Abstract
In this issue of Molecular Cell, Day et al. (2012) reveal a surprising benefit of peroxiredoxin inactivation at high H2O2, showing that in Schizosaccharomyces pombe turning off peroxide defenses preserves the pool of reduced thioredoxin for repairing proteins vital to survival.
Hydrogen peroxide (H2O2) is most generally known as a bacteriocide for cleaning cuts—though it stings like blazes—but it is also widely promoted for its “miraculous” curative properties. This Yin and Yang of hydrogen peroxide is also seen in eukaryotic cell biology, as it causes oxidative damage and cell death at high concentrations, but is also synthesized purposefully by cells at low levels to regulate growth and differentiation. Catalase and glutathione peroxidase had long been considered the primary enzymes dealing with hydrogen peroxide, but peroxiredoxins (Prx) are now known as the dominant cellular peroxide-reducing enzymes (Winterbourn, 2008). Their importance is unarguable, as knockouts of the most highly expressed Prxs are associated with decreases in genome stability and accelerated aging, and Prx overexpression is associated with various difficult to treat cancers (referenced in Day et al., 2012). A “whodunit” mystery was initiated in 2003 with the discovery that the most highly expressed Prxs in eukaryotes have an evolutionarily honed sensitivity to be oxidatively inactivated by their own substrate (Wood et al., 2003). The hyperoxidation is reversed by the tightly regulated enzyme sulfiredoxin (Srx), but nevertheless the circumstances under which this inactivation—which makes Prxs worse peroxidases—is advantageous has been a mystery (Hall et al., 2009). In this study, Day et al. (2012) discovered an unanticipated advantage of the sensitivity when they tracked down how the fission yeast Schizosaccharomyces pombe survives exposure to high (≥ 1 mM) concentrations of H2O2 (Day et al., 2012).
S. pombe has a single Prx, Tpx1, which is sensitive to hyperoxidation, and Day et al. (2012) show that thioredoxin (Trx1) serves as its primary reductant. With exposure to low (0.2 mM) H2O2, things work as expected for oxidative stress (Figure 1A): the Trx1 pool becomes largely oxidized as it rapidly supplies reducing equivalents to Tpx1, and a redox-sensitive transcription factor, Pap1, also becomes oxidized and migrates to the nucleus where it induces an adaptive response of transcription of further antioxidant enzymes. At high concentrations of H2O2 (>1 mM), things take a surprising twist (Figure 1B): the Tpx1 pool is rapidly hyperoxidized, so it neither degrades peroxide nor aids in the oxidation of Pap1. Since the Tpx1 cycle is not active, this leaves substantial reduced Trx1 in the cell, which reduces any Pap1 that had been oxidized so no adaptive transcriptional response occurs. Yet, counterintuitively, the survival rate is higher! Day et al. (2012) hypothesize that the better survival occurs because the remaining pool of reduced Trx1 can drive the repair of vital enzymes needed for cell survival. As one example, they show this in fact occurs for methionine sulfoxide reductase, Mxr1, for which the repair activity correlates with the availability of a pool of reduced Trx1. Among many controls, a striking one shows that yeast having a Tpx1 mutant that cannot be easily hyperoxidized (i.e., is more active in combating oxidative stress) strongly depletes the reduced Trx1 pool and leads to as low a survival rate with high peroxide exposure as is observed with a Trx1 deletion strain.
Figure 1. Two Modes for Oxidative Stress Responses in Schizosaccharomyces pombe.
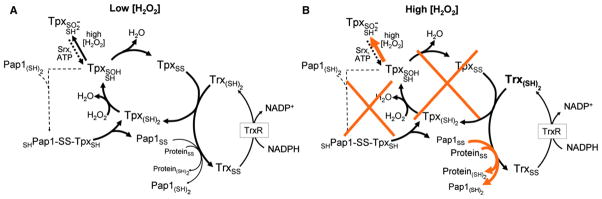
(A) H2O2 concentrations of up to 0.2 mM are efficiently scavenged by the peroxiredoxin (Tpx), which is recycled by thioredoxin (Trx) and the associated thioredoxin reductase (TrxR) and NADPH system. On the higher end of these H2O2 concentrations (e.g., 0.2 mM), some Pap1 oxidation is observed, which is mediated by Tpx and leads to a nuclear localization of the activated Pap1 transcriptional regulator and upregulation of protective antioxidant proteins.
(B) At high H2O2, Tpx hyperoxidation, which can be reversed by the action of sulfiredoxin (Srx), is augmented, eliminating both the H2O2 detoxification and the Pap1 oxidation catalyzed by this protein (orange Xs). Instead (orange arrows), any oxidized Pap1 and other vital proteins such as methionine sulfoxide reductase (Mxr1) are actively reduced by the now more available reduced Trx pool and this promotes survival.
This work shows convincingly that when a very strong oxidative assault is present, it can be effective not to fight the assault—and rapidly exhaust one’s resources—but to instead use resources to maintain vital systems in hopes that the assault will pass. But an open question is whether this yeast truly has given up the fight or just changed weapons. Similar to this story, Seaver and Imlay (2001) had already noted that E. coli “cannot provide enough NADH to rapidly degrade large amounts of H2O2,” and that although an E. coli Prx (known as AhpC) is critical for keeping resting levels of hydrogen peroxide low, it becomes saturated when peroxide levels reach ~10 μM (Seaver and Imlay, 2001). In contrast, catalase has a very high KM, so it becomes increasingly important as H2O2 levels rise, and because catalase dismutates the peroxide, it conserves cellular redox resources, essentially using one H2O2 molecule to reduce another. The situation in bacteria is not precisely equivalent to that in yeast, however. As noted by Day et al. (2012), catalysis by AhpC is supported primarily by a specialized flavoprotein reductase known as AhpF and is not expected to drain reducing equivalents from the Trx pool; moreover, the AhpC/AhpF system depends on NADH rather than NADPH, further insulating the Trx pool from depletion by high peroxide levels (Poole, 2005). The present study does not address the role of catalase in S. pombe, but a catalase-expressing gene is in the genome (ctt1), and it would be of interest to see how knocking out that gene impacts survival under these conditions.
The behavior of the Tpx1 enzyme in this system (which could be considered a peroxide bath) can be likened to that of redox cyclers, compounds that can be oxidized directly by oxygen and reduced by a cellular enzyme system (Figure 2). Upon introduction to the cell, redox cyclers continuously navigate a futile cycle back and forth between oxidized and reduced states, burning up the cells’ redox reserves (e.g., Buchholz et al., 2008). Such compounds are well known to negatively impact the health of a cell by depleting its redox power, and this is a common mode of action of pharmaceuticals.
Figure 2. Redox Cycling and Tpx Hyperoxidation to the Rescue.
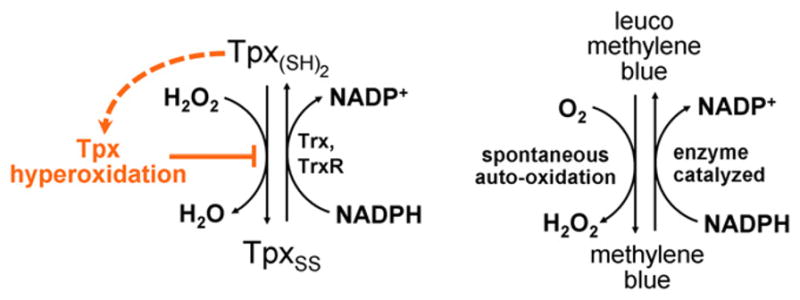
The oxygen-driven redox cycling of the antimalarial methylene blue (Buchholz et al., 2008) is analogous to the H2O2-driven redox cycling of Tpx1 seen by Day et al. (2012). Hyperoxidation of Tpx1 blocks the cycle (orange), avoiding the depletion of cellular redox resources by this pathway.
For this reason, if cells were regularly exposed to such high (~mM) levels of H2O2, the ability for Prxs to be easily inactivated by hyperoxidation could indeed be a quite important evolutionary advantage. But that is a big “if,” because it has been argued that eukaryotic cells within multicellular organisms in natural settings are rarely, if ever, exposed to H2O2 concentrations that approach even 1 μM on a cell-wide basis (Stone and Yang, 2006). Thus, although this work elegantly proves that the sensitivity of Tpx1 to hyperoxidation does help S. pombe better survive exposure to high H2O2, it is not so clear that this observation is part of a viable explanation for why the sensitivity of Prxs originated and has been conserved throughout eukarya. If such high H2O2 concentrations have not regularly occurred in the history of yeast and/or other eukarya, then this response is unlikely to have promoted the evolution of sensitivity to hyperoxidation, and the fact that it helps yeast survive under these conditions would instead be a very interesting—but accidental—benefit of the sensitivity. Nevertheless, the principle of conserving the reduced Trx pool may indeed be an important advantage provided by hyperoxidation even at lower H2O2 concentrations.
So the whodunit mystery introduced above—what is or are the useful purpose or purposes of the hyperoxidative inactivation of Prxs that have led to its development and conservation in eukarya—remains unsolved. In considering possible answers to this question, it is worth distinguishing between advantages related to oxidative signaling events that are part of normal cell growth and development versus advantages related to defense against or responses to pathological oxidative stress conditions (Stone and Yang, 2006; Hall et al., 2009). These two types of processes are often not clearly differentiated in discussions, because responses to oxidative stress do include signaling events (such as the oxidative activation of the transcription factor Pap1 in S. pombe that induces an antioxidant response) that are legitimately called “oxidative signaling.” For this reason, we suggest adoption of the more explicit descriptors of “non-stress-related” versus “stress-related” oxidative signaling (Hall et al., 2009). We note that whereas both prokaryotes and eukaryotes carry out stress-related oxidative signaling, non-stress-related oxidative signaling appears to be unique to eukarya, is important for their growth and development, and involves hydrogen peroxide, making it an attractive hunting ground for finding the answer(s) to the whodunit mystery. Among the explanations so far put forth (Figure 3), only the floodgate hypothesis specifically addresses a positive role for Prx hyperoxidation in non-stress-related oxidative signaling, but all are advantages that hyperoxidation brings. The Day et al. (2012) work emphasizes well the important role that Prxs play in cells, highlighting the interconnectedness of the many redox active components in a cell and the extent to which their relative levels and interactions influence a cell’s fate. It also is a delightful reminder that for cells, as for relationships, the most effective response to a given circumstance may not be the obvious one.
Figure 3. Proposed Roles for Prx Hyperoxidation in Biological Processes.
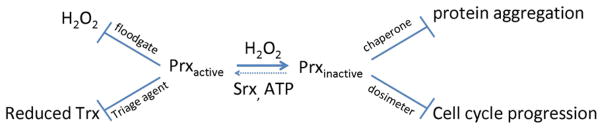
Three oxidative stress related roles that have been proposed for the biological importance of Prx hyperoxidation are gain-of-function activities as a molecular chaperone (Jang et al., 2004) or as a peroxide dosimeter that regulates the cell cycle (Phalen et al., 2006), and a loss-of-function activity as a molecular triage agent conserving reduced Trx, as presented by Day et al. (2012). A fourth proposed role is encompassed by the floodgate hypothesis (Wood et al., 2003), in which active Prxs normally keep H2O2 low (i.e., a closed floodgate) but, under signaling conditions that cause a loss in function via hyperoxidation in a localized region of the cell, allows H2O2 to build up locally (i.e., be released by an open floodgate) for signaling purposes (Hall et al., 2009).
References
- Buchholz K, Schirmer RH, Eubel JK, Akoachere MB, Dandekar T, Becker K, Gromer S. Antimicrob Agents Chemother. 2008;52:183–191. doi: 10.1128/AAC.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AM, Brown JD, Taylor SR, Rand JD, Morgan BA, Veal EA. Mol Cell. 2012;45:398–408. doi: 10.1016/j.molcel.2011.11.027. this issue. [DOI] [PubMed] [Google Scholar]
- Hall A, Karplus PA, Poole LB. FEBS J. 2009;276:2469–2477. doi: 10.1111/j.1742-4658.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HH, Lee KO, Chi YH, Jung BG, Park SK, Park JH, Lee JR, Lee SS, Moon JC, Yun JW, et al. Cell. 2004;117:625–635. doi: 10.1016/j.cell.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Phalen TJ, Weirather K, Deming PB, Anathy V, Howe AK, van der Vliet A, Jönsson TJ, Poole LB, Heintz NH. J Cell Biol. 2006;175:779–789. doi: 10.1083/jcb.200606005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole LB. Arch Biochem Biophys. 2005;433:240–254. doi: 10.1016/j.abb.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Seaver LC, Imlay JA. J Bacteriol. 2001;183:7173–7181. doi: 10.1128/JB.183.24.7173-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JR, Yang S. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Poole LB, Karplus PA. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]


