Abstract
Background
We have recently shown that post-ischemic administration of intralipid protects the heart against ischemia/reperfusion injury. Here we compared the cardioprotective effects of intralipid with cyclosporine-A, a potent inhibitor of the mitochondrial permeability transition pore opening.
Methods
In-vivo rat hearts or isolated Langendorff-perfused mouse hearts were subjected to ischemia followed by reperfusion with Intralipid (0.5%, 1% and 2% ex-vivo and 20% in-vivo), cyclosporine-A (0.2μM, 0.8μM and 1.5μM ex-vivo and 10mg/kg in-vivo) or vehicle. The hemodynamic function, infarct size, calcium retention capacity, mitochodrial superoxide production and phosphorylation levels of Akt/GSK-3β were measured. The values are mean±SEM.
Results
Administration of intralipid at reperfusion significantly reduced myocardial infarct size compared with cyclosporine-A in-vivo ((infarct size/area at risk)%: 22.9±2.5% vs. 35.2±3.5%; p=0.030, n=7/group). Postischemic administration of intralipid at its optimal dose (1%) was more effective than cyclosporine-A (0.8μM) in protecting the ex-vivo heart against ischemia/reperfusion injury as the rate pressure product at the end of reperfusion was significantly higher (mmHg*beats/min:12740±675(n=7) vs. 9203±10781(n=5), p=0.024), and the infarct size was markedly smaller (17.3±2.9(n=7) vs. 29.2±2.7(n=5), p=0.014). Intralipid was as efficient as cyclosporine-A in inhibiting the mPTP opening (calcium retention capacity=280±8.2 vs. 260.3±2.9nmol/mg-mitochondria-protein in cyclosporine-A, p=0.454, n=6) and in reducing cardiac mitochondrial superoxide production. Unlike intralipid, which increased phosphorlyation of Akt (6-fold) and GSK-3β (5-fold), cyclosporine-A had no effect on the activation of these pro-survival kinases.
Conclusions
Although intralipid inhibits the opening of the mitochondrial permeability transition pore as efficiently as cyclosporine-A, intralipid is more effective in reducing the infarct size and improving the cardiac functional recovery.
1. Introduction
Acute myocardial infarction is responsible for the death of millions of people worldwide each year. Although early reperfusion is the only way to salvage an ischemic organ, during the crucial early moments of reperfusion significant reversible and irreversible organ damage is initiated, a process referred to as reperfusion injury. The reperfusion injury oftentimes is even more damaging than the ischemia itself due to oxidative damage caused by free radicals and calcium overload as a result of reintroduction of blood to the tissue1. Pharmacological postconditioning has been used to protect the heart against ischemia/reperfusion (I/R) injury. Cardioplegic arrest and cardiopulmonary bypass are also other key triggers of myocardial injury during cardiac surgery, and multiple techniques have been used to protect the heart during the surgical requirement for global or regional ischemia. Many pharmacological agents have been shown in experimental studies to have the ability to induce a polarized arrest and to better protect the heart, such as a cardio-selective β1-blocker2 and the adenosine triphosphate-sensitive potassium channel openers3,4. However, none of pharmacological candidates have been widely accepted5. Recently we have shown that postischemic treatment with Intralipid, the first safe fat emulsion for human use, improves the cardiac functional recovery of isolated Langendorff-perfused mouse hearts by ∼4 fold and results in 70% reduction in the myocardial infarct size6.
The mitochondrial permeability transition pore (mPTP) is a large non-selective conductance pore located in the inner membrane of mitochondria. Although the exact molecular identity of mPTP has been questioned recently, cychlophilin-D (CypD) is still an established component of mPTP7;8.The mPTP remains closed during ischemia, but opens during the reperfusion period9;10. Opening of the mPTP is favored by events occurring during ischemia and reperfusion, including overproduction of reactive oxygen species and accumulation of Ca2+ in the mitochondrial matrix10. Delaying the opening of the mPTP upon reperfusion has been a potential target to reduce myocardial injury. To date, the most specific inhibitor of the mPTP is cyclosporine-A11, which acts by directly inhibiting the peptidyl-prolyl cis-trans isomerase activity of CypD, which is a key component of the mPTP12,13. Recently we demonstrated that intralipid inhibits the opening of the mPTP and protects the heart by recruiting the reperfusion injury salvage kinases pathway Phosphatidylinositol 3-kinase (PI3K)/Akt/ERK1 and leading to phosphorylation of GSK-3β6.
Here we compared the cardioprotective effect of intralipid with cyclosporine-A when administrated at the onset of reperfusion. Our data revealed that although intralipid inhibits the mPTP opening as efficiently as cyclosporine-A, intralipid is more effective in protecting the heart against I/R injury than cyclosporine-A both in-vivo and ex-vivo.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats 250-300 g and 3 month old wild type male mice (C57BL/6) were used. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institute of Health (Publication No. 85-23, revised 1996). The animal protocol received institutional approval by the Animal Research Committee of University of California Los Angeles.
2.1. Left anterior descending coronary artery occlusion and measurement of infarct size
Male Sprague-Dawley rats were anesthetized with ketamine (80 mg/kg, intraperitoneally) and xylazine (8 mg/kg, intraperitoneally). The rats were intubated and ventilated with a ventilator (CWE SAR-830/P, Ardmore, PA). A catheter filled with heparinized saline was placed into the right carotid artery for the measurement of blood pressure and heart rate. Pressure and heart rate were monitored using a pressure transducer (Power Lab, ADInstruments, Colorado Springs, CO) throughout the experiment. The hearts were exposed through a left thoracotomy in the fourth intercostal space. The pericardium was opened, and a 5.0 Prolene suture was tightened around the proximal left anterior descending coronary artery. Ischemia was confirmed by ST elevation in electrocardiogram.
At the end of the experiment, 2.5 ml of 1% Evans Blue dye was injected into the femoral vein and the myocardial ischemic area at risk was identified as the region lacking blue staining. The ventricles of the hearts were sliced transversely into 2mm thick slices. The slices were incubated in 1% triphenyltetrazolium chloride at 37°C for 15 min to identify the non-infarcted and infarcted areas. The infarcted area was displayed as the area unstained by triphenyltetrazolium chloride. Infarct size was expressed as a percentage of the area at risk.
2.2. Langendorff preparation
Mice were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally) and heparin (200 IU/kg) was injected to prevent blood coagulation. The heart was quickly removed and placed in ice-cold Krebs-Henseleit buffer solution (KH, in mM): glucose 11.1, NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25.0, CaCl2 2 at pH 7.4 bubbled with 95% O2/5% CO2 at 37 °C.
2.3. Experimental protocol
In-vivo
We used the well established method to induce I/R in vivo14;15. The heart was subjected to 30 min of ischemia by ligating the proximal left anterior descending coronary artery, followed by 180 min of reperfusion, which was achieved by releasing the tension on the ligature. A bolus of intralipid (20%, 5ml/kg body weight) or cyclosporine-A (10mg/kg body weight) were applied via the femoral vein 5 minutes before reperfusion (Fig.1A). These doses of intralipid and cyclosporine-A have been already used in vivo by our group or others6;16;17. The rats that died during 180 min reperfusion were excluded from the study (2 only in the control (CTRL) group due to the technical problem with ventilator).
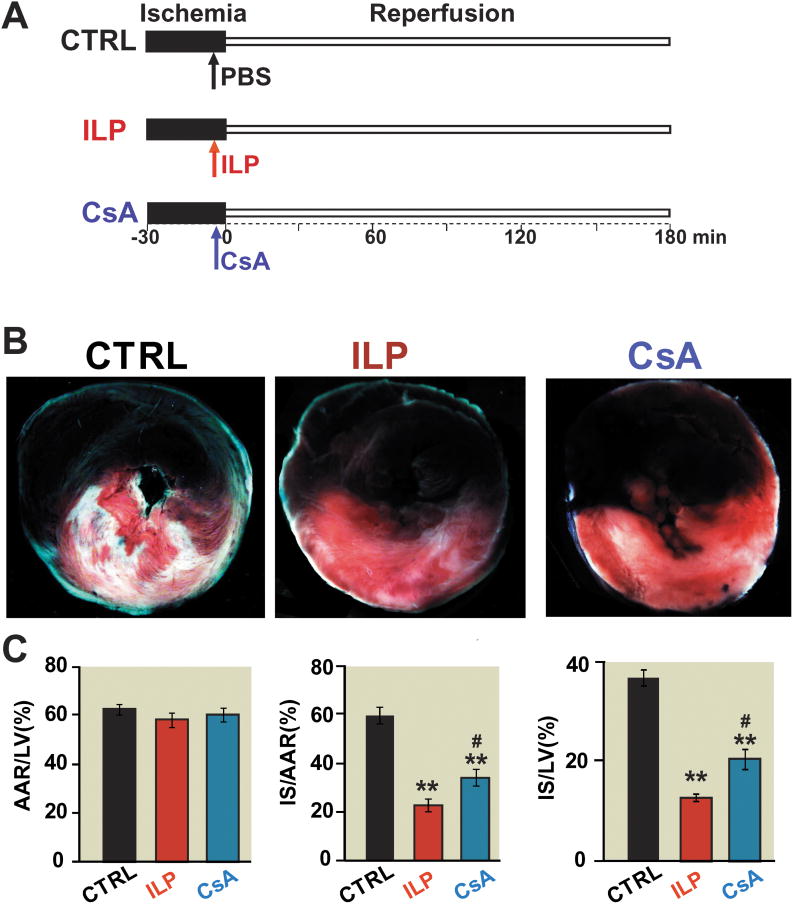
Figure 1. Intralipid reduces the infarct size more efficiently than Cyclosporine-A in the in vivo ischemia/reperfusionrat model.
A. The left coronary artery was occluded for 30 minutes followed by 3 hr of reperfusion. One single IV bolus of 20% Intralipid (5ml/kg body weight, intralipid group), Cyclosporine-A(10mg/kg body weight, cyclosporine-A group) and phosphate buffered saline (control group, CTRL) was administered 5 min before reperfusion. B. representativetriphenyltetrazolium chloride-stained heart slices from from control, intralipid(ILP) and cyclosporine-A(CsA) group. The white area represents show infracted area (Corresponding drawings), blue area shows noneinfarct area red and white areas show risk area. C. Percentage of area at risk (AAR) divided by left ventricle (LV) (B), infarct size (IS) divided by AAR (C), and infarct size (IS) divided by left ventricle in CTRL(n=7), intralipid group(n=7) and cyclosporine-A group (n=7). **p<0.001 vs. CTRL, #p=0.030 vs. CsA.
Ex-vivo
We used the well-established protocol to induce I/R injury in isolated mouse hearts as shown by our group and others6;18;19. The heart was connected to the perfusion cannula via the aorta and perfused with KH solution. Once the equilibration was achieved, the aorta was clamped for 20 min to induce global normothermic (37°C) ischemia (the heart was immersed in the 37°C Krebs solution during ischemia), followed by reperfusion for 40 min with KH alone (CTRL group), or with additional of intralipid or cyclosporine-A. The ex-vivo hearts that during stabilization had i) poor contractile function (<60 mmHg developed pressure after stabilization), 2) bradycardic (heart rate < 140 beats/min) or 3) sign of arrhythmia, were not even subjected to ischemia and were excluded from the study.
To obtain the optimal doses of intralipid and cyclosporine-A in ex-vivo, different doses of intralipid (0.5, 1% and 2% intralipid) or cyclosporine-A (0.2, 0.8 and 1.5 μM) were applied at the onset of reperfusion. The lower concentration of intralipid or cyclosporine-A which resulted in higher heart functional recovery and smaller infract size was used as the optimal concentration. The ex-vivo doses of intralipid between 0.5 and 2% were based on the in-vivo dose of 5ml/kg of 20% following these assumptions: i) for a given rat of 250 g, the volume of the intralipid bolus in in-vivo is 1.25 ml of intralipid-20%; ii) The blood volume in a rat is estimated to be between 4.3-8 times the body weight20, thus, the maximum blood volume is about 8×0.250g=20 ml; iii) The intralipid will be diluted in the blood, to 1.25ml/(1.25+20)ml=0.058 of 20%, which is equal to 1.17% or intralipid=∼1%. Our ex-vivo doses of cyclosporine-A between 0.2 and 1.5 μM were based on previous in-vitro studies showing that Cyclosporine-A inhibits mitochondrial swelling and loss of membrane potential in a dose-dependent manner with partial inhibition at ∼0.2 μM and full inhibition at ≥1 μM21. Cyclosporine-A also protects against apoptosis in a dose-dependent fashion between 0.5 and 2 μM, cyclosporine-A start to present cell toxicity at concentrations greater than 2μM22.
2.4. Cardiac functional measurements
A catheter (1.4F Millar SPR-671 Colorado Springs, CO) connected to a pressure transducer was directly inserted into the left ventricle (LV) to measure left ventricular systolic pressure, left ventricular end-diastolic pressure and heart rate. The LV developed pressure (LVDP) was calculated as LVDP = left ventricular systolic pressure – left ventricular end-diastolic pressure and rate pressure product (RPP) = heart rate×LVDP. The maximum rate of LV pressure rise (dP/dtmax) and decline (-dP/dtmin) were directly calculated from the selected stable recordings.
2.5. Myocardial necrosis
At the end of the reperfusion, the hearts were cut into four transverse slices and myocardial necrosis was assessed by measurement of the infarct size using triphenyltetrazolium chloride staining. The slices were fixed in 4% paraformaldehyde, and the area of necrosis was quantified by Photoshop (Adobe Systems Incorporated, San Jose, CA) and expressed as the percentage of total ventricular area.
2.6. Ca2+-induced mitochondrial permeability transition
Preparation of isolated mitochondria
Mitochondria was prepared from the hearts reperfused with KH, 1% intralipid, or 1.5 μM cyclosporine-A for 10 min. Briefly, myocardial sections were placed in isolation buffer A containing (mM: 70 sucrose, 210 mannitol, 1 EDTA and 50 Tris-HCl, pH 7.4 at 4°C. The tissue was finely minced with scissors and homogenized in the same buffer A (1 ml buffer/0.1g of tissue) using Kontes and Potter-Elvehjem tissue grinders (Fisher Scientific, Pittsburgh, PA). The homogenate was centrifuged at 1,300 g for 3 min; the supernatant was filtered through a cheesecloth and centrifuged at 10,000 g for 10 min. The mitochondrial pellet was resuspended in isolation buffer B containing in mM 70 sucrose, 210 mannitol, 0.1 EDTA and 50 Tris-HCl, pH 7.4. Mitochondrial protein concentration was measured using the Bradford assay method.
Calcium Retention Capacity (CRC)
Ca2+ accumulation during reperfusion is one of the major triggers of the opening of the mPTP. Therefore we measured Calcium Retention Capacity (CRC) in the mitochondria isolated from the hearts subjected to I/R. The onset of the mPTP opening was assessed following in-vitro Ca2+ overload as previously described6. Free Ca2+ concentration outside the mitochondria was recorded with 0.5 μM CaCl2 pulses were applied every 60 sec in the spectrofluorometer. The Ca2+ pulses induced a peak of extra-mitochondrial Ca2+ concentration that returned to near-baseline level as Ca2+entered the mitochondrial matrix via the Ca2+ uniporter. With increasing Ca2+ loading, the extra-mitochondrial Ca2+ concentration started accumulating, reflecting a lower capacity for mitochondria Ca2+ uptake, which was followed by a sustained Ca2+ increase indicating a massive release of the mitochondria Ca2+ by the mPTP opening. The CRC was defined as the amount of Ca2+ required to trigger this massive Ca2+ release which was used here as an indicator of the mPTP sensitivity to Ca2+. CRC was expressed as nmol of CaCl2 per mg of mitochondrial protein.
2.7. Production of reactive oxygen species in the heart tissue and in isolated cardiac mitochondria
Reactive oxygen species (ROS) production during reperfusion triggers the opening of the mPTP. We therefore measured ROS production in the hearts subjected to 20 min ischemia followed by reperfusion for 5 min with KH, 1% intralipid, or 1.5 μM cyclosporine-A.
Dihydroethidium staining to measure ROS production in the heart tissue sections
Freshly opti-mum cutting temperature compound-embedded 6μm heart tissue sections obtained from. that were incubated with 10μM dihydroethidium in Krebs-HEPES buffer (containing in mM: 99 NaCl, 4.69 KCl, 25 NaHCO3, 1.03 KH2PO4, 5.6 D-Glucose, 20 Na-HEPES, 2.5 CaCl2 and 1.2 MgSO4) for 2 hr in the dark at room temperature. The sections were then washed 3 times for 1.5 hrs in the dark with Krebs-HEPES buffer and mounted with Prolong Antifade Reagent (Invitrogen Carlsbad, CA). Images were acquired with a confocal microscope (Olympus Fluoview, Hicksville, NY).
Electron spin resonance to quantify O2•− production in isolated myocardial mithochondria
Superoxide was detected by electron spin resonance as previously reported23-26. The superoxide (O2•−) spin probe methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine (0.5 mmol/L, Alexis, San Diego, CA) solution was prepared freshly in nitrogen gas bubbled Krebs-HEPES buffer containing diethyldithiocarbamic acid (5 μmol/L, Sigma, St. Louis, MO) and deferoxamine (25 μmol/L, Sigma, St. Louis, MO). Freshly isolated mitochondria were mixed with the O2•−-specific spin probe methoxyc, rbonyl-2,2,5,5-tetramethyl-pyrrolidine in the presence or absence of 100 U/ml of superoxide dismutases and loaded in glass capillaries for analysis of O2•− production kinetically for 10 min. The electron spin resonance settings used were as follows: center field, 3475; sweep width, 9G; static field, 3484.981; microwave frequency, 9.75 GHz; microwave power 21.02 mW; modulation frequency 86 KHz; modulation amplitude, 2.47 G; resolution in X, 512; and number of x-scans, 10. The superoxide dismutases inhibitable O2•− signals at 10 min time point, normalized by protein concentrations, were compared among different experimental groups.
2.8. Western blot analysis
Intralipid-induced cardioprotection is associated with increased phosphorylation levels of Akt and GSK-3β6. Therefore we performed Western blot to examine the possible involvements of Akt and GSK-3β in cardioprotection offered by cyclosporine-A. The entire ex-vivo hearts which were reperfused with KH, 1% intralipid, or 1.5 μM cyclosporine-A for 10 min were used for making whole heart lysates, since in this model the whole heart is considered to be the area at risk. Hearts were homogenized at 4°C in (mM): 150 NaCl, 50 Tris-HCl, 1 EGTA. 1 EDTA, 1 NaF, 1 PMSF, 1 Na3VO4, 1% NP-40, 0.1% SDS and 0.5% Sodium Deoxycholate (pH 7.4) supplemented with Protease and Phospatase Inhibitor cocktails (Roche, San Francisco, CA). The samples were centrifuged at 12,000 g for 10 min and the supernatants were collected. Protein concentration was measured and 100 μg of total protein was loaded on a 4-20% gradient Tris/HCl SDS polyacrylamide gel, electrotransferred to nitrocellulose paper, blocked with 5% nonfat dry milk in 20 mM Tris-buffered saline with 0.1% Tween and 0.5% Triton-X100 and incubated with primary antibodies. Blots were then indirectly labeled using infrared fluorophore-conjugated secondary antibodies for 1 h at room temperature, and visualized with the Odyssey™ Imaging System (Li-Cor, Lincoln, NE). Equal loading of protein onto each lane in the gel was confirmed with Vinculin. The proteins were first normalized to their corresponding Vinculin and then the phosphorylated proteins were normalized to their corresponding total protein levels.
2.9. Statistics
For cardiac infarct size, calcium retention capacity, ROS production means were compared between groups using one-way ANOVA. For ex-vivo cardiac function, means were compared among doses and over time using two-way repeated measure ANOVA where both dose and time are repeated factors. Pairwise mean comparisons were judged significant using the Tukey studentized range criterion. For dose comparisons over time, contrasts were computed under a given ANOVA model to test for trends. A priori sample size/power analysis was not carried out. However, our sample size was intuitively based on our previous experience and our published results6. All statistical analyses were performed using SPSS 13.0 (SPSS Inc, Chicago, IL) or SAS 9.3 (SAS Institute, Cary NC). As all outcomes were continuous, results were summarized with means ± standard errors of the mean (SEM). All p values are two sided, P < 0.05 was considered statistically significant.
3. Results
3.1 Intralipid protects the heart against I/R injury more efficiently than cyclosporine-A in the in-vivo rat model
The cardioprotective effect of intralipid was compared with cyclosporine-A in an in-vivo rat model of I/R injury. The area at risk to LV ratio was similar in all groups (62.4±2.0 in CTRL (n=7), 58.0±3.0 in intralipid group (n=7) and 59.4±2.8 in cyclosporine-A group(n=7)), indicating that all three groups were subjected to a comparable degree of ischemic risk . However, the ratio of infarct size to area at risk was significantly smaller in the intralipid group compared to cyclosporine-A (22.9±2.5 in intralipid group vs. 35.2±3.5 in cyclosporine-A group, p=0.030) (Fig. 1B,C). Intralipid and cyclosporine-A both reduced the infarct size significantly compared with the CTRL group (59.8±3.3 in CTRL, p<0.001). The differences in the infarct size was not due to the hemodynamic changes as the heart rate and mean arterial pressure were not significantly different among the three groups at baseline, during ischemia and at reperfusion (Table 1).
Table 1. Systemic Hemodynamics in in-vivo model of ischemia/reperfusioninjury.
| Baseline | Ischemia | Reperfusion 1hr |
Reperfusion 2hr |
Reperfusion 3hr |
|
|---|---|---|---|---|---|
|
| |||||
| Heart rate(beats/min) | |||||
| CTRL (n=5) | 296±15 | 280±10 | 292±26 | 286±13 | 278±19 |
| ILP (n=5) | 311±16 | 285±16 | 291±19 | 301±10 | 304±11 |
| CsA(n=4) | 325±11 | 296±15 | 298±17 | 299±25 | 302±22 |
| MAP(mmHg) | |||||
|
| |||||
| CTRL (n=5) | 97±4 | 75±4 | 79±4 | 82±13 | 79±12 |
| ILP (n=5) | 92±5 | 79±4 | 83±6 | 85±7 | 83±6 |
| CsA (n=4) | 93±6 | 77±6 | 84±6 | 88±4 | 85±3 |
The heart rate and mean arterial pressure (MAP) in CTRL, ILP and CsA group at baseline, during ischemia and at reperfusion of 1hr, 2hr and 3hr. Data are mean ± SEM.CsA: cyclosporine-A; CTRL: control; ILP: intralipid.
3.2. Dose-response curves to obtain the optimal dose of postischemic administration of intralipid and cyclosporine-A
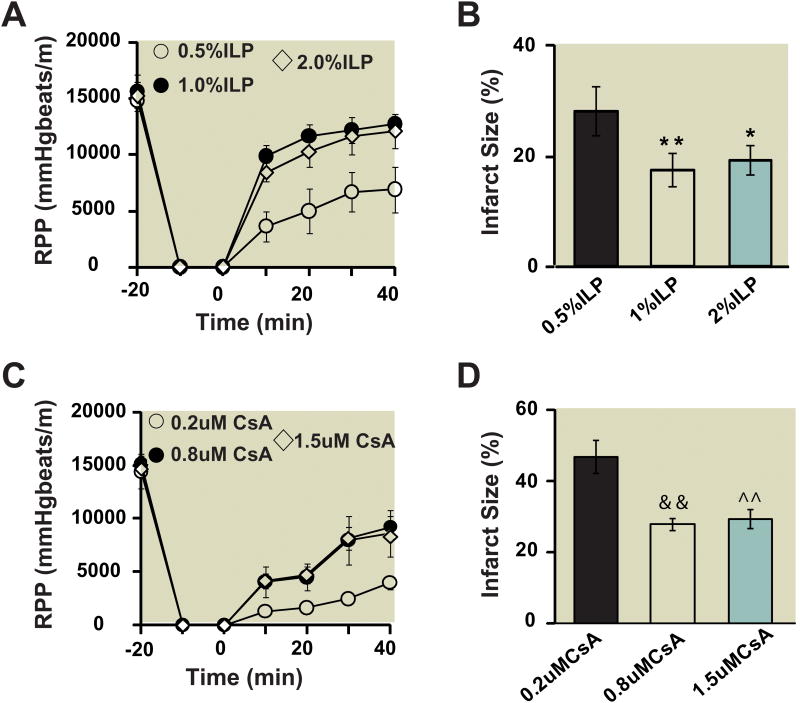
Next we compared the cardioprotective effect of intralipid with cyclosporine-A in isolated Langendorff perfused hearts. Fig. 2 shows the RPP and infarct size as a function of intralipid (Fig. 2A,B) and cyclosporine-A (Fig. 2C,D) concentrations. Although 0.5% intralipid induced some degree of protection (RPP=6909±2055 mmHg*beats/min (n=5) at 40 min of reperfusion, Infarct size=28.1±4.4 (n=5), the cardioprotection achieved by 1% and 2% intralipid were similar and was significantly better than 0.5% (1% intralipid: RPP=12740±675mmHg*beats/min (n=7) at the end of reperfusion, Infarct size=17.3±2.9 (n=7); 2% intralipid: RPP=12117±1527mmHg*beats/min (n=5), Infarct size=19.1±2.6 (n=5)). Cyclosporine-A at 0.2 μM had no apparent cardioprotective effect (RPP=3968.6±624 mmHg*beats/min (n=5) at end of reperfusion, Infarct size=46.7±4.6 (n=5). Postischemic administration of 0.8 μm cyclosporine-A significantly improved the cardiac functional recovery and reduced the infarct size compared to 0.2 μM cyclosporine-A (RPP=9203±1078mmHg*beats/min (n=5) at end of reperfusion, Infarct size=27.8±1.6 (n=5)). Increasing the concentration of cyclosporine-A from 0.8 to 1.5 μM did not result in further cardioprotection as the RPP and the infarct size for 1.5μM were not significantly different than 0.8 μM (RPP=8652±2182 mmHg*beats/min (n=5) at end of reperfusion, Infarct size=28.9±2.5 (n=5).
Figure 2. Dose response of intralipid and cyclosporine-A in ex vivo ischemia/reperfusionmouse model.
A. Rate pressure product (RPP) as a function of time in 0.5%, 1% and 2% intralipid (n=7). B. The area of necrosis as the percentage of total ventricular area in 0.5%, 1% and 2% intralipidgroup(ILP). C. Rate pressure product as a function of time in 0.2μM, 0.8μM and 1.5μM cyclosporine-A(CsA)( n=5). D. The area of necrosis as the percentage of total ventricular area in control group(CTRL), 0.5%, 1% and 2% intralipid group. **p=0.004, 1% ILP vs. 0.5% ILP; *p=0.022, 2%ILP vs. 0.5% ILP; &&p=0.008, 0.8μMCsA vs. 0.2μM CsA; ^^p=0.009, 1.5μM CsA vs. 0.2μM CsA.
3.3 Intralipid is more effective than cyclosporine-A in protecting the heart against I/R injury in ex-vivo mouse model
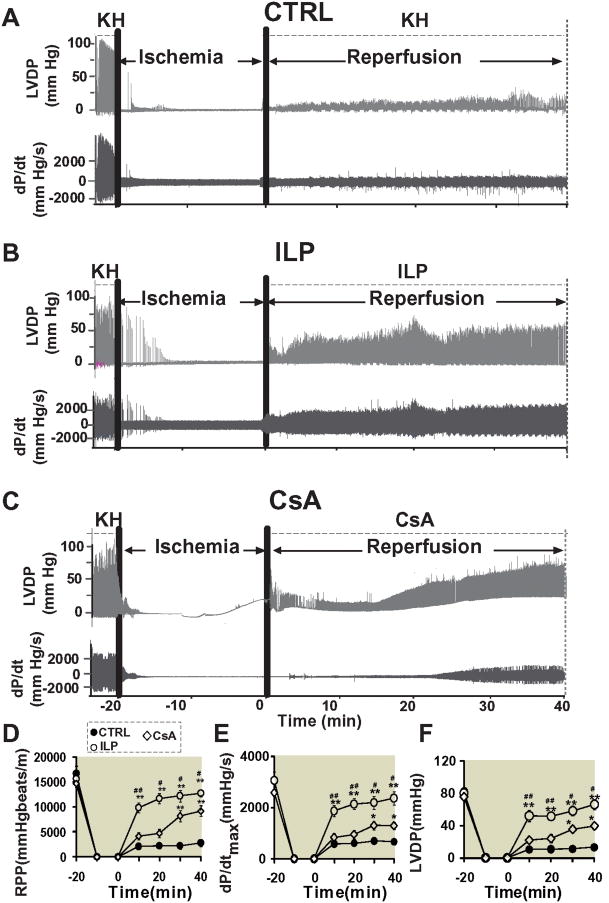
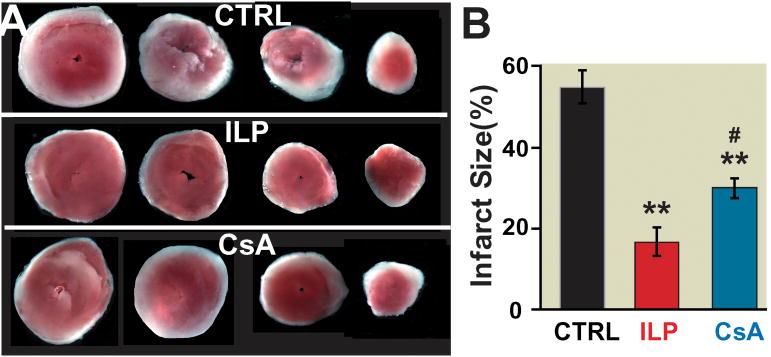
We compared the effect of intralipid and cyclosporine-A on the cardiac functional recovery and infarct size at their optimal dose of 1% and 0.8 μM, respectively. Typical examples of LVDP and dP/dt are shown in Fig. 3A-C. Although the baseline RPP before ischemia was similar in all groups, the functional recovery was very poor in the CTRL group; RPP was 2077±100 mmHg*beats/min(n=7) after 10 min of reperfusion and did not change significantly throughout the 40 min reperfusion (RPP=2791±758 mmHg*beats/min (n=7) at 40 min of reperfusion, Fig. 3D). The functional recovery in the intralipid group was much higher, as the RPP was 9873±995 mmHg*beats/min(n=7) after 10 min of reperfusion (recovery of 63%), improved further to 11705±1021 mmHg*beats/min(n=7) at 20 min of reperfusion (recovery of 75%), and to 12217±1123 mmHg*beats/min(n=7) at 30 min and to 12740±675 mmHg*beats/min (n=7) after 40 min (recovery of 81%, Fig. 3D). The intralipid group also showed a much better LV dP/dtmax and LV dP/dtmin, and LVDP compared to CTRL hearts (Fig. 3E, F). In the cyclosporine-A group, the RPP was 4159±739 mmHg*beats/min (n=5) at 10 min of reperfusion (recovery of 28%) and 4728±823 mmHg*beats/min (n=5) at 20 min of reperfusion (recovery of 32%), improved to 8188±1087 mmHg*beats/min(n=5) at 30 min (recovery of 55%) and 9203 ±1078 mmHg*beats/min at 40 min, which was significantly lower than the intralipid group (p=0.024, Fig. 3D). The LV dP/dtmax (2373±261 in intralipid group(n=7) vs. 1286±147 mmHg/s in cyclosporine-A group(n=5), p=0.031) and LVDP (66±6 in intralipid group(n=7) vs. 39±4 mmHg in cyclosporine-A group(n=5), p=0.005) were also significantly lower than intralipid at the end of 40 min reperfusion (Fig. 3E,F). Consistent with the cardiac function, the infarct size was also significantly smaller in the intralipid group compared to the cyclosporine-A group (17.3± 2.9 % in intralipid (n=7) vs.27.8±1.6 % in cyclosporine-A group (n=5), 54.4±3.8 % in CTRL (n=7, p<0.001 vs. CTRL, Fig. 4).
Figure 3. Administration of intralipid at reperfusion improves heart functional recovery against reperfusion injury more efficiently than cyclosporine-A.
Representatives of the left ventricular developed pressure (LVDP) and dP/dtMax and dP/dtMin as a function of time in control group(CTRL)(A), 1%intralipid(ILP) (B) and 0.8μM cyclosporine-A(CsA)(C). Rate pressure product (D), dP/dtMax(E) and LVDP (F) as a function of time in CTRL (filled circles, n=7), ILP (open circles, n=7) and cyclosporine-A group (CsA, dimanods, n=5). *p<0.05 and **p< 0.001 vs. CTRL, #p< 0.05, ##p< 0.01 vs.CsA.
Figure 4. The infarct size is significantly smaller in intralipid than cyclosporine-A group.
Four slices of the same heart after 2,3,5-triphenyltetrazolium chloride (TTC) staining in control group(CTRL)(n=7) (A), 1% intralipid group(ILP) (n=7) (B) and 0.8 μM cyclosporine-A group(n=5) (C). The white area represents the infarct zone and the red shows the viable area. E. The area of necrosis as the percentage of total ventricular area in CTRL (black), intralipid (red) and cyclosporine-A group(blue). **p< 0.001 vs. CTRL, #p=0.014 vs. ILP.
3.4. Intralipid inhibits the opening of mPTP as efficiently as cyclosporine-A after I/R
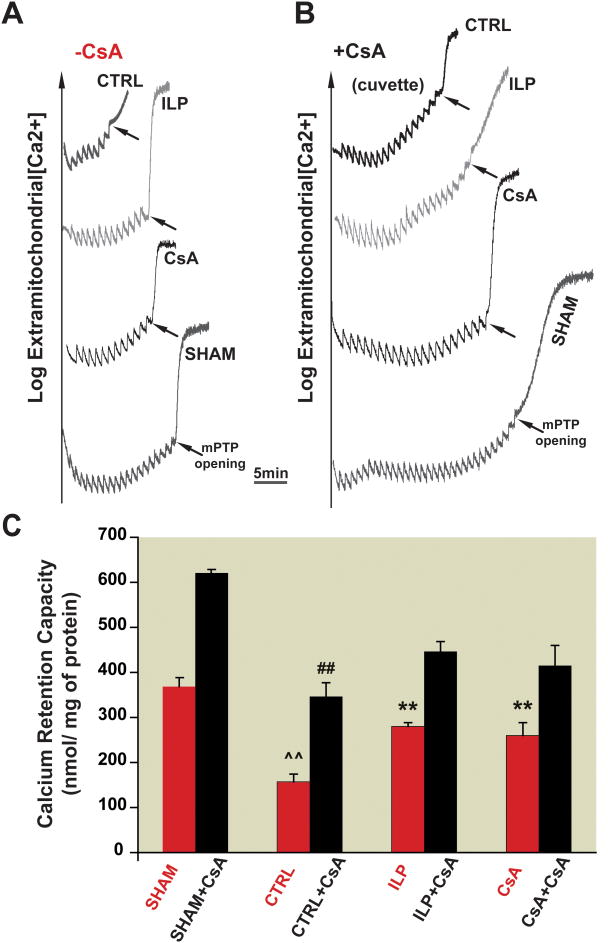
Delaying the opening of the mPTP upon reperfusion has been a potential target to reduce myocardial injury. Fig. 5A, B represents a typical recording of fluorescence showing the time course of Ca2+ concentration in the mitochondrial external medium. In the CTRL group, 8 pulses were sufficient to trigger the opening of mPTP. Interestingly, the number of calcium pulses required for opening of the mPTP in both intralipid and cyclosporine-A groups were increased to 14 and 13, respectively (Fig. 5A). These data suggest that post-ischemic treatment of intralipid increases the resistance of the mPTP to Ca2+ overload to a comparable level with the cyclosporine-A group. In fact, the CRC was about two fold higher in intralipid and cyclosporine-A groups compared to CTRL (280±8.2 in intralipid and 260±29 in cyclosporine-A vs. 157±25nmol/mg protein in CTRL, n=6, p<0.001, Fig. 5C). As expected, sham hearts had much higher CRC (370±20 nmol/mg protein).
Figure 5. Postischemic treatment of intralipid increases mitochondrial CRC after I/R as cyclosporine-A in a CypD-dependent manner. A, B.
Typical recordings of the mitochondrial permeability transition pore opening in isolated mitochondria from control group (CTRL), 1% intralipid (ILP) and 1.5 μM cyclosporine-A groups (CsA) subjected to 20 min of global ischemia followed by 10 min of reperfusion as well as sham hearts before (A) and after addition of 1.5μM cyclosporine-A directly in the cuvette (B). C. CRC in the absence of cyclosporine-A (red bars) and after addition of cyclosporine-A in the cuvette (black bars). **p<0.001 vs. CTRL; ##p<0.001 vs. sham+ cyclosporine-A group; ^^p<0.001 vs. sham (n=6).
3.5. Inhibition of mPTP opening by intralipid is CypD-dependent
We examined the involvement of CypD in intralipid-induced inhibition of mPTP opening by comparing the CRC after addition of 1.5μM of cyclosporine-A directly in the cuvette in CTRL, intralipid and cyclosporine-A groups. Administration of exogenous cyclosporine-A increased the number of Ca2+ pulses required to trigger the opening of mPTP in all groups, but to different extents (Fig. 5B, C). In sham and CTRL groups, the addition of cyclosporine-A to the cuvette greatly increased the CRC (from 370±20 to 620±10 nmol/mg protein in sham and from 157±25 to 347±30 nmol/mg protein in CTRL, n=6, p<0.001). In intralipid and cyclosporine-A groups, however, the CRC increased mildly to 445±25 and 415±45 nmol/mg protein, respectively, in the presence of cyclosporine-A.
3.6. Post-ischemic treatment of intralipid and cyclosporine-A decreases ROS production after I/R
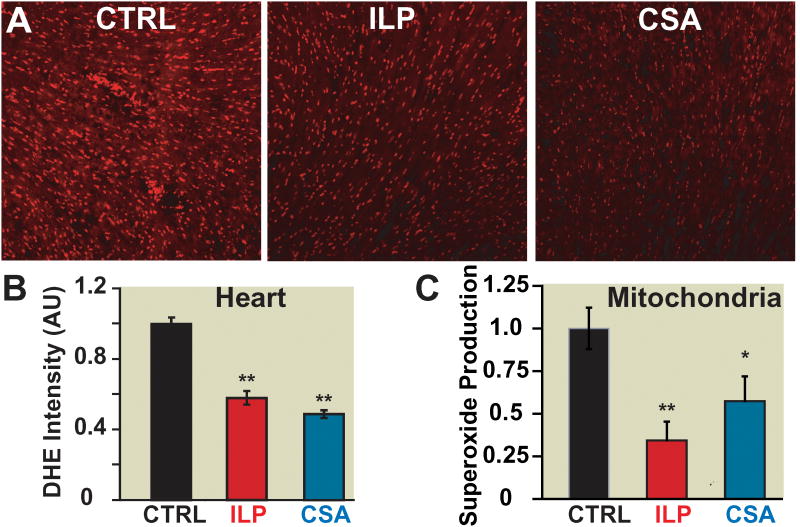
Next we investigated whether the delay of the mPTP opening in intralipid and cyclosporine-A groups is in part due to decreased ROS/superoxide generation. Dihydroethidium staining of heart sections revealed a significantly lower ROS production in the intralipid and cyclosporine-A groups when compared to CTRL (normalized to CTRL, 0.57±0.04 in intralipid group and 0.49±0.01 in cyclosporine-A, p<0.001 vs. CTRL, Fig. 6A, B). We then examined the superoxide production in isolated cardiac mitochondria from intralipid and cyclosporine-A. The mitochondrial superoxide production was significantly lower in the intralipid and cyclosporine-A groups compared to CTRL (normalized to CTRL: 0.34±0.11 in intralipid group, 0.57±0.14 in cyclosporine-A group; n=4, p=0.005 intralipid group vs. CTRL; p=0.041 cyclosporine-A group vs. CTRL, Fig. 6C).
Figure 6. Post-ischemic treatment of intralipid and cyclosporine-A decreases cardiac reactive oxygen species generation as well as mitochondrial superoxide production.
A. Representative dihidroethidium staining of transverse heart sections subjected to 20min of global ischemia followed by 5min reperfusion with phosphate buffered saline (control group, CTRL) (black), 1% intralipidgroup(ILP) (red) and 1.5 μM cyclosporine-A group(CsA)(blue). B. Fluorescence quantification of dihydroethidium (DHE) staining: average intensity represents area×fluorescence intensity and is normalized to CTRL (**p<0.001 vs. CTRL, n=3). C. Superoxide production in isolated mitochondria using electron spin resonance in CTRL (black), 1% intralipid (red) and 1.5 μMcyclosporine-A group(blue). **p=0.005 ILP vs. CTRL; *p=0.041 CsA vs. CTRL (n=4).
3.7. Unlike intralipid, cyclosporine-A does not induce Akt and GSK-3β phosphorlyation
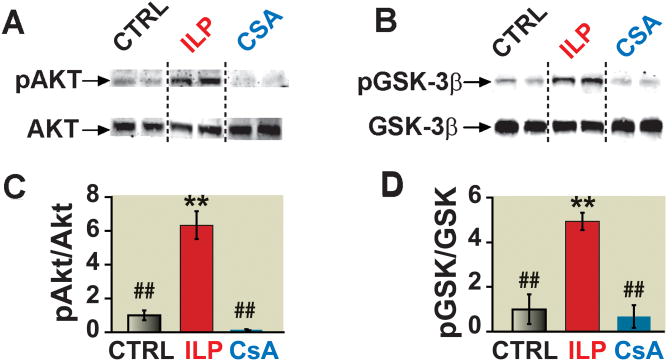
Intralipid-induced cardioprotection is associated with increased phosphorylation levels of Akt and GSK-3β6. Here we examined the possible involvements of Akt and GSK-3β in cardioprotection offered by cyclosporine-A. Western Blot analysis revealed that Intralipid-induced cardioprotection was associated with ∼6 fold increase in phosphorylation of Akt, and ∼5 fold increase in phosphorylation of GSK-3β. Cyclosporine-A however, did not affect the phosphorylation levels of Akt and GSK-3β (Fig. 7), as there were no significant differences between the phosphorylation levels of these pro-survival kinases in cyclosporine-A and CTRL groups.
Figure 7. The lack of involvement of Akt/GSK pathways in cyclosporine-A-induced protection.
A, B. representative immunoblots of pAkt and total Akt (A), and pGSK-3β and total GSK-3β (C) in heart homogenates subjected to I/R from control group(CTRL), 1% intralipid, or 1.5 μM cyclosporine-A group. C, D. Western blot analysis of pAkt protein to total Akt (B) and pGSK-3β to total GSK-3b (D) ratios in CTRL (black bars), intralipid group (ILP, white bars) and cyclosporine-A group(gray bars). **p<0.001 vs. CTRL; ##p<0.001 vs. ILP (n=4−6/group).
4. Discussion
Our previous study has shown that post-ischemic administration of Intralipid can protect the heart against I/R injury in both the in-vivo and ex-vivo models. Here we compared the cardioprotective effect of intralipid with cyclosporine-A. Our data demonstrate that intralipid, a safe fat emulsion for human use, is more effective than cyclosporine-A in protecting the heart against isc I/R injury. A bolus of intralipid right before the onset of reperfusion resulted in smaller infarct size of ∼40% compared to cyclosporine-A in the in-vivo rat model. Postischemic administration of intralipid was also more efficient than cyclosporine-A in improving the heart functional recovery and reducing the infarct size in isolated Langendorff perfused ex-vivo hearts. Intralipid is as effective as cyclosporine-A in inhibiting the mPTP opening, most likely by increasing mitochondrial resistance to Ca2+ overload and reducing mitochondrial superoxide production during the first few minutes of reperfusion.
4.1.Iintralipid is more effective than cyclosporine-A in protecting the heart against I/R injury
We report that intralipid was more effective than cyclosporine-A in protecting the heart against I/R injury as the infarct size was significantly smaller and the heart functional recovery indices were all significantly better. Although the optimal concentration of cyclosporine-A, if applied before ischemia, has been shown to be 0.2μM27;28, here we found that cyclosporine-A at this concentration does not induce cardioprotection if applied at the onset of reperfusion. Interestingly, administration of 1% intralipid at the onset of reperfusion rapidly restored heart function, as 40-60% recovery was observed in the RPP and LVDP parameters within 10 min of reperfusion. On the other hand, the cardiac functional recovery in the cyclosporine-A group during the first 10 min of reperfusion was only 20-30%. Since the first few minutes of reperfusion are critical in myocardial protection, intralipid could be an ideal safe pharmacological agent to rapidly restore the heart function resulting in smaller infarct size.
Our data demonstrate that intralipid is a very powerful postischemic pharmacological agent reducing infarct size (both in-vivo and ex-vivo) and improving cardiac contractility6. The role of intralipid in preconditioning, however, seems to be controversial. In the ex-vivo model of I/R injury, intralipid failed to protect the myocardium from contractile dysfunction when administered 10 min before the onset of ischemia and throughout the reperfusion phase29. Administration of intralipid before ischemia in an in-vivo model of myocardial I/R injury was also not able to reduce the infarct size30;31. However, in another work from the same group, intralipid was shown to reduce the infarct size in the ex-vivo model of I/R in rats if administered before ischemia32. Further studies are required to clarify the role of intralipid in preconditioning.
4.2.Iintralipid is as effective as cyclosporine-A in inhibiting mPTP opening by increasing mitochondrial resistance to Ca2+ overload and reducing mitochondrial superoxide production
The opening of the mPTP during reperfusion has been implicated in cell death33;34. Ca2+ accumulation and overproduction of ROS during reperfusion are the two major triggers of the opening of the mPTP. It has been demonstrated that ischemic preconditioning and postconditioning induce cardioprotection by increasing mitochondrial resistance to Ca2+ overload35. Mitochondria are also the major source of ROS generation through their respiratory chain and are also the target organelle of oxidative damage35. Decreasing ROS generation during reperfusion has been considered to induce cardioprotection against I/R injury36. Recently we showed that intralipid inhibits the opening of the mPTP6. Here our data demonstrate that intralipid is as efficient as cyclosporine-A in increasing the mitochondrial calcium uptake for the opening of the mPTP compared to CTRL (Fig. 5). We propose that intralipid enhances the homeostasis of cardiomyocytes in the same manner as cyclosporine-A to better regulate calcium overload and therefore increase the threshold for opening of the mPTP. Intralipid exerts its action in a CypD dependent manner as cyclosporine-A, which results in attenuation of the interaction between CypD with mPTP and therefore increases the mitochondrial CRC.
We also found that ROS generation in the heart tissue, as well as the production of superoxide in cardiac mitochondria during the first 5 min of reperfusion, was significantly reduced by post-ischemic administration of intralipid. In fact, intralipid was as effective as cyclosporine-A, if not better, in reducing the production of superoxide in mitochondria. Since it is well accepted that overproduction of ROS in the mitochondria is one of the triggers of the opening of the mPTP, reduced superoxide production in the mitochondria by intralipid could delay the opening of mPTP. Up to now, cyclosporine-A is the most specific inhibitor of the mPTP and has been demonstrated in many species including pigs, as well as in clinical trails, to be cardioprotective37;38. However, cyclosporine-A is known to have undesirable side effects. Intralipid, which has been in clinical use for more than four decades with no known side effects, is as effective as cyclosporine-A in inhibiting the mPTP opening, most likely by increasing mitochondrial resistance to Ca2+ overload and reducing mitochondrial superoxide production during the first few minutes of reperfusion.
4.3. Unlike intralipid, cyclosporine-A -induced cardioprotection is not mediated via Akt/GSK-3β
Phosphatidylinositol 3-kinase-protein kinase B(PKB)/Akt pathway plays an important role in reperfusion injury39-41. GSK-3β phosphorylation has emerged as an end effector step where multiple protective signaling pathways converge39-42. We have recently shown that postischemic administration of intralipid increases the phosphorylation levels of Akt and GSK. Here we report that cyclosporine-A-induced cardioprotection is not mediated through Akt/GSK, as cyclosporine-A had no effect on the phosphorylation levels of these kinases. The cyclosporine-A -induced protection of mesenchymal stem cells against apoptosis has also been reported to be independent of Akt/ERK as cyclosporine-A did not promote the phosphorylation of these prosurvival proteins43. The lack of involvement of the Akt/GSK pathway in the cyclosporine-A -induced cardioprotection could be one of the reasons behind the slow action of cyclosporine-A in improving the postischemic heart function.
4.4 Limitations
To date, Cyclosporine-A11 is known to be the most specific inhibitor of the mPTP. It exerts its action by directly inhibiting the peptidyl-prolyl cis-trans isomerase activity of CypD, which is a key component of the mPTP12,13. Here we report that intralipid inhibits the opening of the mPTP as efficiently as cyclosporine-A. Our previous study demonstrated that intralipid increases the phosphorylation of GSK3β, leading to inhibition of the mPTP opening6;18;19. However, it is not clear if intralipid directly inhibits the mPTP in a similar manner as cyclosporine-A. Further experiments using genetically modified mice of mPTP component are required to demonstrate a possible direct action of intralipid on the mPTP.
Our data demonstrate that cyclosporine-A-induced cardioprotection is most likely not mediated via the Akt/GSK-3β pathway. However, we did not examine the involvement of the protein kinase G nor the survivor activating factor enhancement pathway in cyclosporine-A-induced cardioprotection. Since the activation of the reperfusion injury salvage kinases pathway does not seem to be crucial for postconditioning in pigs44, it remains to be seen whether reperfusion injury salvage kinases, protein kinase G or the survivor activating factor enhancement pathways play a role in cyclosporine-A or intralipid-induced cardioprotection in larger animals.
4.5. Clinical Perspectives
Acute myocardial infarction is still a major cause of mortality despite advances in the management of patients. While prompt reperfusion is critical in salvaging the ischemic heart, it also has the potential to induce reperfusion injury. Currently, there is no effective postischemic pharmacological agent for protecting the heart against the detrimental effects of lethal myocardial reperfusion injury. Delaying the opening of the mPTP upon reperfusion has been a potential target to reduce myocardial injury. Cyclosporine-A, which is one of the most potent inhibitor of mptp opening, is emerging as a new postconditioning cardioprotective agent and it has been used recently in small clinical trials both in Europe and United states. Chronic treatment with cyclosporine-A, however, has been associated with a number of potentially serious adverse drug reactions such as high blood pressure, potassium retention, and possibly hyperkalemia, kidney and liver dysfunction and an increased vulnerability to opportunistic fungal and viral infections. Therefore due to cyclosporine-A undesirable side effects, it is questionable whether cyclosporine-A is an ideal pharmacological postconditioning agent. Intralipid, on the other hand, is a clinically safe lipid emulsion which is generally well tolerated and is widely used in different clinical settings for parenteral nutrition for almost four decades. Our results demonstrate that not only intralipid is as potent as cyclosporine-A in inhibiting mPTP opening, intralipid is in fact more effective that cyclosporine-A in reducing the infarct size. Therefore, intralipid could be a better alternative and an ideal safe pharmacological post-conditioning agent for targeting the critical first few minutes of reperfusion to rapidly restore the heart function and therefore reduce the infarct size. Although, our in-vivo dose of intralipid at 5ml/kg is within the range that has been recommended by American Society of Regional Anesthesia and Pain Medicine for rescuing bupivacaine cardiotoxicity in patients, the exact dose of intralipid still needs to be determined in clinical trails for reperfusion injury. Our findings raise the intriguing concept that intralipid could serve as a novel therapeutic agent for acute myocardial infarction patients which certainly warrants further investigation in the human heart.
MS #201109076 Final Box Summary.
What We Already Know about This Topic
Previous studies have demonstrated that post-ischemic administration of intralipid protects the heart against ischemia/reperfusion injury.
This study compared the cardioprotective effect of intralipid with cyclosporine-A when administered at the onset of myocardial reperfusion.
What This Article Tells Us That Is New
Intralipid is more effective than cyclosporine-A in reducing myocardial infarct size and improving cardiac functional recovery after ischemia/reperfusion.
Acknowledgments
The authors would like to thank Dr. Jeffrey Gornbein, DrPH Biostatistics, Senior Statistician, from Department of Biomathematics at University of California Los Angeles, Los Angeles, CA, for the statistical assistance.
Funding: Mansoureh Eghbali is supported by National Institutes of Health grants HL089876 and HL089876S1 (Bethesda, Maryland), and Hua Cai is supported by National Institutes of Health grants HL077440, HL088975 and HL101228.
References
- 1.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorczynski RJ. Basic pharmacology of esmolol. Am J Cardiol. 1985;56:3F–13F. doi: 10.1016/0002-9149(85)90910-5. [DOI] [PubMed] [Google Scholar]
- 3.Sugimoto S, Puddu PE, Monti F, Schiariti M, Campa PP, Marino B. Pretreatment with the adenosine triphosphate-sensitive potassium channel opener nicorandil and improved myocardial protection during high-potassium cardioplegic hypoxia. J Thorac Cardiovasc Surg. 1994;108:455–66. [PubMed] [Google Scholar]
- 4.Dorman BH, Hebbar L, Zellner JL, New RB, Houck WV, Acsell J, Nettles C, Hendrick JW, Sampson AP, Mukherjee R, Spinale FG. ATP-sensitive potassium channel activation before cardioplegia. Effects on ventricular and myocyte function. Circulation. 1998;98(II):176–83. [PubMed] [Google Scholar]
- 5.Cour M, Gomez L, Mewton N, Ovize M, Argaud L. Postconditioning: From the bench to bedside. J Cardiovasc Pharmacol Ther. 2011;16:117–30. doi: 10.1177/1074248410383174. [DOI] [PubMed] [Google Scholar]
- 6.Rahman S, Li J, Bopassa JC, Umar S, Iorga A, Partownavid P, Eghbali M. Phosphorylation of GSK-3beta Mediates Intralipid-induced Cardioprotection against Ischemia/Reperfusion Injury. Anesthesiology. 2011;115:242–53. doi: 10.1097/ALN.0b013e318223b8b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res Cardiol. 2009;104:181–8. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: The Holy Grail of cardioprotection. Basic Res Cardiol. 2010;105:151–4. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 9.Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta. 1998;1366:79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 10.Lemasters JJ, Qian T, He L, Kim JS, Elmore SP, Cascio WE, Brenner DA. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid Redox Signal. 2002;4:769–81. doi: 10.1089/152308602760598918. [DOI] [PubMed] [Google Scholar]
- 11.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255:357–60. [PMC free article] [PubMed] [Google Scholar]
- 12.Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J. 1990;268:153–60. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffiths EJ, Halestrap AP. Further evidence that cyclosporin A protects mitochondria from calcium overload by inhibiting a matrix peptidyl-prolyl cis-trans isomerase. Implications for the immunosuppressive and toxic effects of cyclosporin Biochem J. 1991;274(Pt 2):611–4. doi: 10.1042/bj2740611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Q, Nguyen T, Ogbi M, Caldwell RW, Johnson JA. Differential loss of cytochrome-c oxidase subunits in ischemia-reperfusion injury: Exacerbation of COI subunit loss by PKC-epsilon inhibition. Am J Physiol Heart Circ Physiol. 2008;294:H2637–45. doi: 10.1152/ajpheart.91476.2007. [DOI] [PubMed] [Google Scholar]
- 15.Yin T, Hou R, Liu S, Lau WB, Wang H, Tao L. Nitrative inactivation of thioredoxin-1 increases vulnerability of diabetic hearts to ischemia/reperfusion injury. J Mol Cell Cardiol. 2010;49:354–61. doi: 10.1016/j.yjmcc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Gomez L, Li B, Mewton N, Sanchez I, Piot C, Elbaz M, Ovize M. Inhibition of mitochondrial permeability transition pore opening: Translation to patients. Cardiovasc Res. 2009;83:226–33. doi: 10.1093/cvr/cvp063. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg GL, Di GG, Ripper R, Kelly K, Massad M, Edelman L, Schwartz D, Shah N, Zheng S, Feinstein DL. Resuscitation with lipid versus epinephrine in a rat model of bupivacaine overdose. Anesthesiology. 2008;108:907–13. doi: 10.1097/ALN.0b013e31816d91d2. [DOI] [PubMed] [Google Scholar]
- 18.Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;298:H16–H23. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin ZQ, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation. 2004;110:1980–9. doi: 10.1161/01.CIR.0000143632.06471.93. [DOI] [PubMed] [Google Scholar]
- 20.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72–6. [PubMed] [Google Scholar]
- 21.Waldmeier PC, Feldtrauer JJ, Qian T, Lemasters JJ. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol Pharmacol. 2002;62:22–9. doi: 10.1124/mol.62.1.22. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz-Meana M, Abellan A, Miro-Casas E, Garcia-Dorado D. Opening of mitochondrial permeability transition pore induces hypercontracture in Ca2+ overloaded cardiac myocytes. Basic Res Cardiol. 2007;102:542–52. doi: 10.1007/s00395-007-0675-y. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Cai H. Netrin-1 prevents ischemia/reperfusion-induced myocardial infarction via a DCC/ERK1/2/eNOS s1177/NO/DCC feed-forward mechanism. J Mol Cell Cardiol. 2010;48:1060–70. doi: 10.1016/j.yjmcc.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2005;102:9056–61. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oak JH, Cai H. Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes. 2007;56:118–26. doi: 10.2337/db06-0288. [DOI] [PubMed] [Google Scholar]
- 26.Youn JY, Wang T, Cai H. An ezrin/calpain/PI3K/AMPK/eNOSs1179 signaling cascade mediating VEGF-dependent endothelial nitric oxide production. Circ Res. 2009;104:50–9. doi: 10.1161/CIRCRESAHA.108.178467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem. 2002;277:34793–9. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- 28.Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–9. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 29.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–24. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao Y, Wang YL, Zhang WS, Liu J. Emulsified isoflurane produces cardiac protection after ischemia-reperfusion injury in rabbits. Anesth Analg. 2008;106:1353–9. doi: 10.1213/ane.0b013e3181679347. [DOI] [PubMed] [Google Scholar]
- 31.Hu ZY, Luo NF, Liu J. The protective effects of emulsified isoflurane on myocardial ischemia and reperfusion injury in rats. Can J Anaesth. 2009;56:115–25. doi: 10.1007/s12630-008-9016-3. [DOI] [PubMed] [Google Scholar]
- 32.Liu SL, Wang Y, Wang RR, Chai YF, Wu W, Huang H, Liu J. Protective effect of intralipid on myocardial ischemia/reperfusion injury in isolated rat heart. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2008;20:227–30. [PubMed] [Google Scholar]
- 33.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341(Pt 2):233–49. [PMC free article] [PubMed] [Google Scholar]
- 34.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–76. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 35.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 36.Ambrosio G, Zweier JL, Jacobus WE, Weisfeldt ML, Flaherty JT. Improvement of postischemic myocardial function and metabolism induced by administration of deferoxamine at the time of reflow: The role of iron in the pathogenesis of reperfusion injury. Circulation. 1987;76:906–15. doi: 10.1161/01.cir.76.4.906. [DOI] [PubMed] [Google Scholar]
- 37.Mewton N, Croisille P, Gahide G, Rioufol G, Bonnefoy E, Sanchez I, Cung TT, Sportouch C, Angoulvant D, Finet G, ndre-Fouet X, Derumeaux G, Piot C, Vernhet H, Revel D, Ovize M. Effect of cyclosporine on left ventricular remodeling after reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:1200–5. doi: 10.1016/j.jacc.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 38.Skyschally A, Schulz R, Heusch G. Cyclosporine A at reperfusion reduces infarct size in pigs. Cardiovasc Drugs Ther. 2010;24:85–7. doi: 10.1007/s10557-010-6219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–7. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsui T, Tao J, del MF, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–5. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 41.Yang XM, Krieg T, Cui L, Downey JM, Cohen MV. NECA and bradykinin at reperfusion reduce infarction in rabbit hearts by signaling through PI3K, ERK, and NO. J Mol Cell Cardiol. 2004;36:411–21. doi: 10.1016/j.yjmcc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–76. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 43.Wang JA, Chen TL, Jiang J, Shi H, Gui C, Luo RH, Xie XJ, Xiang MX, Zhang X. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol Sin. 2008;29:74–82. doi: 10.1111/j.1745-7254.2008.00716.x. [DOI] [PubMed] [Google Scholar]
- 44.Skyschally A, van CP, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G. Ischemic postconditioning in pigs: No causal role for RISK activation. Circ Res. 2009;104:15–8. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]