Abstract
Background
The Veterans Health Administration, the American Cancer Society, and the American Geriatrics Society recommend colorectal cancer screening for older adults unless they are unlikely to live 5 years or have significant comorbidity that would preclude treatment.
Objective
To determine whether colorectal cancer screening is targeted to healthy older patients and is avoided in older patients with severe comorbidity who have life expectancies < 5 years.
Design
Cohort study.
Setting
Minneapolis, Durham, Portland, and West LA VA’s with linked national VA and Medicare administrative claims.
Patients
27,068 patients > 70 years who had an outpatient visit in 2000 and an outpatient visit at 1 of 4 VA’s during 2001–2002 and due for screening.
Measurements
The main outcome was receipt of fecal occult blood testing (FOBT), colonoscopy, sigmoidoscopy, or barium enema during 2001–2002 based on national VA and Medicare claims. Charlson comorbidity scores were used to stratify patients into 3 groups ranging from no comorbidity (score=0) to severe comorbidity (score > 4) and 5-year mortality was determined for each group.
Results
46% of patients were screened during 2001–2002. Only 47% of patients with no comorbidity were screened despite having life expectancies > 5 years (5-year mortality=19%). While the incidence of screening declined with age and worsening comorbidity, it was still 41% for patients with severe comorbidity who had life expectancies < 5 years (5-year mortality=55%). The number of VA outpatient visits predicted screening independent of comorbidity, such that patients with severe comorbidity and > 4 visits had similar or higher screening rates than healthier patients with fewer visits.
Limitations
Some tests may have been performed for non-screening reasons. The generalizability of findings to persons who do not use the VA is uncertain.
Conclusions
Advancing age was inversely associated with colorectal cancer screening while comorbidity was a weaker predictor. More attention to comorbidity is needed to better target screening to older patients with substantial life expectancies and avoid screening older patients with limited life expectancies.
INTRODUCTION
Colorectal cancer screening guidelines recommend screening older adults who have substantial life expectancies according to age and/or comorbid conditions.1 For example, the US Preventive Services Task Force recommends routine screening until age 75 years, while the Veterans Health Administration, the American Cancer Society, and the American Geriatrics Society recommend colorectal cancer screening for older adults unless they are unlikely to live 5 years or have significant comorbidity that would preclude treatment.2–5 Targeting screening to healthy persons who are likely to live at least 5 years is recommended because randomized trials of fecal occult blood testing (FOBT) suggest that a difference in colorectal cancer mortality between screened and unscreened persons does not become noticeable until at least 5 years after screening.6,7 Therefore, persons with a life expectancy < 5 years are not likely to benefit from screening but remain at risk for harms which may occur immediately (e.g. complications from procedures and treatment of clinically unimportant disease).8,9 However, it remains unclear whether screening is being targeted to healthy older persons with substantial life expectancies and avoided in older persons with significant comorbidity for whom the risks of screening outweigh the benefits.
Prior studies evaluating associations between age, comorbidity, and receipt of cancer screening have found that age is a stronger determinant of screening than comorbidity. For example, while advancing age is consistently associated with lower screening rates, worsening comorbidity has had little impact on the use of screening mammography, Papanicolaou smears, or prostate-specific antigen (PSA).10–12 Previous studies of the relationship of colorectal cancer screening and comorbidity have been limited by their small sample size, short follow-up times, and their focus on FOBT rather than measuring all types of colorectal cancer screening tests.13,14 In addition, previous VA studies have not measured colorectal cancer screening performed outside the VA health care system in Medicare.14–16 Having a better understanding of how comorbidity and age impact overall screening use is particularly important for colorectal cancer screening because tests and follow-up procedures are often more invasive than those for other cancers and may result in substantial harms (e.g., major bleeding, colon perforation, stroke), especially in elders with limited life expectancies.8,17,18
Therefore, we examined VA data and Medicare claims for patients > 70 years seen at 4 geographically diverse VA facilities to characterize the use of colorectal cancer screening across a prognostic spectrum of advancing age and comorbidity. Specifically, we determined 2-year screening rates and 5-year mortality rates for subgroups of older patients without significant comorbidity for whom guidelines recommend screening as well as for subgroups of older patients with severe comorbidity for whom most guidelines agree the risks of screening outweigh the benefits.
METHODS
Data Sources and Subjects
We identified a cohort of screen-eligible patients on 1/1/01 and followed them for 2 years for the performance of colorectal cancer screening. Data for this cohort study were obtained from 1) National VA Data Systems, 2) clinical data extracted from 4 VA’s (Minneapolis, Durham, Portland, and West Los Angeles) electronic record system, VISTA (Veterans Health Information Systems and Technology Architecture), and 3) Medicare claims. National VA data included the National Patient Care Database (which captures all inpatient and outpatient claims within the VA), Fee Basis Files (which capture claims for non-VA services paid for by the VA) and the Vital Status File (which captures mortality data for veterans).20 Clinical data extracted from the 4 VA’s included text data entered by clinicians in response to computerized clinical reminders about colorectal cancer screening.21 We used linked Medicare claims () from the VA Information Resource Center to capture services provided to our cohort by Medicare. 19
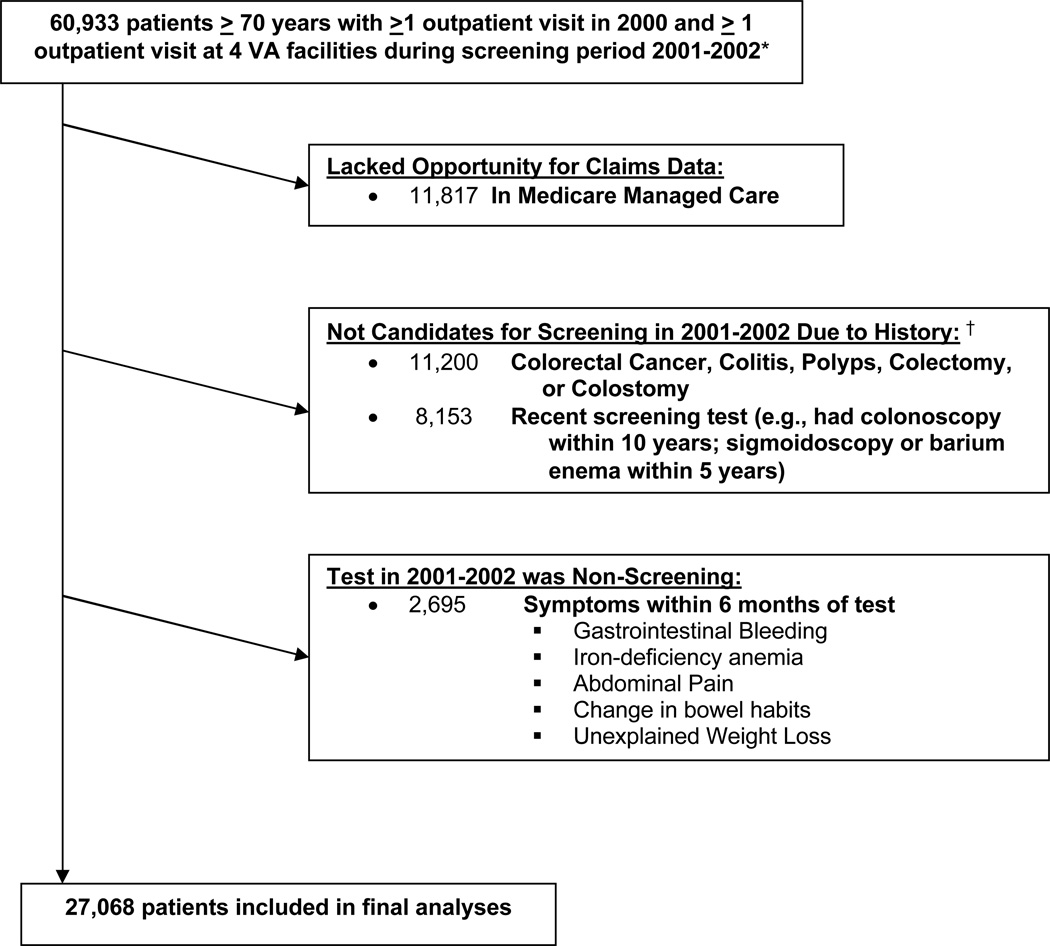
Based on these data sources we identified a cohort of 60,933 patients aged > 70 years who had at least 1 outpatient visit within the VA healthcare system or Medicare between 1/1/00–12/31/00 (the period during which we measured comorbidity) and at least 1 outpatient visit at 1 of the 4 VA’s between 1/1/01–12/31/02 (the period during which we measured the performance of colorectal cancer screening)(See Figure 1). The 4 VA’s were selected for geographic diversity. We excluded 11,817 (19%) patients who were enrolled in Medicare managed care at any point during 1/1/00 to 12/31/02 because they lacked Medicare claims. In addition, for inclusion in our cohort patients had to be eligible for screening. Therefore, we used VA and Medicare inpatient and outpatient claims during the 8-year period prior to the start of 2001 (dating back to 10/1/92 for VA claims and 1/1/99 for Medicare claims) to exclude 11,200 (18%) patients with a history of colorectal cancer, colitis, colorectal polyps, colectomy or colostomy and 8,153 (13%) patients who had a history of a colonoscopy or had had a sigmoidoscopy or barium enema within 5 years and therefore were not due for screening at the start of 2001. We also used claims during the 6 months before their index test to exclude 2,695 (4%) of patients who had signs or symptoms that would justify the performance of a test for non-screening purposes (see Figure 1). This left a final screen-eligible cohort of 27,068.
Figure 1. Criteria used to define the final cohort of elderly patients eligible for a colorectal cancer screening during 2001–2002.
*Eligibility criteria included having been seen in an outpatient clinic at 1 of 4 VA’s during 1/1/01–12/31/02 which indicated that the VA was at least partially responsible for medical care, but data on colorectal cancer screening was gathered during the entire 2-year interval from both national VA and Medicare claims. Additional eligibility criteria included having at least 1 outpatient visit during 1/1/00–12/31/00 to define comorbidity as of 1/1/01.
†History was defined by searching VA and Medicare inpatient and outpatient claims prior to 1/1/01, dating as far back as 10/1/92 for VA claims and 1/1/99 for Medicare claims.
Data Collection and Measurement
Outcome Variables
Receipt of colorectal cancer screening between 1/1/01–12/31/02 was assessed for our cohort across the VA healthcare system and Medicare because many elderly veterans use more than one VA, and most are enrolled in Medicare.22 Colorectal cancer screening was identified in National VA Data Systems and linked Medicare payment data (hospital outpatient and physician/supplier files) using International Classification of Disease, Ninth Revision (ICD-9) codes, Current Procedural Terminology (CPT) codes, and Level II Healthcare Common Procedure Coding System (HCPCS) codes for 1) FOBT (CPT: 82270, 82273, 82274; HCPCS: G0107), 2) colonoscopy (ICD-9: 45.22, 45.23, 45.25, 45.41, 45.42, 45.43; CPT: 44388–44394, 45355, 45378–45385; HCPCS: G0105, G0121), 3) sigmoidoscopy (ICD-9: 45.24, 48.22–48.24, 48.26, 48.35, 48.36; CPT: 45300, 45303, 45305, 45308, 45309, 45315, 45320, 45330–45334,45337–45339; HCPCS: G0104), or 4) barium enema (ICD-9: 87.64; CPT: 74270, 74280; HCPCS:G0106, G0120, G0122).23–26 Patients were assigned to one of the four screening procedures based on their first test during 2001–2002. A 2-year screening period was chosen to allow sufficient time for screening tests to be scheduled and performed, and this is the screening interval used in randomized trials of FOBT.6,7
Vital status through 12/31/05 was obtained from the VA Vital Status File in order to determine 5-year mortality rates. The VA Vital Status File is highly comparable to the National Death Index in terms of accuracy and completeness.27 5-year mortality rates were used descriptively to identify conditions associated with having a life expectancy < 5 years (i.e., 5-year mortality rate > 50%).
Predictor Variables
Age on 1/1/01 was categorized into 3 groups: 70–74 years, 75–79 years, and > 80 years. Burden of comorbidity was defined by the Charlson-Deyo Comorbidity Index, which is a summary measure of 19 chronic disease diagnoses from administrative data selected and weighted according to their association with mortality.28,29 Charlson scores were calculated from VA and Medicare inpatient and outpatient claims during the 12 months prior to the start of 2001. Patients were categorized as having no significant comorbidity if they had a Charlson score = 0, average comorbidity if they had a Charlson score = 1–3, and severe comorbidity if they had a Charlson score > 4. These cutoffs were chosen a priori to assess how extremes in comorbidity influence screening and have been used in prior studies.12,30 Another measure of illness included being home-bound as defined by enrollment in VA Home-Based Primary Care at the start of 2001. We also measured whether a clinician responded to VA clinical reminders that colorectal cancer screening was “refused or contraindicated” during 2001–2002.31
Other factors known to influence the use of cancer screening were also ascertained from VA and Medicare administrative data and linkage to the 2000 US Census (Table 1).32 The number of outpatient visits during the 2-year screening interval was based on primary care, gastroenterology, or general surgery clinic codes from national VA data. We included General Medicine, Cardiology, Endocrinology, Diabetes, Hypertension, Pulmonary, and Women’s Clinic as primary care clinics because they are defined as such by the VA colorectal cancer screening quality indicator. The Committee on Human Research at the University of California, San Francisco, and the Committee for Research and Development at the San Francisco VA approved the study. The funding source had no role in the design, conduct, or analysis of this study or in the decision to submit the manuscript for publication.
Table 1.
Baseline Characteristics of Study Participants (N=27,068)
| Characteristic | N (%) |
|---|---|
| Age, years | |
| 70–74 | 10091 (37) |
| 75–79 | 10234 (38) |
| ≥ 80 | 6743 (25) |
| Gender | |
| Men | 26119 (96) |
| Women | 949 (4) |
| Race/Ethnicity | |
| White | 23595 (87) |
| Black | 2394 (9) |
| White Hispanic | 261 (1) |
| Other | 545 (2) |
| Charlson Score | |
| 0 (no comorbidity) | 9698 (36) |
| 1–3 (average comorbidity) | 14126 (52) |
| ≥ 4 (severe comorbidity) | 3244 (12) |
| Selected Charlson Comorbidities* | |
| Congestive Heart Failure | 3420 (13) |
| Peripheral Vascular Disease | 2366 (9) |
| Cerebrovascular Disease | 3114 (12) |
| Dementia | 586 (2) |
| Chronic Obstructive Pulmonary Disease | 5765 (21) |
| Diabetes | 6623 (24) |
| Diabetes with Complications | 1673 (6) |
| Chronic Renal Failure | 1031 (4) |
| Metastatic Solid Tumor | 343 (1) |
| Home-bound† | 858 (3) |
| Married | |
| Yes | 17161 (63) |
| No | 9907 (37) |
| Lived in ZCTA in which ≥ 25% of Adults Had a College Education‡ |
|
| Yes | 9847 (36) |
| No | 17221 (64) |
| Median Income of ZCTA‡ | |
| Highest tertile (≥$29,194) | 8729 (32) |
| Middle tertile | 8977 (33) |
| Lowest tertile (≤$21,169) | 8721 (32) |
| VA Site | |
| Minneapolis | 10911 (40) |
| Durham | 5651 (21) |
| Portland | 4158 (15) |
| West Los Angeles | 6348 (23) |
| Distance to nearest VA clinic§ | |
| <10 miles | 13052 (48) |
| 10–49 miles | 12655 (47) |
| ≥50 miles | 1312 (5) |
The categories are not mutually exclusive because patients could have more than 1 condition
Enrolled in VA Home Based Primary Care at the start of 2001.
ZCTA = Zip Code Tabulation Area
Distance to the nearest VA clinic was measured as a straight-line distance between the location of the VA clinic and the center of the zip code of the patient’s residence. The following variables had missing data: Distance to nearest VA clinic (0.2%); Income (2.4%); Education (2.4%); Married (3.3%); Race/Ethnicity (1.0%).
Analyses
For all estimates of colorectal cancer screening incidence, patients were observed from baseline (1/1/01) until the first screening test, death, or the end of the screening period (12/31/02). Patients were not censored for reasons other than death or the end of the screening period because there was no reason to assume that screening would not be recorded otherwise, since we had access to both VA and Medicare claims for all patients up until these times. We first used Kaplan-Meier analysis to estimate the unadjusted 2-year cumulative incidence and 95% confidence intervals of colorectal cancer screening according to baseline characteristics. Next, we used Cox regression models to estimate 2-year cumulative incidence of colorectal cancer screening with bootstrapped 95% confidence intervals adjusting for age, gender, race, marital status, VA site, education, income, Charlson comorbidity category and an age-site interaction. We chose not to adjust for number of visits or home-bound status because we felt these were likely in the causal pathway for how age and comorbidity impact screening. In addition, to describe the association of age and comorbidity combined, we categorized patients into 9 subgroups on the basis of age (3 categories) and Charlson score (3 categories) and plotted the Kaplan-Meier estimates of 5-year mortality versus screening incidence for each age-Charlson subgroup. We used logrank and trend tests where appropriate when comparing Kaplan-Meier rates across categories. SAS statistical software (version 9.1) and Stata/SE (version 10.0) were used for all analyses.
RESULTS
Participant Characteristics
Baseline characteristics of the 27,068 patients are presented in Table 1. Consistent with the elderly veteran population 96% were men and 87% were white. The median age was 77 years (IQR=73–80). The median Charlson score was 1.0 (IQR=0–2) with scores ranging from 0 to 16. 36% of patients had a Charlson score=0 and 12% of patients had a Charlson score > 4 (severe comorbidity). Only 261 (1%) patients in our cohort would have been defined as inappropriate for screening by the VA colorectal cancer screening quality indicator which only excludes patients with cancer of the liver, pancreas, or esophagus, or hospice eligibility. Based on responses to clinical reminders, only 599 (2%) patients had documentation that screening was refused or contraindicated. 22,253 (82%) patients were seen by VA primary care, gastroenterology, or general surgery during 2001–2002.
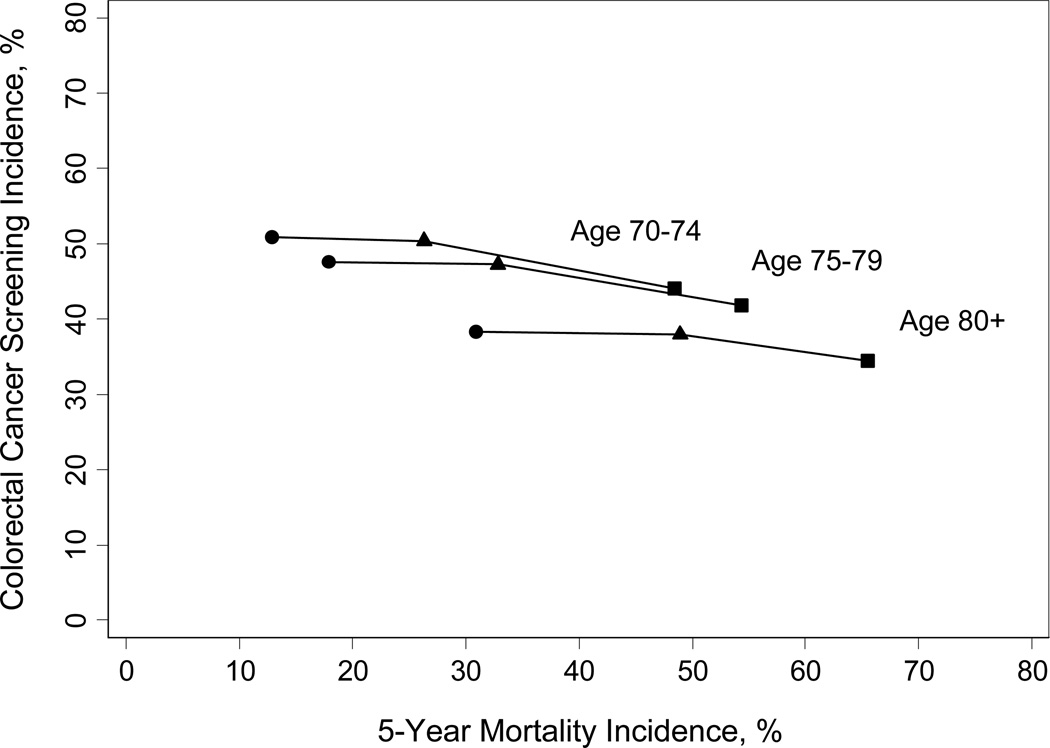
31% of patients (8,518/27,068) died within 5 years (19% of patients with no comorbidity; 55% of patients with severe comorbidity). Combining age and Charlson score resulted in 5-year mortality rates that ranged from 13% (495/3846) for patients aged 70–74 without comorbidity to 66% (585/893) for patients aged > 80 with severe comorbidity (Figure 2). Patients who had congestive heart failure, dementia, chronic renal failure, metastatic cancer, or were home-bound had 5-year mortality rates greater than 50%.
Figure 2. Colorectal cancer screening incidence versus 5-year mortality for different age groups as comorbidity increases (n=27,068).
Circles (●) indicate no comorbidity (Charlson score = 0); Triangles (▲) indicate average comorbidity (Charlson score 1–3); Squares (▪) indicate severe comorbidity (Charlson score > 4).*
*Within each age group screening incidence declined only a small amount with worsening comorbidity. The lines are included in the figure to illustrate the relatively flat incidence of screening between patients without comorbidity (●), average comorbidity (▲), and severe comorbidity (▪) for each age group. This is despite the substantial increase in 5-year mortality that occurs with worsening comorbidity. If screening was targeted to older patients with substantial life expectancies and away from those with severe comorbidity all lines would start much higher and slope down more steeply.
Colorectal Cancer Screening Rates
46% of patients (12,354/27,068) received colorectal cancer screening during the 2-year period from 2001–2002; 31% (8,288/27,068) were screened in the first year. Of those screened, 68% (8,346/12,354) had their index screening test during the 2-year period performed in the VA healthcare system [45% (5,585/12,354) at one of the 4 VA’s and 22% (2,761/12,354) at another VA] whereas 33% (4,008/12,354) were performed within Medicare. Among the 12,354 patients who were screened, 77% had FOBT as their index screening test, whereas 13% had colonoscopy, 8% had sigmoidoscopy, and 2% had a barium enema. 5.4% had both FOBT and sigmoidoscopy.
Advancing age was associated with decreased colorectal cancer screening while worsening comorbidity was a weaker predictor (Table 2). For example, even for elderly patients with severe comorbid conditions, the incidence of screening did not fall below 30% except among the small number of patients who were home-bound or had dementia. On the other hand, even among patients with characteristics predictive of living more than 5 years (e.g., aged 70–74, Charlson score=0) screening incidence was only around 50%. When combining age and Charlson score, screening incidence ranged from 51% in patients aged 70–74 without comorbidity to 34% in patients aged > 80 with severe comorbidity (P < 0.001) (Figure 2). Figure 2 shows that within each age group, screening incidence varied little with worsening comorbidity even over a wide range of 5-year mortality. For example, screening incidence among persons aged > 80 without comorbidity (5-year mortality = 31%) and with severe comorbidity (5-year mortality= 66%) were similar at 38% and 39% respectively (P= 0.064). In multivariate analyses, the association of age and comorbidity on colorectal cancer screening incidence remained similar after adjustment for gender, race, marital status, VA site, education, and income, and an age-site interaction (P<0.001) which reflects that the incidence of screening at the LA site did not differ as much across age groups as it did at the other sites (Table 2).
Table 2.
Rates of colorectal cancer screening in persons 70 years of age or older according to patient characteristics (N=27,068)
| Characteristic | Colorectal Cancer Screening 2-Year Cumulative Incidence* % (95% CI) |
Adjusted Colorectal Cancer Screening 2-Year Cumulative Incidence† % (95% CI) |
|---|---|---|
| Age, years | ||
| 70–74 | 49.9 (48.9–50.8) | 47.2 (46.9–47.4) |
| 75–79 | 46.7 (45.7–47.7) | 46.3 (45.8–46.8) |
| ≥ 80 | 37.6 (36.5–38.8) | 43.9 (43.4–44.3) |
| Gender | ||
| Men | 45.9 (45.3–46.5) | 45.7 (45.5–45.8) |
| Women | 38.6 (35.6–41.8) | 44.1 (43.5–44.8) |
| Race/Ethnicity | ||
| White | 46.8 (46.2–47.5) | 46.5 (46.3–46.6) |
| Black | 39.8 (37.9–41.8) | 39.6 (39.1–40.1) |
| White Hispanic | 27.2 (22.2–33) | 30.3 (29.1–31.6) |
| Other | 38.9 (34.9–43.1) | 42.5 (41.3–43.7) |
| Charlson Score | ||
| 0 (best health) | 46.7 (45.7–47.7) | 47.0 (46.8–47.3) |
| 1–3 (average health) | 46.0 (45.2–46.9) | 45.8 (45.6–46.0) |
| ≥ 4 (worst health) | 40.6 (38.9–42.3) | 40.7 (40.4–41.0) |
| Selected Charlson Comorbidities‡ | ||
| Congestive Heart Failure | 39.6 (38–41.2) | 41.2 (40.7–41.7) |
| Peripheral Vascular Disease | 45.7 (43.7–47.7) | 47.2 (46.7–47.7) |
| Cerebrovascular Disease | 42.8 (41.1–44.5) | 44.4 (44.0–44.8) |
| Dementia | 17.4 (14.6–20.7) | 19.2 (18.5–19.9) |
| COPD | 43.2 (41.9–44.5) | 43.8 (43.5–44.1) |
| Diabetes | 46.7 (45.5–47.9) | 47.2 (46.8–47.5) |
| Diabetes with Complications | 46.4 (44.1–48.9) | 48.6 (47.9–49.2) |
| Chronic Renal Failure | 36.4 (33.5– 39.4) | 39.4 (38.6– 40.3) |
| Metastatic Solid Tumor | 33.2 (28.5– 38.5) | 36.9 (35.9– 37.9) |
| Home Bound§ | ||
| No | 46.3 (45.7– 46.9) | 46.1 (46.0– 46.3) |
| Yes | 24.2 (21.5– 27.3) | 26.5 (25.7– 27.2) |
| Married | ||
| No | 38.9 (37.9– 39.9) | 40.2 (40– 40.5) |
| Yes | 49.8 (49.1–50.6) | 48.6 (48.5–48.7) |
| Lived in ZCTA in which ≥ 25% of Adults Had a College Education ¶ |
||
| No | 46.6 (45.9–47.4) | 46.3 (46.1–46.5) |
| Yes | 44.3 (43.3–45.3) | 44.4 (44.2–44.7) |
| Median Income of ZCTA ¶ | ||
| Highest Tertile (> $29,194) | 47.4 (46.4–48.5) | 46.5 (46.2–46.8) |
| Middle Tertile | 45.5 (44.5–46.5) | 45.5 (45.3–45.7) |
| Lowest Tertile (< $21,169) | 44.3 (43.3–45.4) | 44.5 (44.2–44.8) |
| VA Site | ||
| Minneapolis | 47.6 (46.7–48.5) | 46.4 (46.1–46.6) |
| Durham | 51.8 (50.5–53.1) | 52.0 (51.7–52.3) |
| Portland | 43.6 (42.1–45.1) | 43.5 (43.3–43.8) |
| West Los Angeles | 38.0 (36.8–39.2) | 40.6 (40.3–41.0) |
| Distance to Nearest VA Clinic |
||
| < 10 miles | 41.9 (41.1–42.7) | 44.5 (44.3–44.7) |
| 10–49 miles | 49.1 (48.3–50) | 46.7 (46.6–46.9) |
| ≥ 50 miles | 48.6 (46–51.4) | 47.1 (46.6–47.7) |
| Number of VA Outpatient Visits (Primary Care, GI, or Surgery), 2001–2002∥ |
||
| 0 | 23.7 (22.6–25.0) | 23.0 (22.7–23.3) |
| 1 | 45.5 (44.5–46.6) | 43.9 (43.7–44.1) |
| 3 | 52.5 (51.4–53.6) | 52.1 (51.8–52.3) |
| ≥ 4 | 55.1 (53.8–56.5) | 57.4 (57.1–57.6) |
| Type of VA Outpatient Visit, 2001–2002 |
||
| Seen in Primary Care, GI, or Surgery Clinic |
50.1 (50.0–50.3) | 50.1 (50.0–50.3) |
| Never attended Primary Care, GI, or Surgery Clinic |
23.7 (22.6–25.0) | 23.0 (22.7–23.3) |
Cumulative incidence at 2 years converted to percent, estimated from Kaplan-Meier analysis. Complete-case analyses were conducted because missing data were minimal. The following variables had missing data: Distance to nearest VA clinic (0.2%); Income (2.4%); Education (2.4%); Married (3.3%); Race/Ethnicity (1.0%).
Estimated 2-year cumulative incidence from Cox regression models adjusted for age, gender, race, marital status, VA site, education and income categories, Charlson comorbidity category as well as an interaction between age (continuous and centered) and VA site. Adjusted estimates for each site represent the incidence at the mean age of the cohort (77 years).
The comparison group is all patients without the comorbidity of interest.
Enrolled in VA Home Based Primary Care at the start of 2001.
ZCTA = Zip Code Tabulation Area
Defined by VA clinic codes 301, 303, 305, 306, 307, 309, 312, 321–323, and 401 representing primary care clinics subjected to the 2001–2002 VA colorectal cancer screening performance measure (e.g., General Medicine, Cardiology, Endocrinology, Diabetes, Hypertension, Pulmonary, Women’s Clinic) as well as Gastroenterology and General Surgery.
The number of VA outpatient visits to primary care, gastroenterology, or general surgery during the study interval was one of the strongest predictors of screening in unadjusted and adjusted analyses (Table 2). Colorectal cancer screening incidence increased with increasing visits, ranging from 23% for patients with no visits to these clinics to 55% for patients with > 4 visits (P for trend <0.001). Yet, persons with severe comorbidity had more medical visits than those without comorbidity. For example, 43% (1253/2885) of patients with > 4 visits had severe comorbidity. Only 24% (1305/5459) of patients without comorbidity had > 4 visits. Because the number of visits predicted screening independent of comorbidity, screening incidence increased with number of visits even among patients with severe comorbidity (Figure 3). As a result, patients with severe comorbidity and > 4 visits had a screening incidence of 50% which was similar or higher than screening rates for healthier patients with fewer visits.
Figure 3. Patients aged > 70 years who had colorectal cancer screening during 2001–2002, according to comorbidity and number of VA outpatient medical visits (n=27,068)*.
*Number of visits was defined by the number of visits during 1/1/01–12/31/02 to VA primary care, gastroenterology, or general surgery clinics (clinic codes 301, 303, 305, 306, 307, 309, 312, 321–323, and 401).
DISCUSSION
Less than half of veterans aged > 70 years received colorectal cancer screening during the 2-year period from 2001 to 2002. Advancing age was strongly and inversely associated with screening while comorbidity was a weaker predictor. As a result, many healthy older patients with substantial life expectancies are not being screened while some patients with severe comorbidity are being screened. For example, only 47% of patients aged > 70 years without comorbidity were screened despite having a high probability of living > 5 years (5-year survival=81%). In addition, the number of VA outpatient visits was a strong predictor of screening independent of comorbidity, such that patients without comorbidity who did not attend one of the primary care, gastroenterology, or general surgery clinics had a lower incidence of screening than patients with severe comorbidity who attended these clinics.
Prior studies of the associations between age, comorbidity, and receipt of cancer screening have found similar problems with the targeting of cancer screening tests to healthy older patients. We identified English-language literature related to cancer screening, age, and comorbidity published between January 1998 and December 2008 using MEDLINE. While cancer screening generally decreases with advancing age, increasing comorbidity has been shown to have little impact on the relatively high rates of mammography, Papanicolaou smears, and PSA screening. In contrast, colorectal cancer screening rates have been found to be generally low across the U.S., especially in older adults, and the impact of comorbidity on screening had not been well described. 39,40 In our study the use of colorectal cancer screening was based on the actual performance of screening and is lower than that reported in national surveys which frequently overestimate screening due to the desire of patients to report behaviors in a favorable light.41,42 Our screening rates are consistent with those of prior claims-based studies.15,43,44 For example, we found that 31% of veterans > 70 years were screened in 2001, while a study of veterans aged 49–75 years found that annual screening incidences were 19%-31% during 1998–2004.15 An advantage of our study over prior studies using national VA databases is that we were able to identify patients who had screening outside VA paid for by Medicare, which accounted for 32% of veterans screened. Based on this comprehensive view of colorectal cancer screening in elderly veterans we found that, as with other cancer screening tests, age was a stronger predictor of colorectal cancer screening than comorbidity. 33
There are several possible explanations for our findings that colorectal cancer screening is not optimally targeted to healthy older patients or avoided in older patients with severe comorbidity. First, consistent with other studies we found that more frequent medical visits increased the likelihood of receiving cancer screening independent of a patient’s comorbidity.34 This contributes to underscreening of healthy older patients, who see doctors less frequently, and to overscreening of patients with severe comorbidity. Second, clinicians may have difficulty estimating life expectancy because all available methods for predicting an individual’s life expectancy are imperfect. However, even among patients aged 70–74 years without comorbidity, who few clinicians would expect to die in 10 years,8,35 the 2-year cumulative incidence of screening was still only 51%. This may in part be driven by perspectives that colorectal cancer screening is more unpleasant and invasive compared to other cancer screening tests.36,37 For example, annual rates of PSA screening, a blood test, are more than double the rates of colorectal cancer screening among male veterans aged 70–74 years without comorbidity.12 Third, healthy older patients visit clinicians less often and may be less willing to accept recommendations for screening. However, we found that less than 2% of patients in our cohort had documentation that screening was refused. Fourth, quality indicators, which are used extensively by the VA, promote colorectal cancer screening regardless of comorbidity, which may contribute to overscreening patients with severe comorbidity.31,38 For example, the VA’s 2001–2002 colorectal cancer screening quality indicator would have encouraged screening all patients in our cohort seen in primary care clinics except for the 1% of patients who had cancer of the esophagus, liver, or pancreas, or were eligible for hospice.(31) Since almost a third of our cohort died in 5 years this quality indicator encouraged screening in many patients who were unlikely to benefit given their short survival. In fiscal year 2005 an upper age limit of 80 was instituted which appropriately excludes more patients with limited life expectancies.14,35 However, it does not encourage clinicians to better target colorectal cancer screening to healthy older patients. A better quality indicator would encourage high screening rates in older patients without comorbidity and low rates in older patients with severe comorbidity.31
In addition, the USPSTF published colorectal cancer screening guidelines in 2008 which recommend continuing screening only until age 75 years based on a decision model which used chronologic age rather than comorbidity-adjusted life expectancy. The strong relationship between comorbidity and life expectancy and our findings that the use of colorectal cancer screening varied little across dramatically different levels of comorbidity suggest refinements to the guidelines are needed to explicitly encourage or discourage screening based on the severity of comorbidity rather than solely on the age of the patient. Similarly, optimizing the targeting of colorectal cancer screening will likely require changes in how preventive care is delivered. The current strategy relies on finding opportunities for screening when patients visit clinicians for a medical illness, which targets screening to patients with worse comorbidity and misses healthy older patients who see clinicians less frequently.
Our study has several limitations. First, claims data do not give reasons why a test was ordered or the purpose of medical visits such that some tests may have been performed for non-screening reasons. However, we used claims data to exclude patients who had diagnoses or symptoms that would justify a test being performed for a non-screening purpose (Figure 1).15 In addition, FOBT is the predominant mode of screening in the VA and prior studies have found that only 3% of FOBT ordered by VA clinicians were for non-screening reasons.45 Second, while our data sources completely capture screening within the VA healthcare system some tests performed outside the VA may have been missed. For example, Medicare claims do not capture tests paid for by other sources. Third, while the Charlson Comorbidity Index is strongly predictive of 5-year mortality, it does not include all factors that may determine life expectancy, such as functional status.30 However, an advantage of our study is that we calculated actual 5-year mortality rates that confirmed patients without comorbidity had a life expectancy greater than 5 years while those with severe comorbidity had less than a 5-year life expectancy. Fourth, our cohort is primarily men who use the VA, so the generalizability of our findings to persons who do not use the VA is uncertain. However, understanding screening within the VA is important in its own right given the VA is the largest healthcare system in the U.S. and a leader in improving healthcare quality.46
In conclusion, colorectal cancer screening should be better targeted to older patients based on considerations of comorbidity in addition to age. Cancer screening guidelines should be more explicit about which combination of age and comorbid conditions identify older patients who have substantial life expectancies and those who are likely to die within 5 years.47 Clinicians could then consider such characteristics in concert with clinical judgment to estimate an older individual’s potential risks and benefits from screening and use shared decision making to help older patients arrive at an informed screening decision.
ACKNOWLEDGMENTS
Everyone who contributed significantly to this work is listed as an author.
Dr. Walter is supported by a VA Health Services Research and Development grant IIR-04-427 and is a Robert Wood Johnson Physician Faculty Scholar. Dr. Lee is a Hartford Geriatrics Health Outcomes Research Scholar.
Footnotes
AVAILABILITY OF RESEARCH STATEMENTS:
Protocol: Available to interested readers by contacting Dr. Walter at louise.walter@ucsf.edu
Statistical Code: Available to interested readers by contacting Dr. Walter at louise.walter@ucsf.edu
Data: Not available
REFERENCES
- 1.Walter LC, Lewis CL, Barton MB. Screening for colorectal, breast, and cervical cancer in the elderly: a review of the evidence. Am J Med. 2005;118:1078–1086. doi: 10.1016/j.amjmed.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129–131. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 3.Kussman MJ. [Accessed August 17, 2008];VHA Directive 2007–004: Colorectal cancer screening. Available at http://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=1530.
- 4.Smith RA, von Eschenbach AC, Wender R, Levin B, Byers T, Rothenberger D, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. CA Cancer J Clin. 2001;51:38–75. doi: 10.3322/canjclin.51.1.38. [DOI] [PubMed] [Google Scholar]
- 5.AGS Clinical Practice Committee. Colon cancer screening (USPSTF recommendation) J Am Geriatr Soc. 2000;48:333–335. [PubMed] [Google Scholar]
- 6.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 7.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 2996;348:1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 8.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285:2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 9.Ko CW, Sonnenberg A. Comparing risks and benefits of colorectal cancer screening in elderly patients. Gastroenterology. 2005;129:1163–1170. doi: 10.1053/j.gastro.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Burack RC, Gurney JG, McDaniel AM. Health status and mammography use among older women. J Gen Intern Med. 1998;13:366–372. doi: 10.1046/j.1525-1497.1998.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter LC, Lindquist K, Covinsky KE. Relationship between health status and use of screening mammography and Papanicolaou smears among women older than 70 years of age. Ann Intern Med. 2004;140:681–688. doi: 10.7326/0003-4819-140-9-200405040-00007. [DOI] [PubMed] [Google Scholar]
- 12.Walter LC, Bertenthal D, Lindquist K, Konety BR. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296:2336–2342. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 13.Heflin MT, Oddone EZ, Pieper CF, Burchett BM, Cohen HJ. The effect of comorbid illness on receipt of cancer screening by older people. J Am Geriatr Soc. 2002;50:1651–1658. doi: 10.1046/j.1532-5415.2002.50456.x. [DOI] [PubMed] [Google Scholar]
- 14.Fisher DA, Galanko J, Dudley TK, Shaheen NJ. Impact of comorbidity on colorectal cancer screening in the veterans healthcare system. Clin Gastroenterol Hepatol. 2007;5:991–996. doi: 10.1016/j.cgh.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 15.El-Serag HB, Petersen L, Hampel H, Richardson P, Cooper G. The use of screening colonoscopy for patients cared for by the department of veterans affairs. Arch Intern Med. 2006;166:2202–2208. doi: 10.1001/archinte.166.20.2202. [DOI] [PubMed] [Google Scholar]
- 16.Etzioni DA, Yano EM, Rubenstein LV, Lee ML, Ko CY, Brook RH, et al. Measuring the quality of colorectal cancer screening: the importance of follow-up. Dis Colon Rectum. 2006;49:1002–1010. doi: 10.1007/s10350-006-0533-2. [DOI] [PubMed] [Google Scholar]
- 17.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:132–141. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- 18.Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95:230–236. doi: 10.1093/jnci/95.3.230. [DOI] [PubMed] [Google Scholar]
- 19.VA Information Resource Center. [Accessed August 17, 2008];Research findings from the VA Medicare data merge initiative, report to the under secretary for health. 2003 Sep; http://www.virec.research.va.gov/DataSourcesName/VA-MedicareData/USHreport.pdf.
- 20.United States Department of Veterans Affairs. [Accessed August 17, 2008];VA Information Resource Center data sources by name. http://www.virec.research.va.gov/DataSourcesName/DataNames.htm.
- 21.Perlin JB, Kolodner RM, Roswell RH. The Veterans Health Administration: quality, value, accountability, and information as transforming strategies for patient-centered care. Am J Manag Care. 2004;10:828–836. [PubMed] [Google Scholar]
- 22.Shen Y, Hendricks A, Zhang S, Kazis LE. VHA enrollees’ health care coverage and use of care. Med Care Res Rev. 2003;60:253–267. doi: 10.1177/1077558703060002007. [DOI] [PubMed] [Google Scholar]
- 23.Pham HH, Schrag D, Hargraves JL, Bach PB. Delivery of preventive services to older adults by primary care physicians. JAMA. 2005;294:473–481. doi: 10.1001/jama.294.4.473. [DOI] [PubMed] [Google Scholar]
- 24.Cooper GS, Chak A, Koroukian S. The polyp detection rate of colonoscopy: a national study of Medicare beneficiaries. Am J Med. 2005;118:1411–1414. doi: 10.1016/j.amjmed.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Freeman JL, Klabunde CN, Schussler N, Warren JL, Virnig BA, Cooper GS. Measuring breast, colorectal, and prostate cancer screening with Medicare claims data. Med Care. 2002;40:IV-36–IV-42. doi: 10.1097/00005650-200208001-00005. [suppl] [DOI] [PubMed] [Google Scholar]
- 26.Beebe M, Dalton JA, Duffy C, Evans D, Glenn RL, Hayden D, et al. Current Procedural Terminology 2003. Chicago, IL: American Medical Association press; 2002. [Google Scholar]
- 27.Sohn M, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 29.SEER-Medicare Program. [Accessed August 17, 2008];SAS macro for Charlson Comorbidity Index. http://healthservices.cancer.gov/seermedicare/program/charlson.comorbidity.macro.txt.
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Walter LC, Davidowitz NP, Heineken PA, Covinsky KE. Pitfalls of converting practice guidelines into quality measures: lessons learned from a VA performance measure. JAMA. 2004;291:2466–2470. doi: 10.1001/jama.291.20.2466. [DOI] [PubMed] [Google Scholar]
- 32.US Census Bureau. [Accessed August 17, 2008];Census 2000 summary file 3—United States/prepared by the US Census Bureau. 2002 http://www2.census.gov/census_2000/datasets/Summary_File_3/0_National/
- 33.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med. 1993;328:1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 34.Fenton JJ, Cai Y, Weiss NS, Elmore JG, Pardee RE, Reid RJ, et al. Delivery of cancer screening: how important is the preventive health examination? Arch Intern Med. 2007;167:580–585. doi: 10.1001/archinte.167.6.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Center for Health Statistics. [Accessed August 18, 2008];Life Tables of the United States. 2001 Available at: http://www.cdc.gov/nchs/data/dvs/lt2001.pdf.
- 36.Ling BS, Moskowitz MA, Wachs D, Pearson B, Schroy PC. Attitudes toward colorectal cancer screening tests: a survey of patients and physicians. J Gen Intern Med. 2001;16:822–830. doi: 10.1111/j.1525-1497.2001.10337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh JM, Terdiman JP. Colorectal cancer screening: clinical applications. JAMA. 2003;289:1297–1302. doi: 10.1001/jama.289.10.1297. [DOI] [PubMed] [Google Scholar]
- 38.Fisher DA, Judd L, Sanford NS. Inappropriate colorectal cancer screening: findings and implications. Am J Gastroenterol. 2005;100:2526–2530. doi: 10.1111/j.1572-0241.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- 39.Sirovich BE, Schwartz LM, Woloshin S. Screening men for prostate and colorectal cancer in the United States: does practice reflect the evidence? JAMA. 2003;289:1414–1420. doi: 10.1001/jama.289.11.1414. [DOI] [PubMed] [Google Scholar]
- 40.Increase in screening for colorectal cancer in older Americans: results from a national survey. Chen X, White MC, Peipins LA, Seeff LC. J Am Geriatr Soc. 2008:1–6. doi: 10.1111/j.1532-5415.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 41.Jones RM, Mongin SJ, Lazovich D, Church TR, Yeazel MW. Validity of four self-reported colorectal cancer screening modalities in a general population: differences over time and by intervention assignment. Cancer Epidemiol Biomarkers Prev. 2008;17:777–784. doi: 10.1158/1055-9965.EPI-07-0441. [DOI] [PubMed] [Google Scholar]
- 42.Partin MR, Grill J, Noorbaloochi S, Powell AA, Burgess DJ, Vernon SW, et al. Validation of self-reported colorectal cancer screening behavior from a mixed-mode survey of veterans. Cancer Epidemiol Biomarkers Prev. 2008;17:768–776. doi: 10.1158/1055-9965.EPI-07-0759. [DOI] [PubMed] [Google Scholar]
- 43.Ananthakrishnan AN, Schellhase KG, Sparapani RA, Laud PW, Neuner JM. Disparities in colon cancer screening in the Medicare population. Arch Intern Med. 2007;167:258–264. doi: 10.1001/archinte.167.3.258. [DOI] [PubMed] [Google Scholar]
- 44.Cooper GS, Koroukian SM. Geographic variation among Medicare beneficiaries in the use of colorectal carcinoma screening procedures. Am J Gastroenterol. 2004;99:1544–1550. doi: 10.1111/j.1572-0241.2004.30902.x. [DOI] [PubMed] [Google Scholar]
- 45.Fisher DA, Jeffreys A, Coffman CJ, Fasanella K. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomarkers Prev. 2006;15:1232–1235. doi: 10.1158/1055-9965.EPI-05-0916. [DOI] [PubMed] [Google Scholar]
- 46.Jha AK, Perlin JB, Kizer KW, Dudley RA. N Engl J Med. 2003;348:2218–2227. doi: 10.1056/NEJMsa021899. [DOI] [PubMed] [Google Scholar]
- 47.Gross CP, McAvay GJ, Krumholz HM, Paltiel D, Bhasin D, Tinetti ME. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: implications for screening. Ann Intern Med. 2006;145:646–653. doi: 10.7326/0003-4819-145-9-200611070-00006. [DOI] [PubMed] [Google Scholar]