294. doi: 10.1093/nar/gki390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, SaiSree L, Amrutha RN, Reddy M. Three redundant murein endopeptidases catalyse an essential cleavage step in peptidoglycan synthesis of Escherichia coli K12. Mol Microbiol. 2012;86:1036–1051. doi: 10.1111/mmi.12058. [DOI] [PubMed] [Google Scholar]
- Sliusarenko O, Heinritz J, Emonet T, Jacobs-Wagner C. High-throughput, subpixel precision analysis of bacterial morphogenesis and intracellular spatio-temporal dynamics. Mol Microbiol. 2011;80:612–627. doi: 10.1111/j.1365-2958.2011.07579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Penicillin-binding proteins and the antibacterial effectiveness of beta-lactam antibiotics. Rev Infect Dis. 1986;8(Suppl 3):S260–S278. doi: 10.1093/clinids/8.supplement_3.s260. [DOI] [PubMed] [Google Scholar]
- Typas A, Banzhaf M, Gross CA, Vollmer W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol. 2011;10:123–136. doi: 10.1038/nrmicro2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Bernhardt TG. More than just lysins: peptidoglycan hydrolases tailor the cell wall. Curr Opin Microbiol. 2011;14:698–703. doi: 10.1016/j.mib.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Dinh T, Bernhardt TG. LytM-domain factors are required for daughter cell separation and rapid ampicillin-induced lysis in Escherichia coli . J Bacteriol. 2009;191:5094–5107. doi: 10.1128/JB.00505-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Parzych KR, Dinh T, Bernhardt TG. Daughter cell separation is controlled by cytokinetic ring-activated cell wall hydrolysis. EMBO J. 2010;29:1412–1422. doi: 10.1038/emboj.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heijenoort J. Peptidoglycan hydrolases of Escherichia coli . Microbiol Mol Biol Rev. 2011;75:636–663. doi: 10.1128/MMBR.00022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer W. Bacterial growth does require peptidoglycan hydrolases. Mol Microbiol. 2012;86:1031–1035. doi: 10.1111/mmi.12059. [DOI] [PubMed] [Google Scholar]
- Vollmer W, Joris B, Charlier P, Foster S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol Rev. 2008;32:259–286. doi: 10.1111/j.1574-6976.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- Wang RF, Kushner SR. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli . Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- Weidel W, Pelzer H. Bagshaped Macromolecules--a New Outlook on Bacterial Cell Walls. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Weiser JN, Chong ST, Greenberg D, Fong W. Identification and characterization of a cell envelope protein of Haemophilus influenzae contributing to phase variation in colony opacity and nasopharyngeal colonization. Mol Microbiol. 1995;17:555–564. doi: 10.1111/j.1365-2958.1995.mmi_17030555.x. [DOI] [PubMed] [Google Scholar]
- Whatmore AM, Reed RH. Determination of turgor pressure in Bacillus subtilis: a possible role for K+ in turgor regulation. J Gen Microbiol. 1990;136:2521–2526. doi: 10.1099/00221287-136-12-2521. [DOI] [PubMed] [Google Scholar]
- Work E. Reaction of ninhydrin in acid solution with straight-chain amino acids containing two amino groups and its application to the estimation of alpha epsilon-diaminopimelic acid. Biochem J. 1957;67:416–423. doi: 10.1042/bj0670416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff TJ, Taylor JA, Salama NR. Beyond growth: novel functions for bacterial cell wall hydrolases. Trends Microbiol. 2012;20:540–547. doi: 10.1016/j.tim.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Chen S, Recsei P. A dye release assay for determination of lysostaphin activity. Anal Biochem. 1988;171:141–144. doi: 10.1016/0003-2697(88)90134-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.